Hazardous Petroleum Sludge-Derived Nitrogen and Oxygen Co-Doped Carbon Material with Hierarchical Porous Structure for High-Performance All-Solid-State Supercapacitors
Abstract
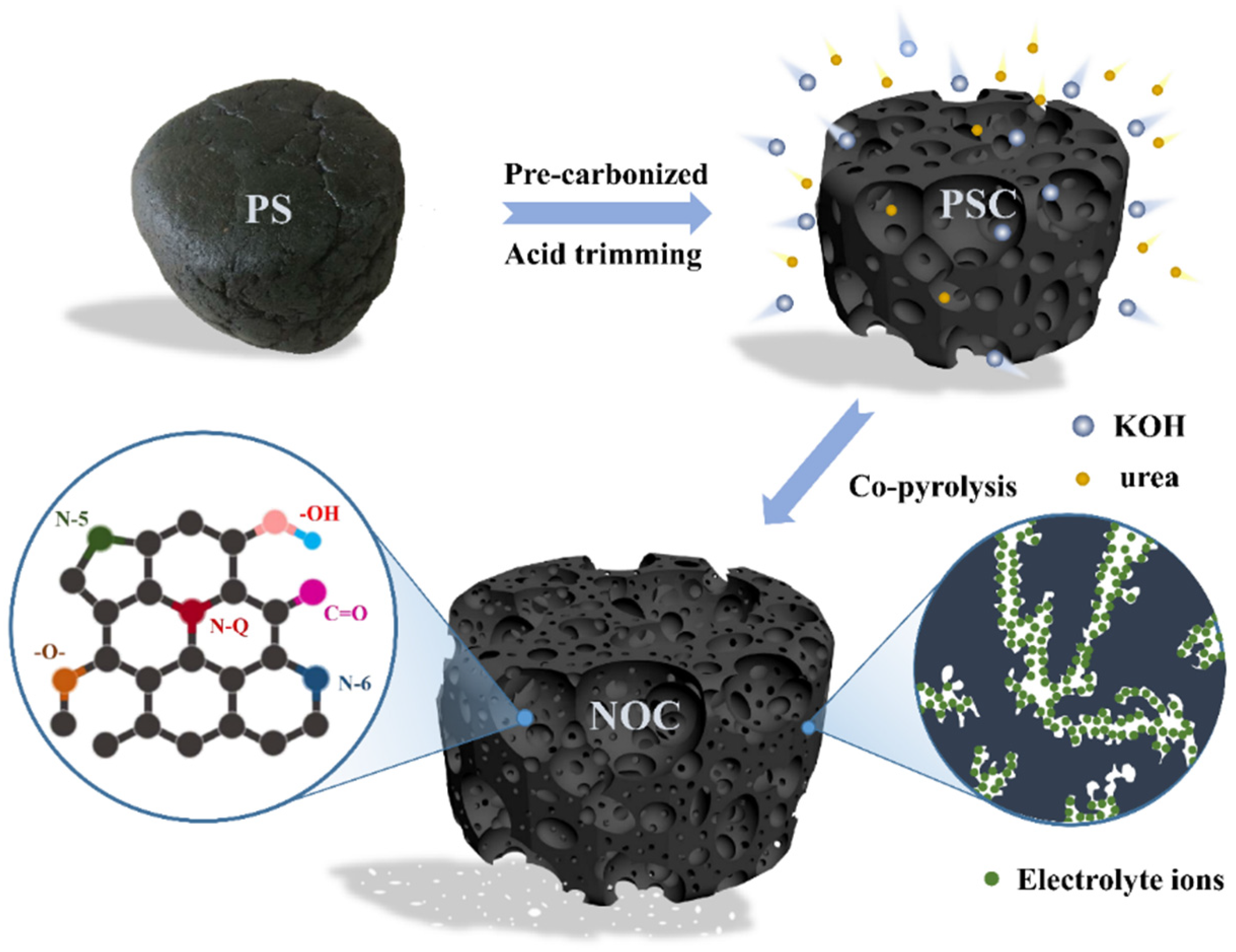
:1. Introduction
2. Materials and Methods
2.1. Materials Preparation
2.2. Electrochemical Test
3. Results
3.1. Structural Characterization
3.2. Capacitance Performance of the NOCs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lee, J.H.; Park, N.; Kim, B.G.; Jung, D.S.; Im, K.; Hur, J.; Choi, J.W. Restacking-Inhibited 3D Reduced Graphene Oxide for High Performance Supercapacitor Electrodes. ACS Nano 2013, 7, 9366–9374. [Google Scholar] [CrossRef]
- Ramakrishnan, P.; Shanmugam, S. Nitrogen-Doped Porous Multi-Nano-Channel Nanocarbons for Use in High-Performance Supercapacitor Applications. ACS Sustain. Chem. Eng. 2016, 4, 2439–2448. [Google Scholar] [CrossRef]
- Kim, N.D.; Buchholz, D.B.; Casillas, G.; Jose-Yacaman, M.; Chang, R.P.H. Hierarchical Design for Fabricating Cost-Effective High Performance Supercapacitors. Adv. Funct. Mater. 2014, 24, 4186–4194. [Google Scholar] [CrossRef]
- Yu, D.; Goh, K.; Zhang, Q.; Wei, L.; Wang, H.; Jiang, W.; Chen, Y. Controlled Functionalization of Carbonaceous Fibers for Asymmetric Solid-State Micro-Supercapacitors with High Volumetric Energy Density. Adv. Mater. 2014, 26, 6790–6797. [Google Scholar] [CrossRef]
- Zhang, L.L.; Zhao, X.S. Carbon-based materials as supercapacitor electrodes. Chem. Soc. Rev. 2009, 38, 2520–2531. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Zhang, L.; Zhang, J. A review of electrode materials for electrochemical supercapacitors. Chem. Soc. Rev. 2012, 41, 797–828. [Google Scholar] [PubMed] [Green Version]
- Hao, L.; Li, X.; Zhi, L. Carbonaceous Electrode Materials for Supercapacitors. Adv. Mater. 2013, 25, 3899–3904. [Google Scholar] [CrossRef]
- Sun, W.; Lipka, S.M.; Swartz, C.; Williams, D.; Yang, F. Hemp-derived activated carbons for supercapacitors. Carbon 2016, 103, 181–192. [Google Scholar]
- John, A.R.; Arumugam, P. Open ended nitrogen-doped carbon nanotubes for the electrochemical storage of energy in a supercapacitor electrode. J. Power Sources 2015, 277, 387–392. [Google Scholar]
- Bulusheva, L.G.; Fedorovskaya, E.O.; Kurenya, A.G.; Okotrub, A.V. Supercapacitor performance of nitrogen-doped carbon nanotube arrays. Phys. Status Solidi B 2013, 250, 2586–2591. [Google Scholar] [CrossRef]
- Chen, L.-F.; Zhang, X.-D.; Liang, H.-W.; Kong, M.; Guan, Q.-F.; Chen, P.; Wu, Z.-Y.; Yu, S.-H. Synthesis of Nitrogen-Doped Porous Carbon Nanofibers as an Efficient Electrode Material for Supercapacitors. ACS Nano 2012, 6, 7092–7102. [Google Scholar] [CrossRef] [PubMed]
