Oscillatory Behaviour of Ni Supported on ZrO2 in the Catalytic Partial Oxidation of Methane as Determined by Activation Procedure
Abstract
1. Introduction
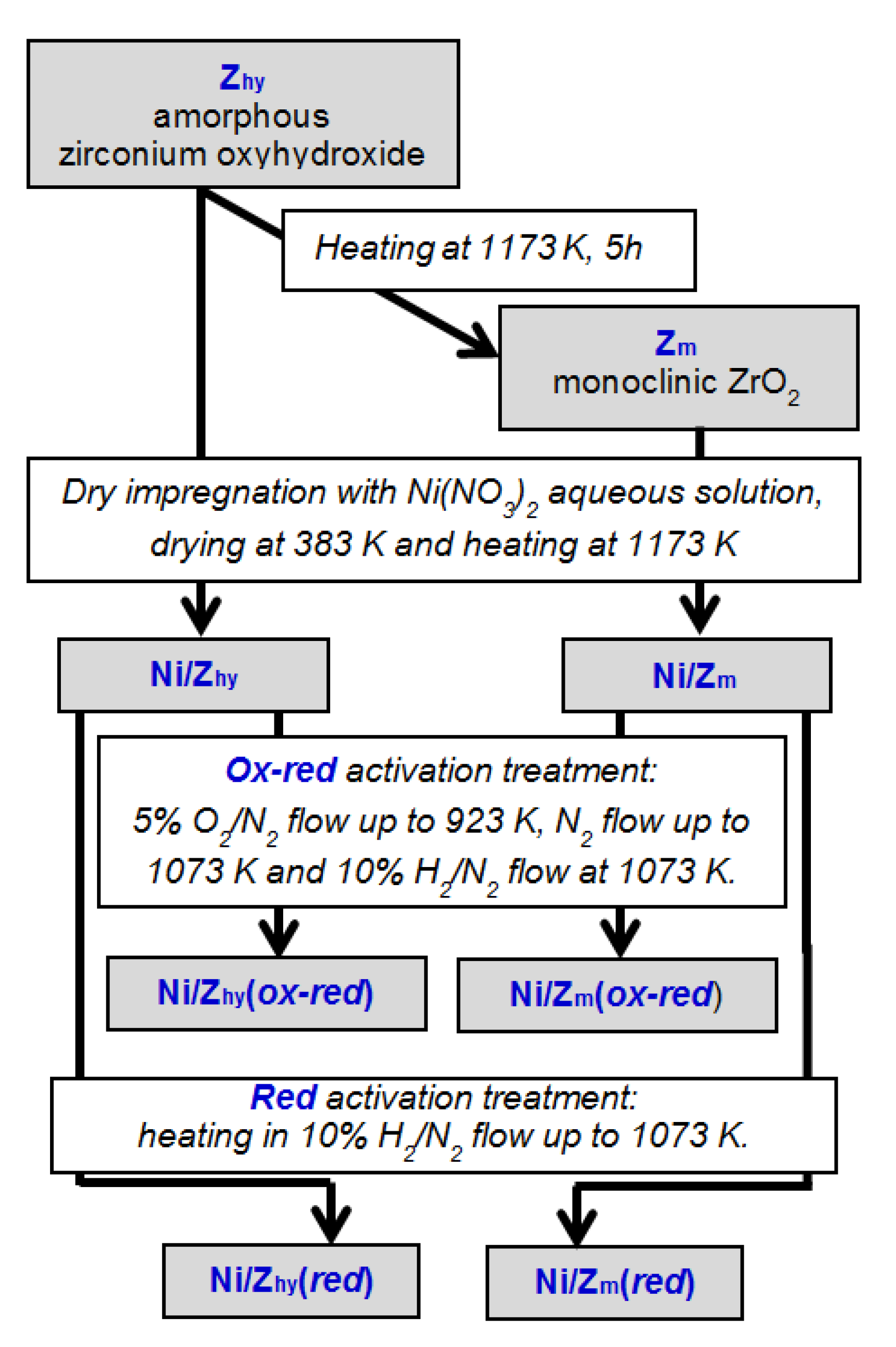
2. Materials and Methods
2.1. Materials
2.2. Characterization
2.3. Catalytic Activity
3. Results and Discussion
3.1. Characterization
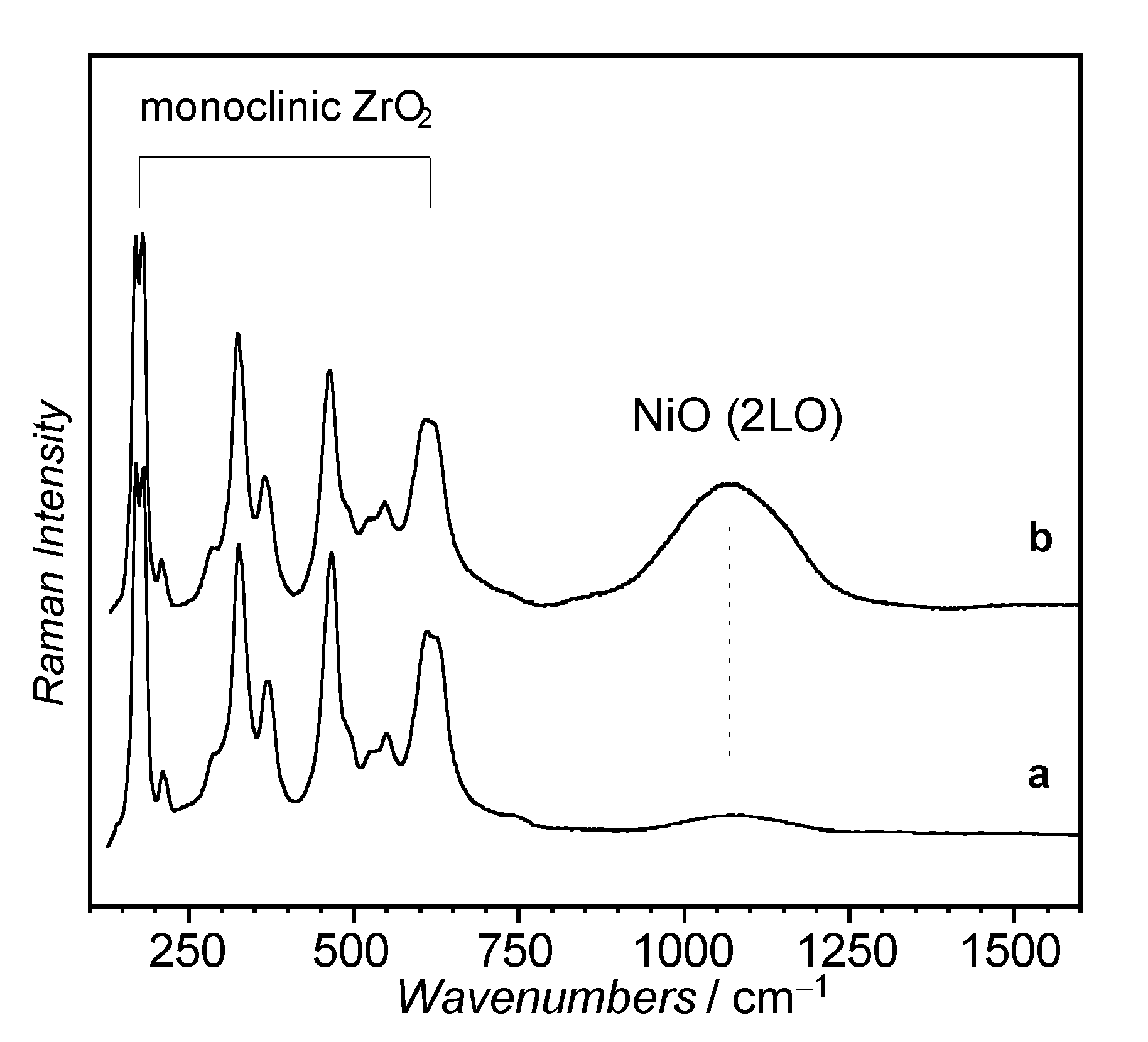
3.1.1. Zirconium Oxide Support
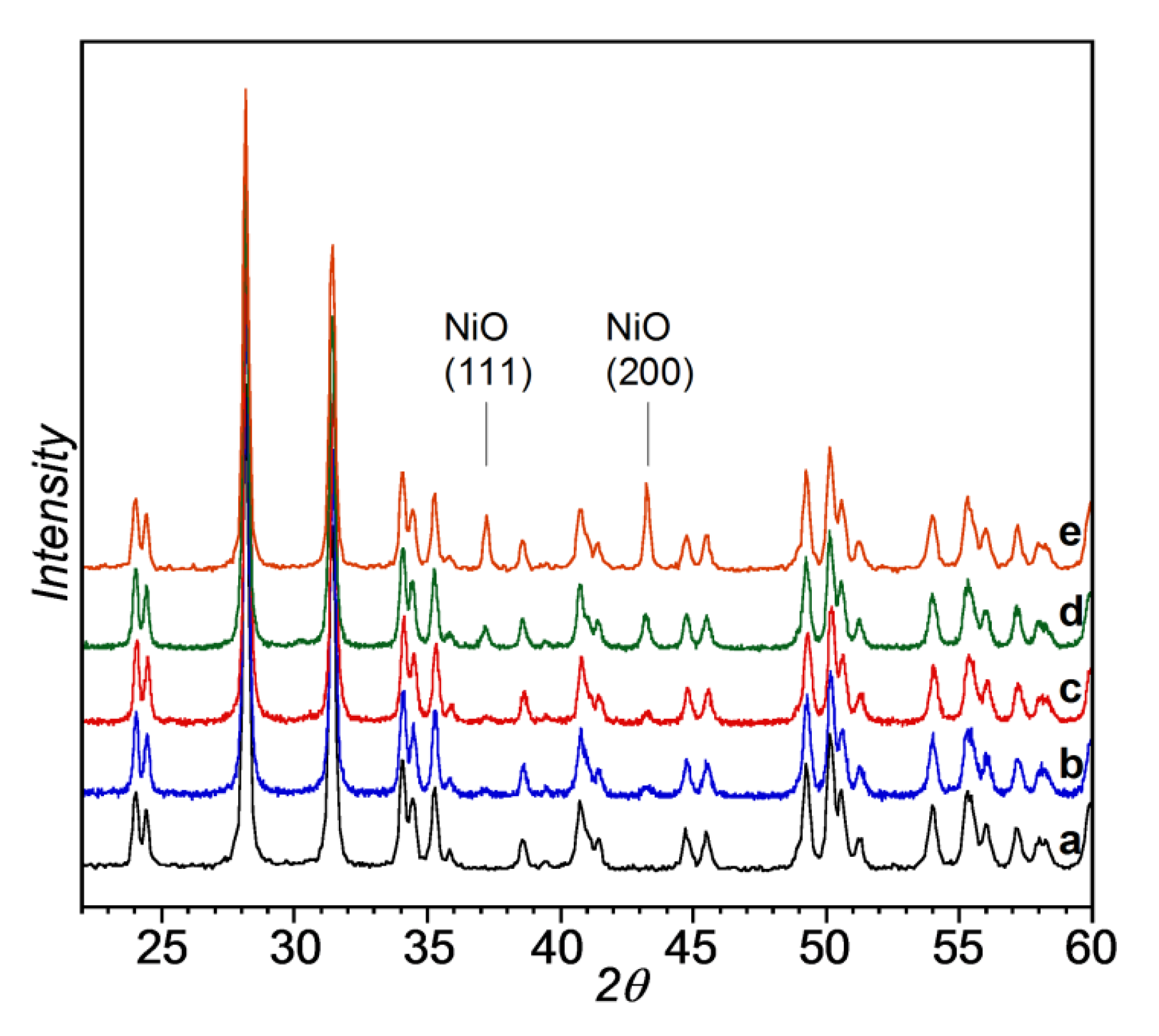
3.1.2. NiO/ZrO2 Catalyst Precursors
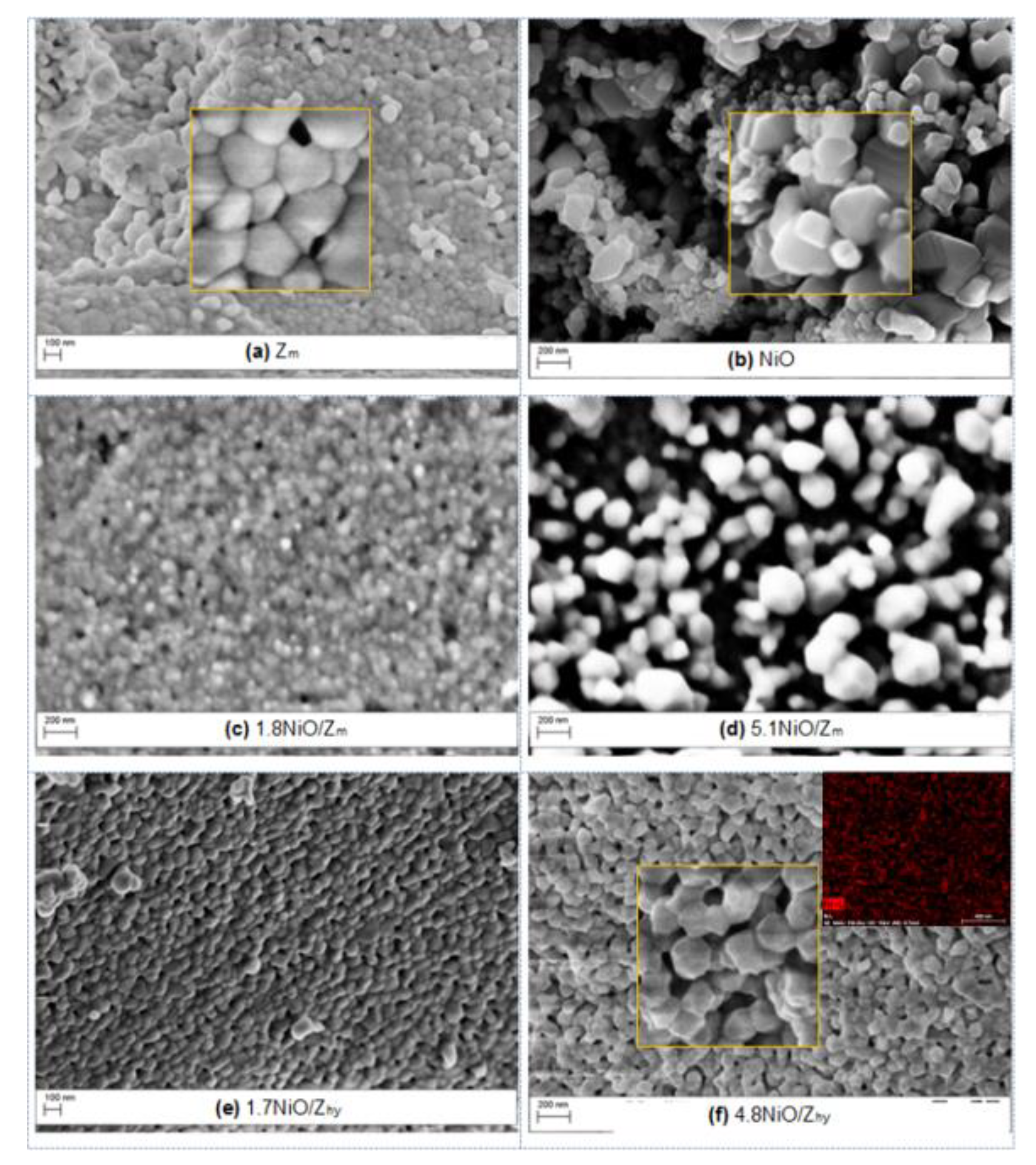
3.1.3. Ni/ZrO2 Catalysts
3.1.4. Dispersion of Ni in the Ni/ZrO2 Catalysts
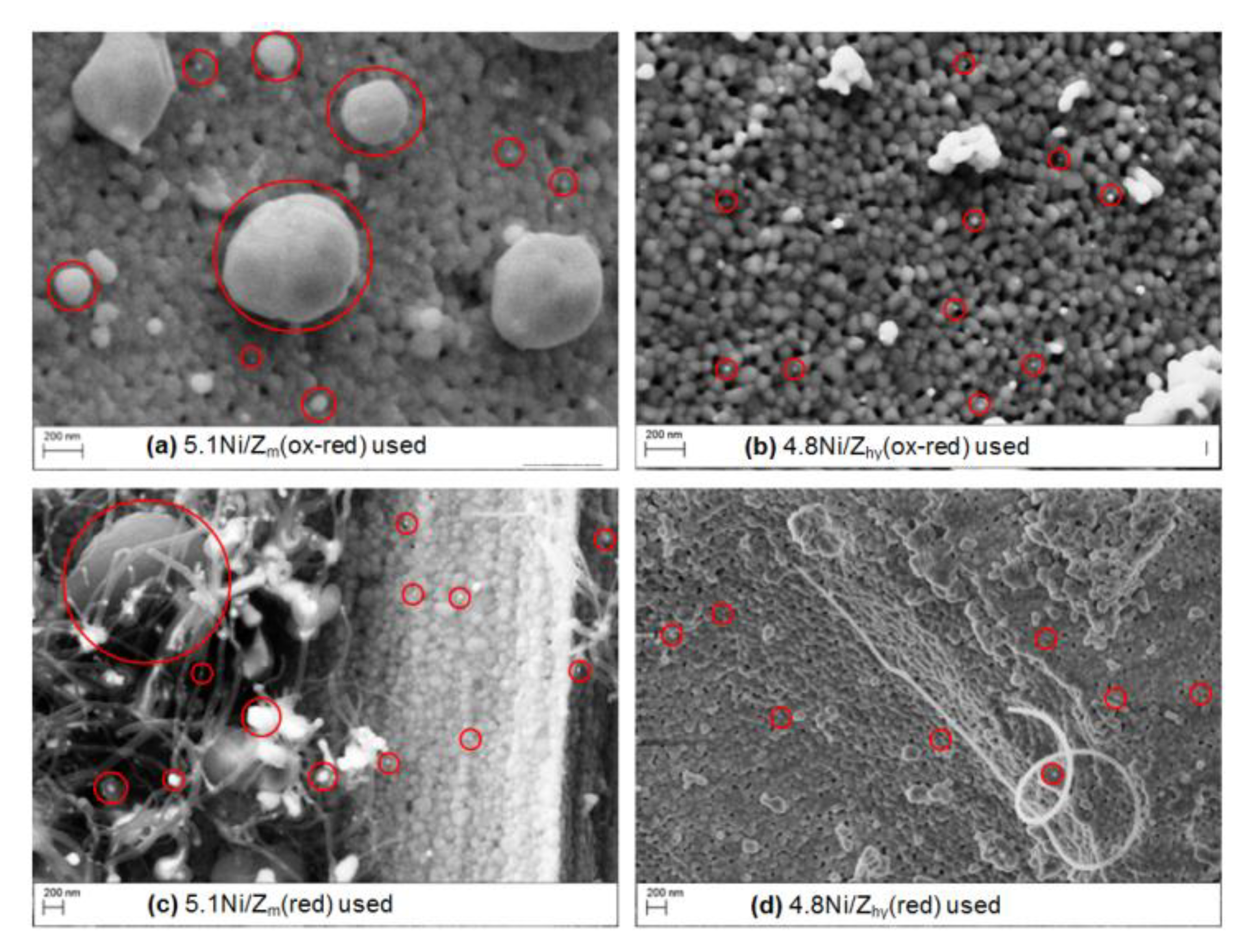
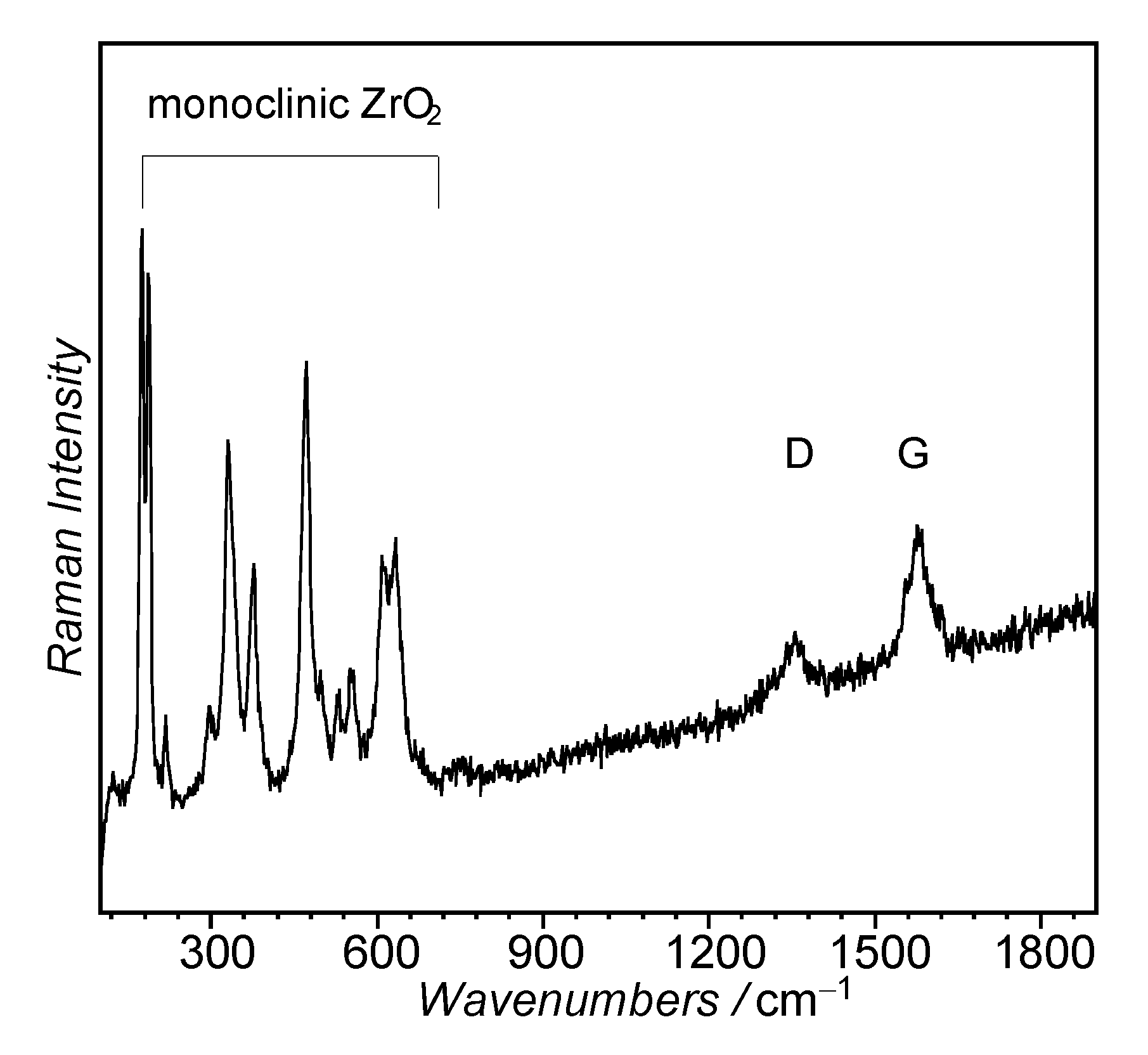
3.1.5. Ni/ZrO2 Used Catalysts
3.2. Catalytic Activity
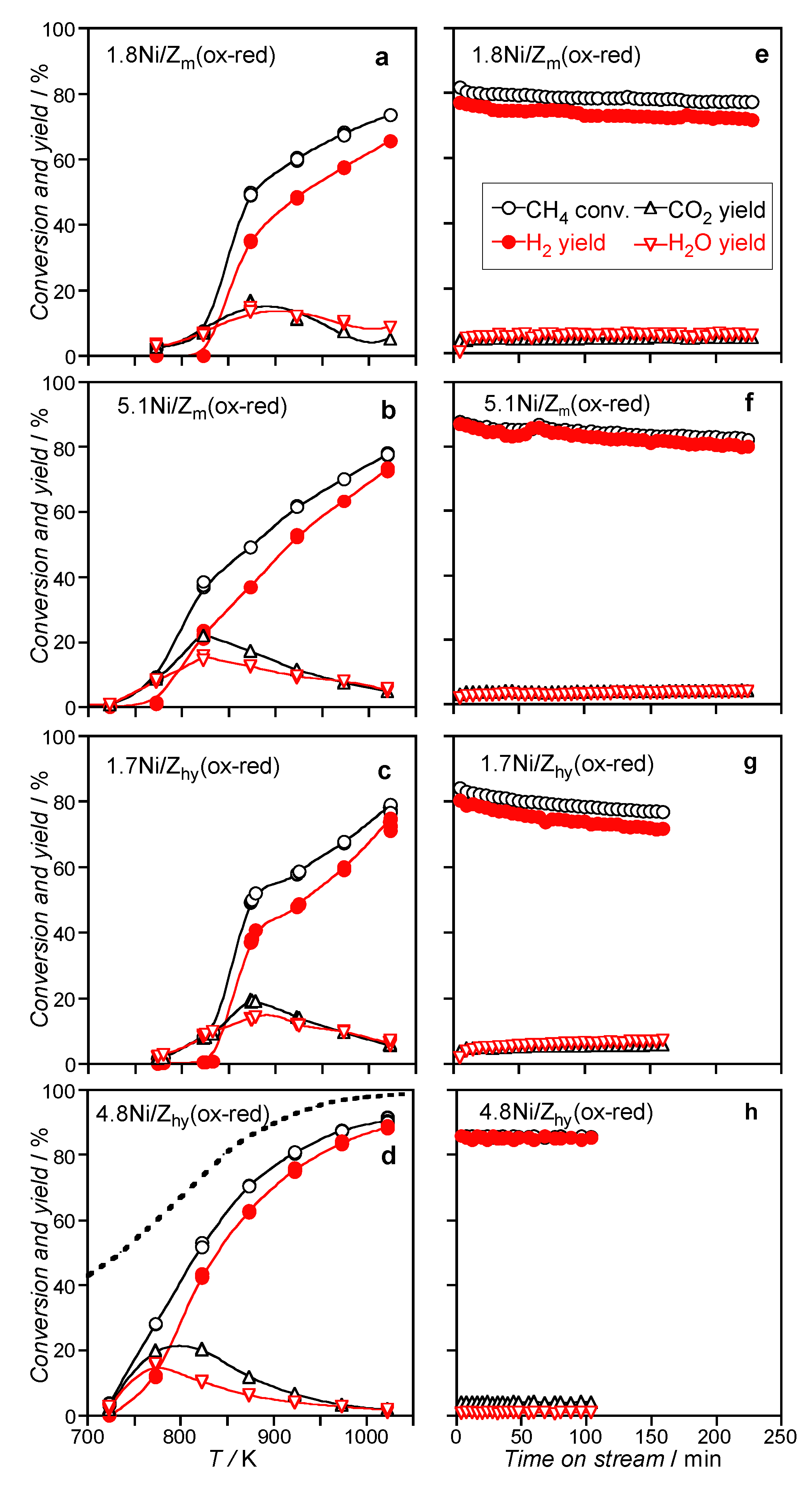
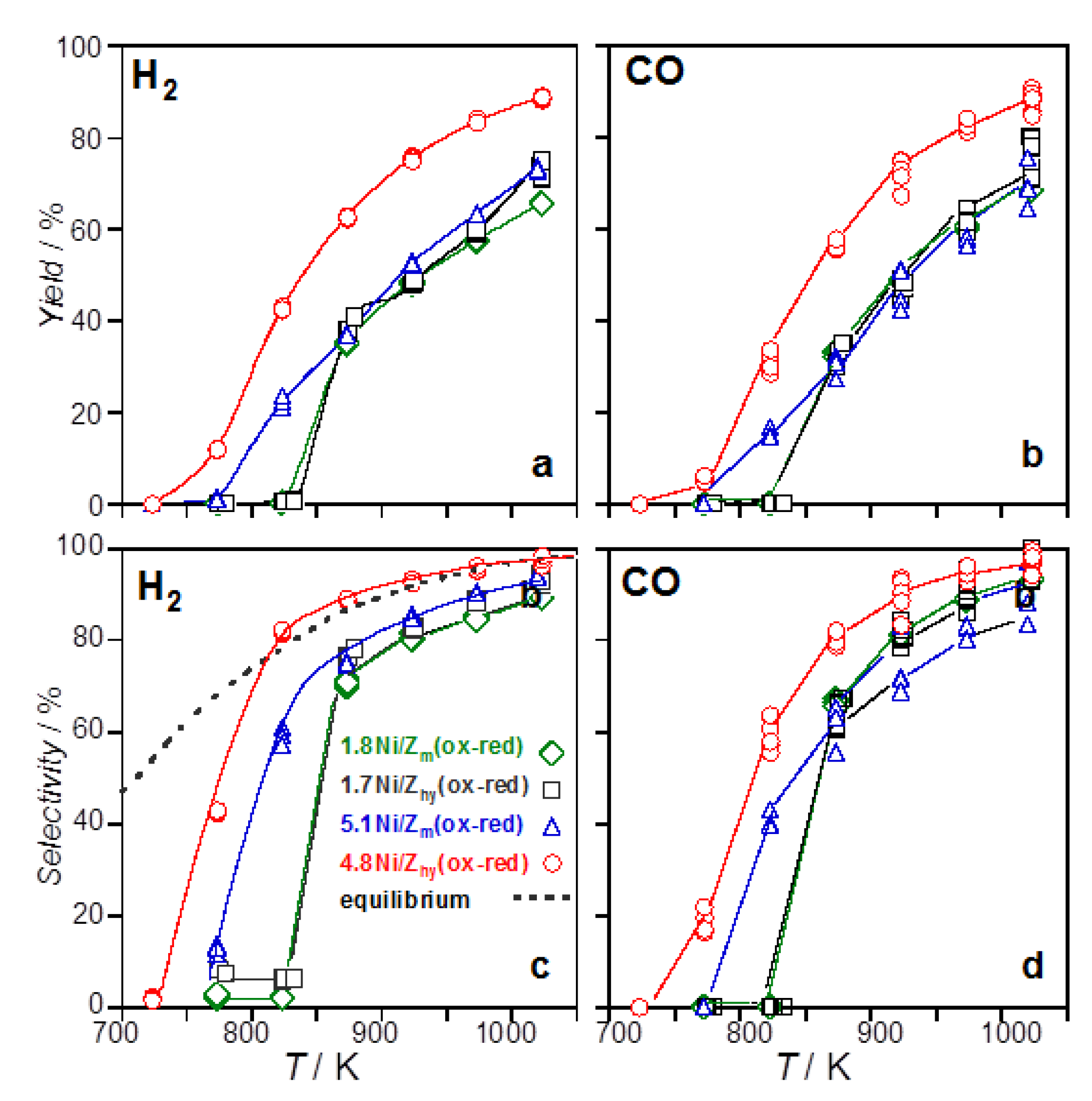
3.2.1. Catalysts Activated by Oxidation–Reduction Treatment
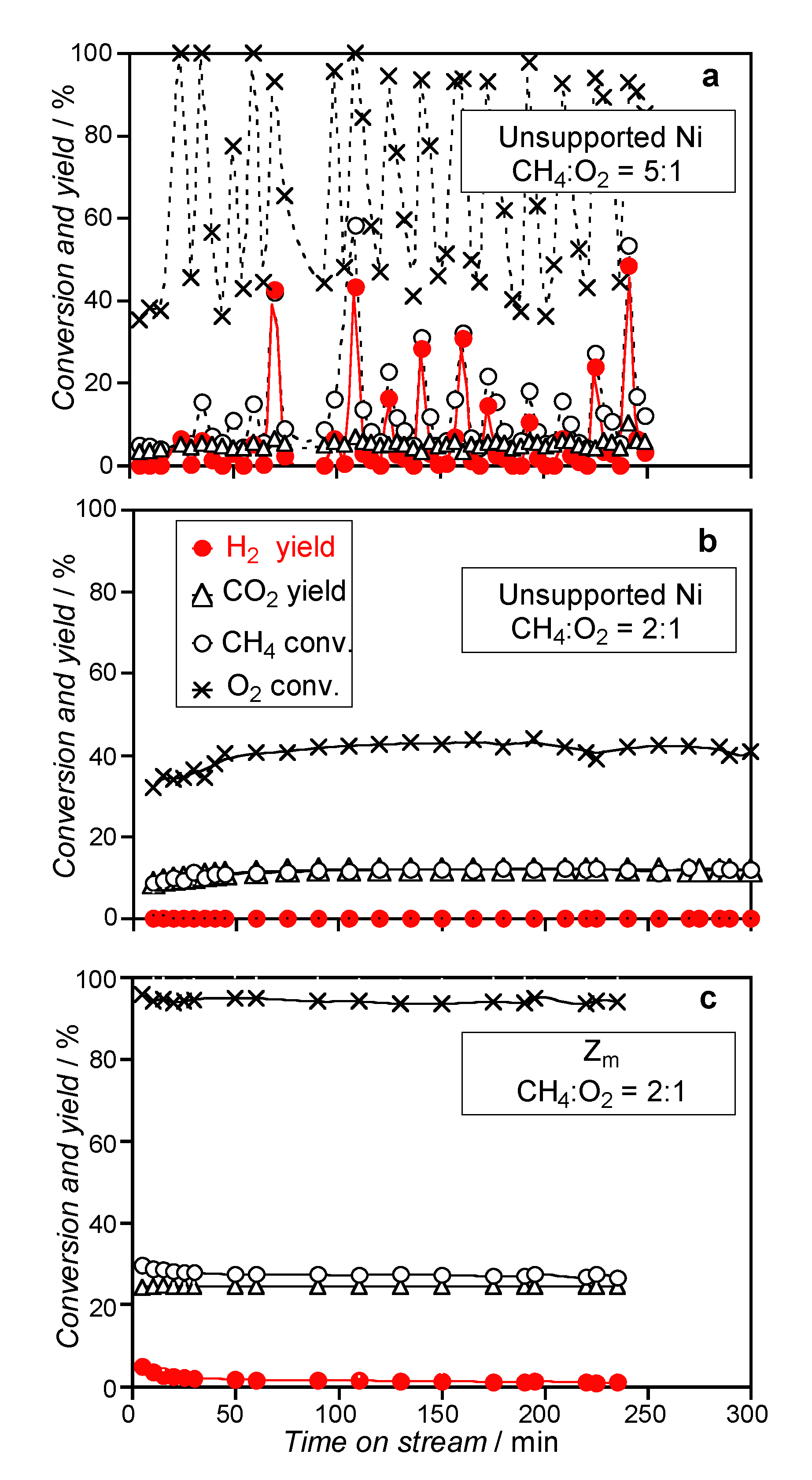
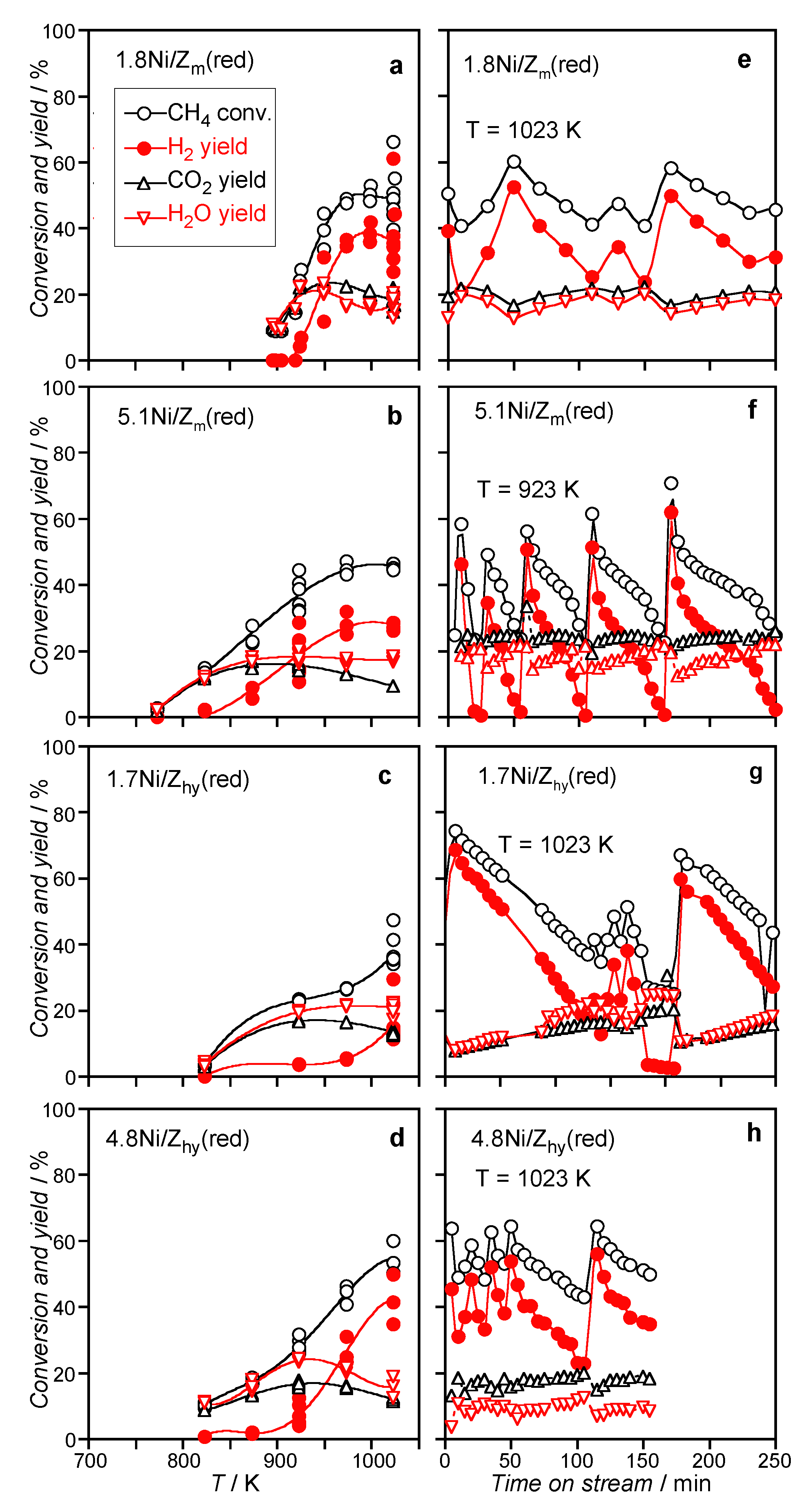
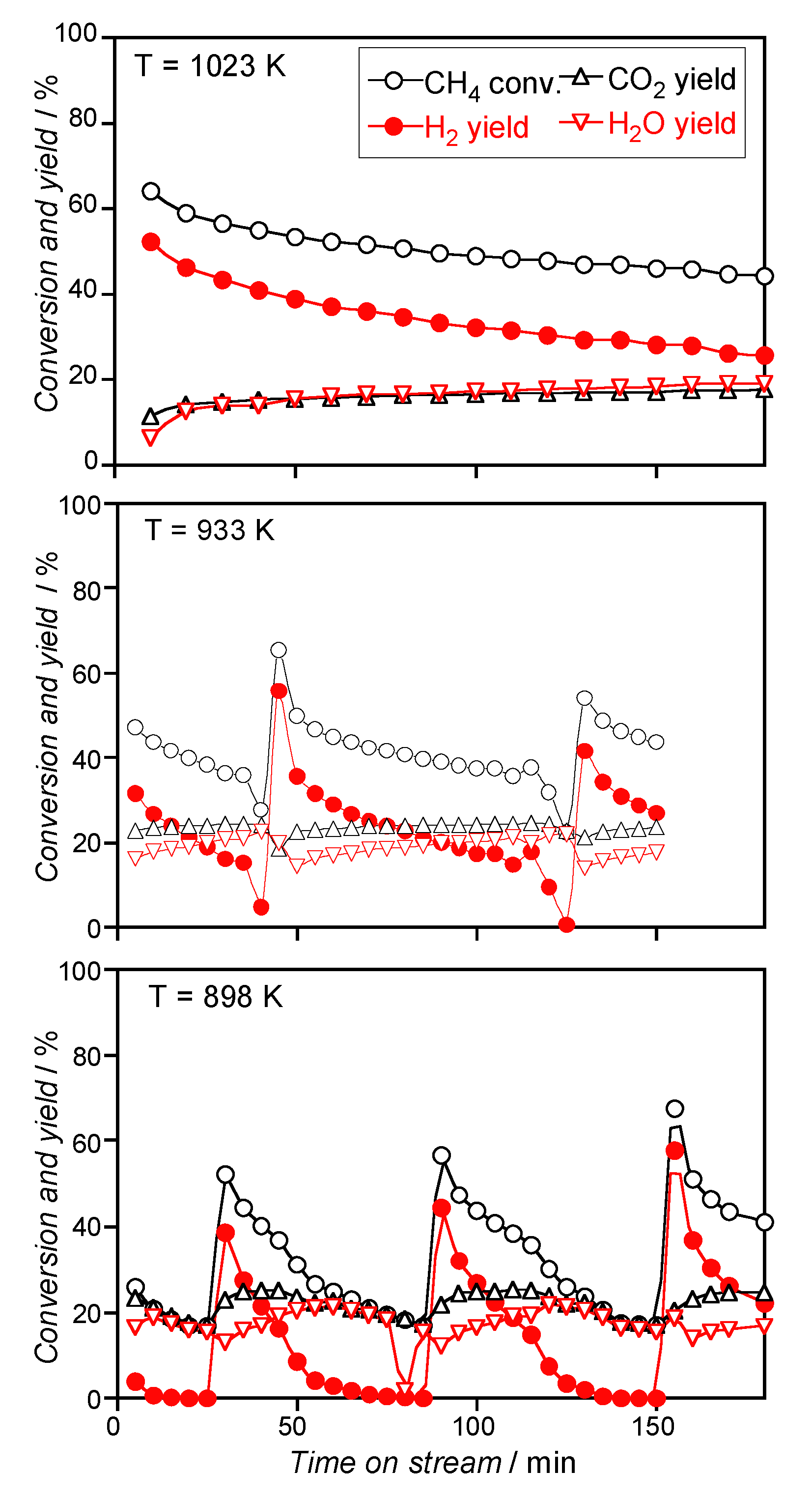
3.2.2. Catalysts Activated by Reduction Treatment
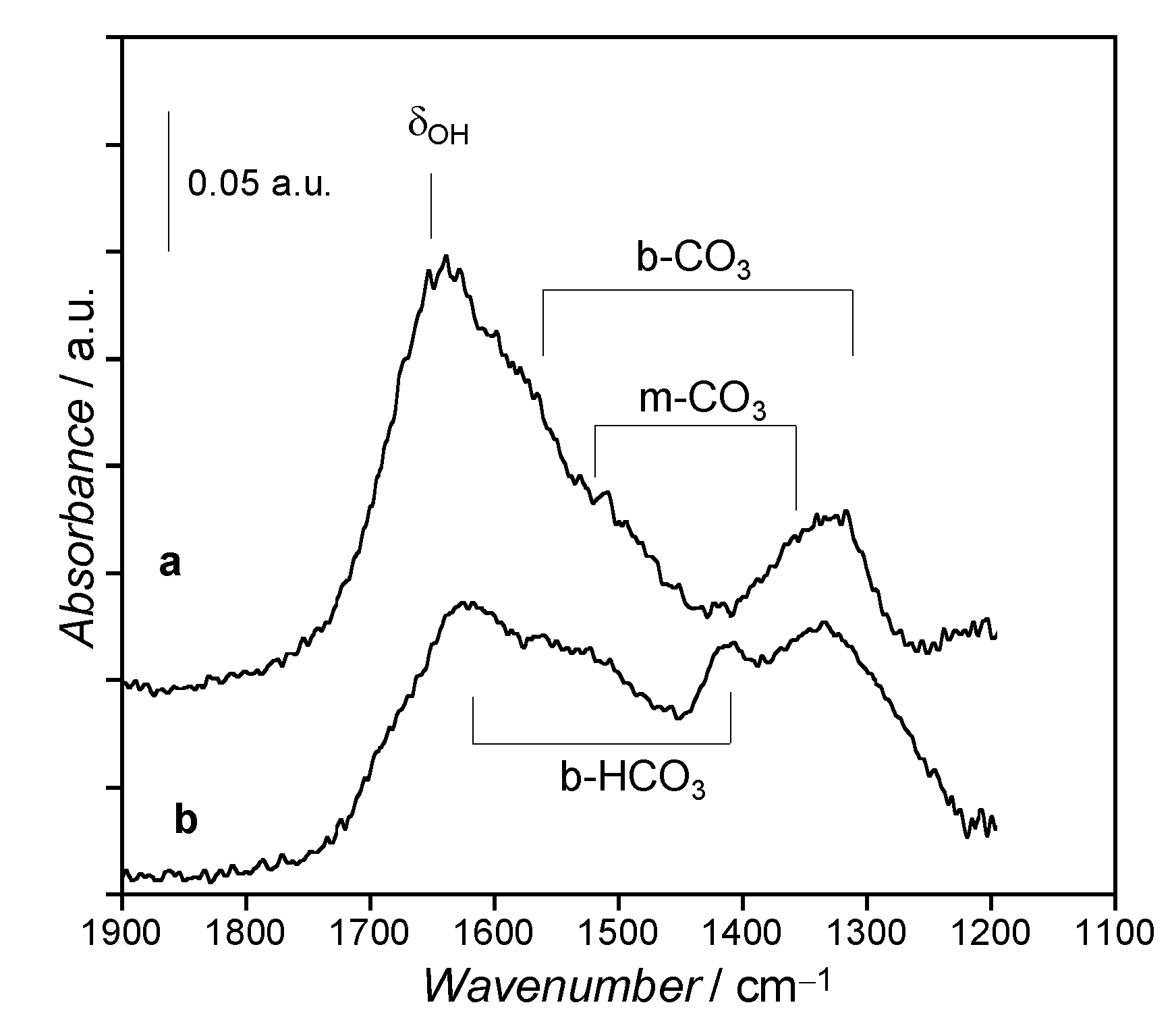
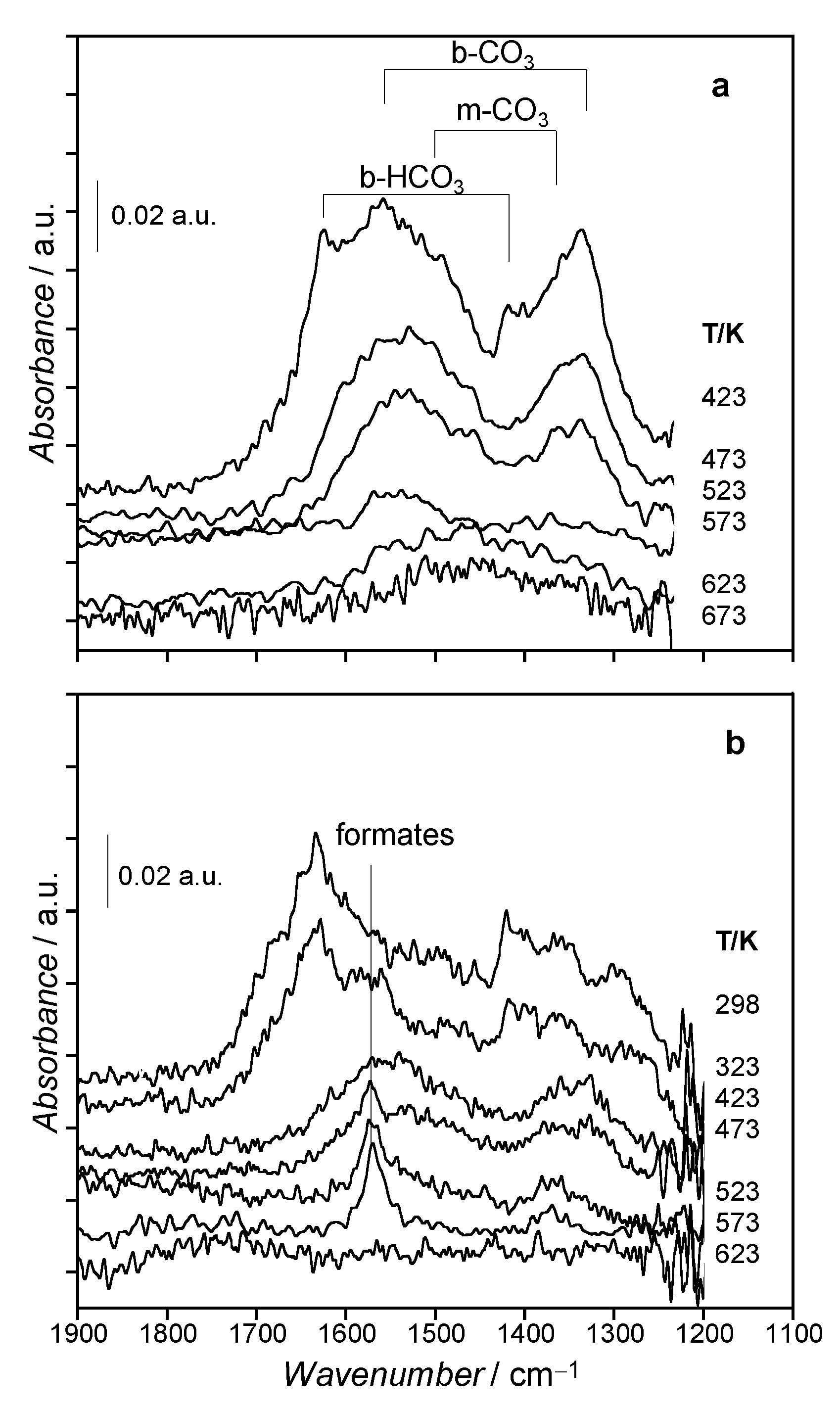
3.3. Surface Species Evolution during Activation Treatments by in situ FTIR
3.4. Hypothesis on Active Sites for the CH4-CPO
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Faramawy, S.; Zaki, T.; Sakr, A.-E. Natural gas origin, composition, and processing: A review. J. Nat. Gas. Sci. Eng. 2016, 34, 34–54. [Google Scholar] [CrossRef]
- Olajire, A.A. Valorization of greenhouse carbon dioxide emissions into value-added products by catalytic processes. J. CO2 Util. 2013, 3-4, 74–92. [Google Scholar] [CrossRef]
- Horn, R.; Schlögl, R. Methane Activation by Heterogeneous Catalysis. Catal. Lett. 2015, 145, 23–39. [Google Scholar] [CrossRef]
- Pen, M.A.; Gomez, J.; Fierro, J. New catalytic routes for syngas and hydrogen production. Appl. Catal. A: Gen. 1996, 144, 7–57. [Google Scholar] [CrossRef]
- Al-Sayari, S.A. Recent Developments in the Partial Oxidation of Methane to Syngas. Open Catal. J. 2013, 6, 17–28. [Google Scholar] [CrossRef]