- Zou, X.; Zhou, Y.; Wang, Z.; Chen, S.; Li, W.; Xiang, B.; Xu, L.; Zhu, S.; Hou, J. Free-standing, layered graphene monoliths for long-life supercapacitor. Chem. Eng. J. 2018, 350, 386–394. [Google Scholar] [CrossRef]
- Zhang, Y.; Wen, G.; Fan, S.; Ma, W.; Li, S.; Wu, T.; Yu, Z.; Zhao, B. Alcoholic hydroxyl functionalized partially reduced graphene oxides for symmetric supercapacitors with long-term cycle stability. Electrochim. Acta 2019, 313, 59–69. [Google Scholar] [CrossRef]
- Liu, B.-T.; Zhao, M.; Han, L.-P.; Lang, X.-Y.; Wen, Z.; Jiang, Q. Three-dimensional nanoporous N-doped graphene/iron oxides as anode materials for high-density energy storage in asymmetric supercapacitors. Chem. Eng. J. 2018, 335, 467–474. [Google Scholar] [CrossRef]
- Jia, S.; Zang, J.; Tian, P.; Zhou, S.; Cai, H.; Tian, X.; Wang, Y. A 3-D covalently crosslinked N-doped porous carbon/holey graphene composite for quasi-solid-state supercapacitors. Microporous Mesoporous Mater. 2020, 293, 109796. [Google Scholar] [CrossRef]
- Zhang, M.; Jin, X.; Wang, L.; Sun, M.; Tang, Y.; Chen, Y.; Sun, Y.; Yang, X.; Wan, P. Improving biomass-derived carbon by activation with nitrogen and cobalt for supercapacitors and oxygen reduction reaction. Appl. Surf. Sci. 2017, 411, 251–260. [Google Scholar] [CrossRef]
- Zeng, D.; Dou, Y.; Li, M.; Zhou, M.; Li, H.; Jiang, K.; Yang, F.; Peng, J. Wool fiber-derived nitrogen-doped porous carbon prepared from molten salt carbonization method for supercapacitor application. J. Mater. Sci. 2018, 53, 8372–8384. [Google Scholar] [CrossRef]
- Zhang, D.; Zhang, Y.; Luo, Y.; Zhang, Y.; Li, X.; Yu, X.; Ding, H.; Chu, P.K.; Sun, L. High-performance asymmetrical supercapacitor composed of rGO-enveloped nickel phosphite hollow spheres and N/S co-doped rGO aerogel. Nano Res. 2017, 11, 1651–1663. [Google Scholar] [CrossRef]
- Li, B.; Cheng, Y.; Dong, L.; Wang, Y.; Chen, J.; Huang, C.; Wei, D.; Feng, Y.; Jia, D.; Zhou, Y. Nitrogen doped and hierarchically porous carbons derived from chitosan hydrogel via rapid microwave carbonization for high-performance supercapacitors. Carbon 2017, 122, 592–603. [Google Scholar] [CrossRef]
- Wang, D.; Fang, G.; Xue, T.; Ma, J.; Geng, G. A melt route for the synthesis of activated carbon derived from carton box for high performance symmetric supercapacitor applications. J. Power Sources 2016, 307, 401–409. [Google Scholar] [CrossRef]