- Ghoneim, S.A.; El-Salamony, R.A.; El-Temtamy, S.A. Review on Innovative Catalytic Reforming of Natural Gas to Syngas. World J. Eng. Technol. 2016, 4, 116–139. [Google Scholar] [CrossRef]
- Aasberg-Petersen, K.; Dybkjær, I.; Ovesen, C.; Schjødt, N.; Sehested, J.; Thomsen, S. Natural gas to synthesis gas – Catalysts and catalytic processes. J. Nat. Gas. Sci. Eng. 2011, 3, 423–459. [Google Scholar] [CrossRef]
- Rostrup-Nielsen, J. Steam reforming and chemical recuperation. Catal. Today 2009, 145, 72–75. [Google Scholar] [CrossRef]
- Meloni, E.; Martino, M.; Palma, V. A Short Review on Ni Based Catalysts and Related Engineering Issues for Methane Steam Reforming. Catalysts 2020, 10, 352. [Google Scholar] [CrossRef]
- Elbadawi, A.H.; Ge, L.; Li, Z.; Liu, S.; Wang, S.; Zhu, Z. Catalytic partial oxidation of methane to syngas: Review of perovskite catalysts and membrane reactors. Catal. Rev. 2021, 63, 1–67. [Google Scholar] [CrossRef]
- Osman, A.I. Catalytic Hydrogen Production from Methane Partial Oxidation: Mechanism and Kinetic Study. Chem. Eng. Technol. 2020, 43, 641–648. [Google Scholar] [CrossRef]
- Choudhary, T.V.; Choudhary, V.R. Energy-Efficient Syngas Production through Catalytic Oxy-Methane Reforming Reactions. Angew. Chem. Int. Ed. 2008, 47, 1828–1847. [Google Scholar] [CrossRef] [PubMed]
- Enger, B.C.; Lødeng, R.; Holmen, A. A review of catalytic partial oxidation of methane to synthesis gas with emphasis on reaction mechanisms over transition metal catalysts. Appl. Catal. A Gen. 2008, 346, 1–27. [Google Scholar] [CrossRef]
- Campa, M.; Ferraris, G.; Gazzoli, D.; Pettiti, I.; Pietrogiacomi, D. Rhodium supported on tetragonal or monoclinic ZrO2 as catalyst for the partial oxidation of methane. Appl. Catal. B Environ. 2013, 142-143, 423–431. [Google Scholar] [CrossRef]
- Liu, H.; He, D. Recent Progress on Ni-Based Catalysts in Partial Oxidation of Methane to Syngas. Catal. Surv. Asia 2012, 16, 53–61. [Google Scholar] [CrossRef]
- Jin, R.; Chen, Y.; Li, W.; Cui, W.; Ji, Y.; Yu, C.; Jiang, Y. Mechanism for catalytic partial oxidation of methane to syngas over a Ni/Al2O3 catalyst. Appl. Catal. A Gen. 2000, 201, 71–80. [Google Scholar] [CrossRef]
- Dong, W.-S.; Jun, K.-W.; Roh, H.-S.; Liu, Z.-W.; Park, S.-E. Comparative Study on Partial Oxidation of Methane over Ni/ZrO2, Ni/CeO2 and Ni/Ce–ZrO2 Catalysts. Catal. Lett. 2002, 78, 215–222. [Google Scholar] [CrossRef]
- Yan, Q.G.; Weng, W.Z.; Wan, H.L.; Toghiani, H.; Toghiani, R.K.; Pittman, C.U., Jr. Activation of methane to syngas over a Ni/TiO2 catalyst. Appl. Catal. A 2003, 239, 43–58. [Google Scholar] [CrossRef]
- Enger, B.C.; Lødeng, R.; Holmén, A. Evaluation of reactor and catalyst performance in methane partial oxidation over modified nickel catalysts. Appl. Catal. A Gen. 2009, 364, 15–26. [Google Scholar] [CrossRef]
- Barbero, J.; Peña, M.A.; Campos-Martin, J.M.; Fierro, J.; Arias, P.L. Support Effect in Supported Ni Catalysts on Their Performance for Methane Partial Oxidation. Catal. Lett. 2003, 87, 211–218. [Google Scholar] [CrossRef]
- Alvarez-Galvan, C.; Melian, M.; Ruiz-Matas, L.; Eslava, J.L.; Navarro, R.M.; Ahmadi, M.; Cuenya, B.R.; Fierro, J.L.G. Partial Oxidation of Methane to Syngas Over Nickel-Based Catalysts: Influence of Support Type, Addition of Rhodium, and Preparation Method. Front. Chem. 2019, 7, 104. [Google Scholar] [CrossRef] [PubMed]
- Claridge, J.B.; Green, M.L.H.; Tsang, S.C.; York, A.P.E.; Ashcroft, A.T.; Battle, P.D. A study of carbon deposition on catalysts during the partial oxidation of methane to synthesis gas. Catal. Lett. 1993, 22, 299–305. [Google Scholar] [CrossRef]
- Mahamulkar, S.; Yin, K.; Agrawal, P.K.; Davis, R.J.; Jones, C.W.; Malek, A.; Shibata, H. Formation and Oxidation/Gasification of Carbonaceous Deposits: A Review. Ind. Eng. Chem. Res. 2016, 55, 9760–9818. [Google Scholar] [CrossRef]
- Yan, Q.; Wu, T.; Li, J.; Luo, C.; Weng, W.; Yang, L.; Wa, H. Mechanism study of carbon deposition on a Ni/Al2O3 catalyst during partial oxidation of methane to syngas. J. Nat. Gas Chem. 2000, 9, 89–102. [Google Scholar]