- Feng, H.; Zheng, M.; Dong, H.; Xiao, Y.; Hu, H.; Sun, Z.; Long, C.; Cai, Y.; Zhao, X.; Zhang, H.; et al. Three-dimensional honeycomb-like hierarchically structured carbon for high-performance supercapacitors derived from high-ash-content sewage sludge. J. Mater. Chem. A 2015, 3, 15225–15234. [Google Scholar] [CrossRef]
- Dutta, S.; Bhaumik, A.; Wu, K.C.-W. Hierarchically porous carbon derived from polymers and biomass: Effect of interconnected pores on energy applications. Energy Environ. Sci. 2014, 7, 3574–3592. [Google Scholar] [CrossRef]
- Huang, G.; Geng, Q.; Kang, W.; Liu, Y.; Li, Y.; Xing, B.; Liu, Q.; Zhang, C. Hierarchical porous carbon with optimized mesopore structure and nitrogen doping for supercapacitor electrodes. Microporous Mesoporous Mater. 2019, 288, 109576. [Google Scholar] [CrossRef]
- Hu, F.; Wang, J.; Hu, S.; Li, L.; Shao, W.; Qiu, J.; Lei, Z.; Deng, W.; Jian, X. Engineered Fabrication of Hierarchical Frameworks with Tuned Pore Structure and N,O-Co-Doping for High-Performance Supercapacitors. ACS Appl. Mater. Interfaces 2017, 9, 31940–31949. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Dou, H.; Geng, X.; Han, J.; Chen, L.; Zhu, H. Free standing three-dimensional nitrogen-doped carbon nanowire array for high-performance supercapacitors. Chem. Eng. J. 2017, 308, 222–228. [Google Scholar] [CrossRef]
- Ye, Z.; Wang, F.; Jia, C.; Shao, Z. Biomass-based O, N-codoped activated carbon aerogels with ultramicropores for supercapacitors. J. Mater. Sci. 2018, 53, 12374–12387. [Google Scholar] [CrossRef]
- Zhan, C.; Zhang, P.; Dai, S.; Jiang, D.-E. Boron Supercapacitors. ACS Energy Lett. 2016, 1, 1241–1246. [Google Scholar] [CrossRef]
- Yu, X.; Park, S.K.; Yeon, S.-H.; Park, H.S. Three-dimensional, sulfur-incorporated graphene aerogels for the enhanced performances of pseudocapacitive electrodes. J. Power Sources 2015, 278, 484–489. [Google Scholar] [CrossRef]
- Peng, C.; Zeng, T.; Yu, Y.; Li, Z.; Kuai, Z.; Zhao, W. Fluorine and oxygen co-doped porous carbons derived from third-class red dates for high-performance symmetrical supercapacitors. J. Mater. Sci. Mater. Electron. 2018, 29, 18674–18683. [Google Scholar] [CrossRef]
- Chen, J.; Wei, H.; Chen, H.; Yao, W.; Lin, H.; Han, S. N/P co-doped hierarchical porous carbon materials for superior performance supercapacitors. Electrochim. Acta 2018, 271, 49–57. [Google Scholar] [CrossRef]
- Tang, C.; Liu, Y.; Yang, D.; Yang, M.; Li, H. Oxygen and nitrogen co-doped porous carbons with finely-layered schistose structure for high-rate-performance supercapacitors. Carbon 2017, 122, 538–546. [Google Scholar] [CrossRef]