- Hayek, K.; Kramer, R.; Paál, Z. Metal-support boundary sites in catalysis. Appl. Catal. A Gen. 1997, 162, 1–15. [Google Scholar] [CrossRef]
- Cuenya, B.R. Synthesis and catalytic properties of metal nanoparticles: Size, shape, support, composition, and oxidation state effects. Thin Solid Films 2010, 518, 3127–3150. [Google Scholar] [CrossRef]
- Tanabe, K. Surface and catalytic properties of ZrO2. Mater. Chem. Phys. 1985, 13, 347–364. [Google Scholar] [CrossRef]
- Chuah, G.-K.; Jaenicke, S. The preparation of high surface area zirconia—Influence of precipitating agent and digestion. Appl. Catal. A Gen. 1997, 163, 261–273. [Google Scholar] [CrossRef]
- Jung, K.T.; Bell, A.T. The effects of synthesis and pretreatment conditions on the bulk structure and surface properties of zirconia. J. Mol. Catal. A Chem. 2000, 163, 27–42. [Google Scholar] [CrossRef]
- Hegarty, M.; O’Connor, A.; Ross, J. Syngas production from natural gas using ZrO2-supported metals. Catal. Today 1998, 42, 225–232. [Google Scholar] [CrossRef]
- Song, Y.-Q.; He, D.-H.; Xu, B.-Q. Effects of preparation methods of ZrO2 support on catalytic performances of Ni/ZrO2 catalysts in methane partial oxidation to syngas. Appl. Catal. A Gen. 2008, 337, 19–28. [Google Scholar] [CrossRef]
- Song, Y.-Q.; Liu, H.-M.; He, D.-H. Effects of Hydrothermal Conditions of ZrO2 on Catalyst Properties and Catalytic Performances of Ni/ZrO2 in the Partial Oxidation of Methane. Energy Fuels 2010, 24, 2817–2824. [Google Scholar] [CrossRef]
- Galanov, S.I.; Sidorova, O.I. Effect of a precursor on the phase composition and particle size of the active component of Ni-ZrO2 catalytic systems for the oxidation of methane into syngas. Russ. J. Phys. Chem. A 2014, 88, 1629–1636. [Google Scholar] [CrossRef]
- Xu, B.-Q.; Wei, J.-M.; Yu, Y.-T.; Li, Y.; Li, J.-L.; Zhu, Q.-M. Size Limit of Support Particles in an Oxide-Supported Metal Catalyst: Nanocomposite Ni/ZrO2 for Utilization of Natural Gas. J. Phys. Chem. B 2003, 107, 5203–5207. [Google Scholar] [CrossRef]
- Pedrero, C.M.; Carrazán, S.G.; Ruiz, P. Preliminary results on the role of the deposition of small amounts of ZrO2 on Al2O3 support on the partial oxidation of methane and ethane over Rh and Ni supported catalysts. Catal. Today 2021, 363, 111–121. [Google Scholar] [CrossRef]
- Martins, R.L.; Schmal, M. Activation of Methane on NiO Nanoparticles with Different Morphologies. J. Braz. Chem. Soc. 2014, 25, 2399–2408. [Google Scholar] [CrossRef]
- Yang, J.; Ren, J.; Guo, H.; Qin, X.; Han, B.; Lin, J.; Li, Z. The growth of Nin clusters and their interaction with cubic, monoclinic, and tetragonal ZrO2 surfaces–a theoretical and experimental study. RSC Adv. 2015, 5, 59935–59945. [Google Scholar] [CrossRef]
- Boudjennad, E.; Chafi, Z.; Ouafek, N.; Ouhenia, S.; Keghouche, N.; Minot, C. Experimental and theoretical study of the Ni−(m-ZrO2) interaction. Surf. Sci. 2012, 606, 1208–1214. [Google Scholar] [CrossRef]
- Dacquin, J.-P.; Dujardin, C.; Granger, P. Surface reconstruction of supported Pd on LaCoO3: Consequences on the catalytic properties in the decomposition of N2O. J. Catal. 2008, 253, 37–49. [Google Scholar] [CrossRef]
- Scherrer, P. Bestimmung der grösse und der inneren struktur von kolloidteilchen mittels röntgensrahlen. Nachr. Ges. Wiss. Göttingen 1918, 26, 98–100. [Google Scholar]
- Barrett, E.P.; Joyner, L.G.; Halenda, P.P. The Determination of Pore Volume and Area Distributions in Porous Substances. I. Computations from Nitrogen Isotherms. J. Am. Chem. Soc. 1951, 73, 373–380. [Google Scholar] [CrossRef]
- Gurvitsch, L. Physicochemical attractive force. J. Phys. Chem. Soc. Russ. 1915, 47, 805–827. [Google Scholar]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Bergeret, G.; Gallezot, P. Particle Size and Dispersion Measurements. In Handbook of Heterogeneous Catalysis; Ertl, G., Knözinger, H., Weitkamp, J., Eds.; Wiley-VCH: Weinheim, Germany, 1997; Volume 2, pp. 439–464. [Google Scholar]