- Yang, Z.; Ren, J.; Zhang, Z.; Chen, X.; Guan, G.; Qiu, L.; Zhang, Y.; Peng, H. Recent Advancement of Nanostructured Carbon for Energy Applications. Chem. Rev. 2015, 115, 5159–5223. [Google Scholar] [CrossRef]
- Hu, G.; Li, J.; Zeng, G. Recent development in the treatment of oily sludge from petroleum industry: A review. J. Hazard. Mater. 2013, 261, 470–490. [Google Scholar] [CrossRef]
- Wang, J.; Sun, C.; Lin, B.-C.; Huang, Q.-X.; Ma, Z.-Y.; Chi, Y.; Yan, J.-H. Micro- and mesoporous-enriched carbon materials prepared from a mixture of petroleum-derived oily sludge and biomass. Fuel Process. Technol. 2018, 171, 140–147. [Google Scholar] [CrossRef]
- Meng, F.; Gong, Z.; Wang, Z.; Fang, P.; Li, X. Study on a nitrogen-doped porous carbon from oil sludge for CO2 adsorption. Fuel 2019, 251, 562–571. [Google Scholar] [CrossRef]
- Li, X.; Liu, K.; Liu, Z.; Wang, Z.; Li, B.; Zhang, D. Hierarchical porous carbon from hazardous waste oily sludge for all-solid-state flexible supercapacitor. Electrochim. Acta 2017, 240, 43–52. [Google Scholar] [CrossRef]
- Zhang, W.; Yu, C.; Chang, L.; Zhong, W.; Yang, W. Three-dimensional nitrogen-doped hierarchical porous carbon derived from cross-linked lignin derivatives for high performance supercapacitors. Electrochim. Acta 2018, 282, 642–652. [Google Scholar] [CrossRef]
- Wu, D.; Cheng, J.; Wang, T.; Liu, P.; Yang, L.; Jia, D. A Novel Porous N- and S-Self-Doped Carbon Derived from Chinese Rice Wine Lees as High-Performance Electrode Materials in a Supercapacitor. ACS Sustain. Chem. Eng. 2019, 7, 12138–12147. [Google Scholar] [CrossRef]
- Liu, F.; Wang, Z.; Zhang, H.; Jin, L.; Chu, X.; Gu, B.; Huang, H.; Yang, W. Nitrogen, oxygen and sulfur co-doped hierarchical porous carbons toward high-performance supercapacitors by direct pyrolysis of kraft lignin. Carbon 2019, 149, 105–116. [Google Scholar] [CrossRef]
- Rehman, A.; Park, S.-J. Tunable nitrogen-doped microporous carbons: Delineating the role of optimum pore size for enhanced CO2 adsorption. Chem. Eng. J. 2019, 362, 731–742. [Google Scholar] [CrossRef]
- Liu, B.; Liu, Y.; Chen, H.; Yang, M.; Li, H. Oxygen and nitrogen co-doped porous carbon nanosheets derived from Perilla frutescens for high volumetric performance supercapacitors. J. Power Sources 2017, 341, 309–317. [Google Scholar] [CrossRef]
- Sharifi, T.; Hu, G.; Jia, X.; Wågberg, T. Formation of Active Sites for Oxygen Reduction Reactions by Transformation of Nitrogen Functionalities in Nitrogen-Doped Carbon Nanotubes. ACS Nano 2012, 6, 8904–8912. [Google Scholar] [CrossRef]
- Zhou, Y.; Ren, J.; Xia, L.; Zheng, Q.; Liao, J.; Long, E.; Xie, F.; Xu, C.; Lin, D. Waste soybean dreg-derived N/O co-doped hierarchical porous carbon for high performance supercapacitor. Electrochim. Acta 2018, 284, 336–345. [Google Scholar] [CrossRef]