- Ul-Hamid, A. A Beginners’ Guide to Scanning Electron. Microscopy; Metzler, J.B., Ed.; Springer: Berlin, Germany, 2018; pp. 77–128. [Google Scholar]
- Mironova-Ulmane, N.; Kuzmin, A.; Steins, I.; Grabis, J.; Sildos, I.; Pärs, M. Raman scattering in nanosized nickel oxide NiO. J. Phys. Conf. Ser. 2007, 93, 012039. [Google Scholar] [CrossRef]
- Wang, W.; Liu, Y.; Xu, C.; Zheng, C.; Wang, G. Synthesis of NiO nanorods by a novel simple precursor thermal decomposition approach. Chem. Phys. Lett. 2002, 362, 119–122. [Google Scholar] [CrossRef]
- Bellido, J.D.A.; Assaf, E.M. Nickel catalysts supported on ZrO2, Y2O3-stabilized ZrO2 and CaO-stabilized ZrO2 for the steam reforming of ethanol: Effect of the support and nickel load. J. Power Sources 2008, 177, 24–32. [Google Scholar] [CrossRef]
- Zhao, K.; Wang, W.; Li, Z. Highly efficient Ni/ZrO2 catalysts prepared via combustion method for CO2 methanation. J. CO2 Util. 2016, 16, 236–244. [Google Scholar] [CrossRef]
- Kesavan, J.K.; Luisetto, I.; Tuti, S.; Meneghini, C.; Iucci, G.; Battocchio, C.; Mobilio, S.; Casciardi, S.; Sisto, R. Nickel supported on YSZ: The effect of Ni particle size on the catalytic activity for CO2 methanation. J. CO2 Util. 2018, 23, 200–211. [Google Scholar] [CrossRef]
- Mori, H.; Wen, C.-ju; Otomo, J.; Eguchi, K.; Takahashi, H. Investigation of the interaction between NiO and yttria-stabilized zirconia (YSZ) in the NiO/YSZ composite by temperature-programmed reduction technique. Appl. Catal. A 2003, 245, 79–85. [Google Scholar] [CrossRef]
- Alderliesten, M. Mean Particle Diameters. From Statistical Definition to Physical Understanding. J. Biopharm. Stat. 2005, 15, 295–325. [Google Scholar] [CrossRef][Green Version]
- Ferrari, A.C.; Robertson, J. Interpretation of Raman spectra of disordered and amorphous carbon. Phys. Rev. B 2000, 61, 14095–14107. [Google Scholar] [CrossRef]
- Zhang, X.; Hayward, D.O.; Mingos, D.M.P. Oscillatory behaviour during the partial oxidation of methane over Nickel foils. Catal. Lett. 2002, 83, 149–155. [Google Scholar] [CrossRef]
- Zhang, X.; Hayward, D.O.; Mingos, D.M.P. Further Studies on Oscillations over Nickel Wires during the Partial Oxidation of Methane. Catal. Lett. 2003, 86, 235–243. [Google Scholar] [CrossRef]
- Hu, Y.H.; Ruckenstein, E. Catalyst Temperature Oscillations during Partial Oxidation of Methane. Ind. Eng. Chem. Res. 1998, 37, 2333–2335. [Google Scholar] [CrossRef]
- Wang, M.; Weng, W.; Zheng, H.; Yi, X.; Huang, C.; Wan, H. Oscillations during partial oxidation of methane to synthesis gas over Ru/Al2O3 catalyst. J. Nat. Gas. Chem. 2009, 18, 300–305. [Google Scholar] [CrossRef]
- Zhang, X.; Lee, C.; Hayward, D.; Mingos, D. Oscillatory behaviour observed in the rate of oxidation of methane over metal catalysts. Catal. Today 2005, 105, 283–294. [Google Scholar] [CrossRef]
- Bychkov, V.; Tyulenin, Y.; Slinko, M.; Korchak, V. Nonlinear behaviour during methane and ethane oxidation over Ni, Co and Pd catalysts. Surf. Sci. 2009, 603, 1680–1689. [Google Scholar] [CrossRef]
- Stötzel, J.; Frahm, R.; Kimmerle, B.; Nachtegaal, M.; Grunwaldt, J.-D. Oscillatory behaviour during the catalytic partial oxidation of methane: Following dynamic structural changes of palladium using the QEXAFS technique. J. Phys. Chem. C 2012, 116, 599–609. [Google Scholar] [CrossRef]
- Lashina, E.A.; Kaichev, V.V.; Saraev, A.A.; Vinokurov, Z.S.; Chumakova, N.A.; Chumakov, G.A.; Bukhtiyarov, V.I. Experimental Study and Mathematical Modeling of Self-Sustained Kinetic Oscillations in Catalytic Oxidation of Methane over Nickel. J. Phys. Chem. A 2017, 121, 6874–6886. [Google Scholar] [CrossRef]
- Saraev, A.A.; Vinokurov, Z.S.; Kaichev, V.; Shmakov, A.N.; Bukhtiyarov, V. The origin of self-sustained reaction-rate oscillations in the oxidation of methane over nickel: An operando XRD and mass spectrometry study. Catal. Sci. Technol. 2017, 7, 1646–1649. [Google Scholar] [CrossRef]