- Zhou, Y.; Ren, J.; Yang, Y.; Zheng, Q.; Liao, J.; Xie, F.; Jie, W.; Lin, D. Biomass-derived nitrogen and oxygen co-doped hierarchical porous carbon for high performance symmetric supercapacitor. J. Solid State Chem. 2018, 268, 149–158. [Google Scholar] [CrossRef]
- Shao, J.; Ma, F.; Wu, G.; Geng, W.; Song, S.; Wan, J.; Ma, D. Facile Preparation of 3D Nanostructured O/N co-Doped Porous Carbon Constructed by Interconnected Carbon Nanosheets for Excellent-Performance supercapacitors. Electrochim. Acta 2016, 222, 793–805. [Google Scholar] [CrossRef]
- Zhou, J.-H.; Sui, Z.-J.; Zhu, J.; Li, P.; Chen, D.; Dai, Y.-C.; Yuan, W.-K. Characterization of surface oxygen complexes on carbon nanofibers by TPD, XPS and FT-IR. Carbon 2007, 45, 785–796. [Google Scholar] [CrossRef]
- Lu, Y.; Chen, N.; Bai, Z.; Mi, H.; Ji, C.; Sun, L. Acid-Assisted Strategy Combined with KOH Activation to Efficiently Optimize Carbon Architectures from Green Copolymer Adhesive for Solid-State Supercapacitors. ACS Sustain. Chem. Eng. 2018, 6, 14838–14846. [Google Scholar] [CrossRef]
- Wang, X.; Liu, Y.; Wu, P. Water-soluble triphenylphosphine-derived microgel as the template towards in-situ nitrogen, phosphorus co-doped mesoporous graphene framework for supercapacitor and electrocatalytic oxygen reduction. Chem. Eng. J. 2017, 328, 417–427. [Google Scholar] [CrossRef]
- Liu, M.; Huo, S.; Xu, M.; Wu, L.; Liu, M.; Xue, Y.; Yan, Y.-M. Structural engineering of N/S co-doped carbon material as high-performance electrode for supercapacitors. Electrochim. Acta 2018, 274, 389–399. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, J.; Hou, Q.; Wang, S. Utilization of nutrient rich duckweed to create N, P Co-doped porous carbons for high performance supercapacitors. J. Alloy. Compd. 2019, 771, 1009–1017. [Google Scholar] [CrossRef]
- Wang, Q.; Qin, B.; Zhang, X.; Xie, X.; Jin, L.; Cao, Q. Synthesis of N-doped carbon nanosheets with controllable porosity derived from bio-oil for high-performance supercapacitors. J. Mater. Chem. A 2018, 6, 19653–19663. [Google Scholar] [CrossRef]
- Ghodbane, O.; Pascal, J.L.; Favier, F. Microstructural effects on charge-storage properties in MnO2-based electrochemical supercapacitors. ACS Appl. Mater. Interfaces 2009, 1, 1130–1139. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Cui, X.; Chen, W.; Ivey, D.G. Manganese oxide-based materials as electrochemical supercapacitor electrodes. Chem. Soc. Rev. 2011, 40, 1697–1721. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Zheng, D.; Zhai, T.; Liu, Z.; Huang, Y.; Xie, S.; Tong, Y. Facile synthesis of large-area manganese oxide nanorod arrays as a high-performance electrochemical supercapacitor. Energy Environ. Sci. 2011, 4, 2915–2921. [Google Scholar] [CrossRef]