- Specchia, S.; Vella, L.D.; Montini, T.; Fornasiero, P. Hydrogen Production: Prospects and Processes; Nova Science Publishers Inc.: New York, NY, USA, 2011; pp. 95–139. [Google Scholar]
- Pokrovski, K.; Jung, K.T.; Bell, A.T. Investigation of CO and CO2 Adsorption on Tetragonal and Monoclinic Zirconia. Langmuir 2001, 17, 4297–4303. [Google Scholar] [CrossRef]
- Kouva, S.; Andersin, J.; Honkala, K.; Lehtonen, J.; Lefferts, L.; Kanervo, J. Water and carbon oxides on monoclinic zirconia: Experimental and computational insights. Phys. Chem. Chem. Phys. 2014, 16, 20650–20664. [Google Scholar] [CrossRef] [PubMed]
- Bachiller-Baeza, B.; Rodriguez-Ramos, I.; Guerrero-Ruiz, A. Interaction of Carbon Dioxide with the Surface of Zirconia Polymorphs. Langmuir 1998, 14, 3556–3564. [Google Scholar] [CrossRef]
- Morterra, C.; Orio, L. Surface characterization of zirconium oxide. II. The interaction with carbon dioxide at ambient temperature. Mater. Chem. Phys. 1990, 24, 247–268. [Google Scholar] [CrossRef]
- Bolis, V.; Morterra, C.; Volante, M.; Orio, L.; Fubini, B. Development and suppression of surface acidity on monoclinic zirconia: A spectroscopic and calorimetric investigation. Langmuir 1990, 6, 695–701. [Google Scholar] [CrossRef]
- Ouyang, F.; Nakayama, A.; Tabada, K.; Suzuki, E. Infrared Study of a Novel Acid-Base Site on ZrO2 by Adsorbed Probe Molecules. I.; Pyridine, Carbon Dioxide, and Formic Acid Adsorption. J. Phys. Chem. B 2000, 104, 2012–2018. [Google Scholar] [CrossRef]
- Esconjauregui, S.; Whelan, C.M.; Maex, K. The reasons why metals catalyze the nucleation and growth of carbon nanotubes and other carbon nanomorphologies. Carbon 2009, 47, 659–669. [Google Scholar] [CrossRef]
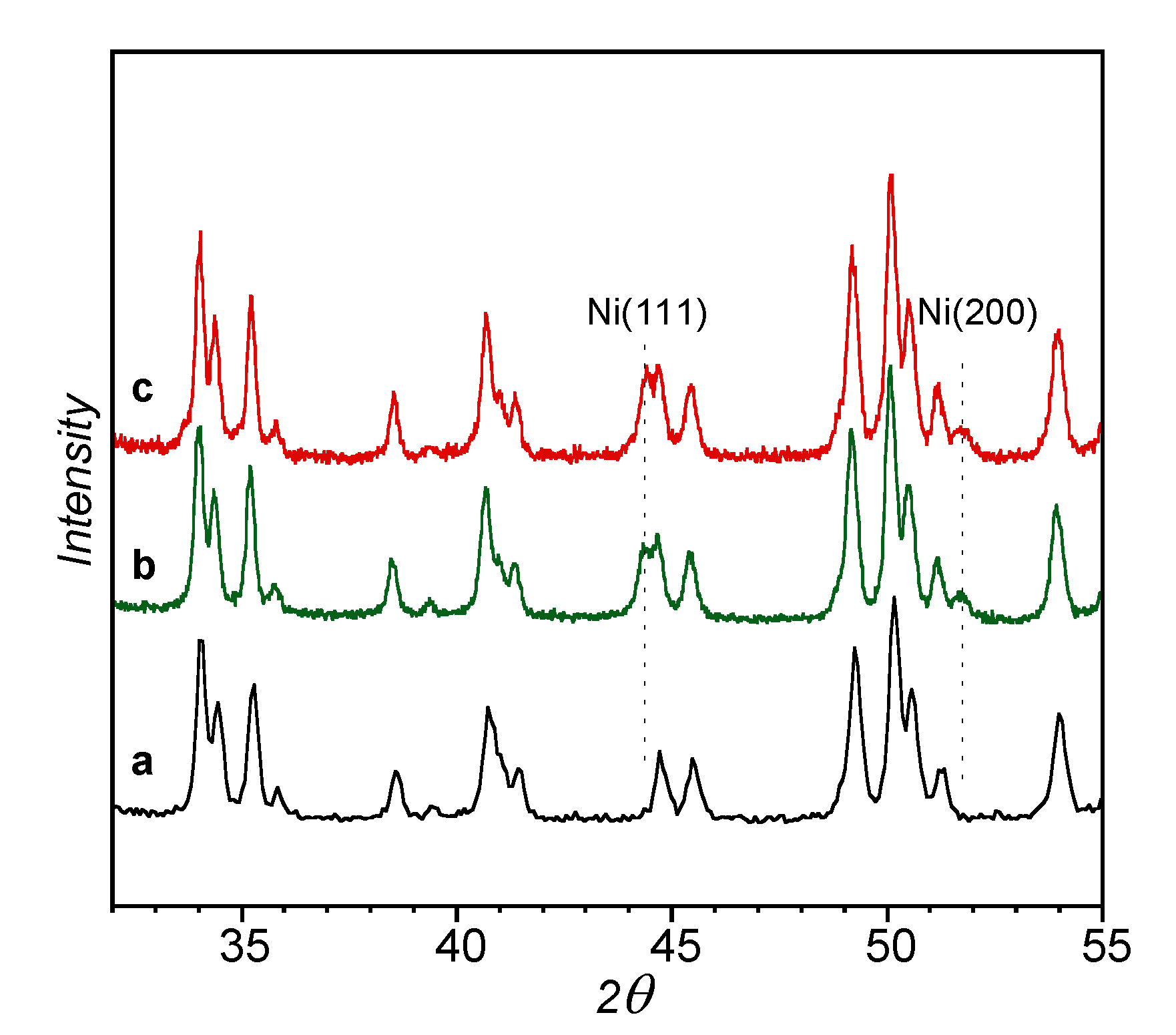

| Precursors | Catalysts | S.A. 1 (m2·g−1) | Vtot 2 (cc·g−1) | Crystallite Size, dXRD (nm) | |||
|---|---|---|---|---|---|---|---|
| ZrO2 | NiO | Ni act 3 Used | |||||
| Zhy | - | 360 | 0.33 | - | - | - | - |
| - | Zm | 5.6 | 0.041 | 49 | - | - | - |
| NiO (unsupported) | - | - | - | - | 70 | - | - |
| - | Ni (unsupported) | - | - | - | - | - | 70 |
| 1.8NiO/