- Xiao, K.; Ding, L.-X.; Chen, H.; Wang, S.; Lu, X.; Wang, H. Nitrogen-doped porous carbon derived from residuary shaddock peel: A promising and sustainable anode for high energy density asymmetric supercapacitors. J. Mater. Chem. A 2016, 4, 372–378. [Google Scholar] [CrossRef]
- Wang, N.; Wang, C.; He, L.; Wang, Y.; Hu, W.; Komarneni, S. Incomplete phase separation strategy to synthesize P/N co-doped porous carbon with interconnected structure for asymmetric supercapacitors with ultra-high power density. Electrochim. Acta 2019, 298, 717–725. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, M.; Pan, D.; Li, Y.; Ma, T.; Xie, J. Nitrogen/sulfur co-doped graphene networks uniformly coupled N-Fe2O3 nanoparticles achieving enhanced supercapacitor performance. Electrochim. Acta 2018, 266, 242–253. [Google Scholar] [CrossRef]
- Le, Q.J.; Huang, M.; Wang, T.; Liu, X.Y.; Sun, L.; Guo, X.L.; Jiang, D.B.; Wang, J.; Dong, F.; Zhang, Y.X. Biotemplate derived three dimensional nitrogen doped graphene@MnO2 as bifunctional material for supercapacitor and oxygen reduction reaction catalyst. J. Colloid Interface Sci. 2019, 544, 155–163. [Google Scholar] [CrossRef]
- Huang, X.; Gou, L.; Yang, L. Enhancement in performance of negative electrode of supercapacitor based on nitrogen doped porous carbon spheres. J. Alloys Compd. 2019, 786, 91–97. [Google Scholar] [CrossRef]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Zhang, M.; Tan, Z.; Gong, Z.; Liu, P.; Wang, Z. Hazardous Petroleum Sludge-Derived Nitrogen and Oxygen Co-Doped Carbon Material with Hierarchical Porous Structure for High-Performance All-Solid-State Supercapacitors. Materials 2021, 14, 2477. https://doi.org/10.3390/ma14102477
Li X, Zhang M, Tan Z, Gong Z, Liu P, Wang Z. Hazardous Petroleum Sludge-Derived Nitrogen and Oxygen Co-Doped Carbon Material with Hierarchical Porous Structure for High-Performance All-Solid-State Supercapacitors. Materials. 2021; 14(10):2477. https://doi.org/10.3390/ma14102477
Chicago/Turabian StyleLi, Xiaoyu, Mingyang Zhang, Zhuowei Tan, Zhiqiang Gong, Peikun Liu, and Zhenbo Wang. 2021. "Hazardous Petroleum Sludge-Derived Nitrogen and Oxygen Co-Doped Carbon Material with Hierarchical Porous Structure for High-Performance All-Solid-State Supercapacitors" Materials 14, no. 10: 2477. https://doi.org/10.3390/ma14102477






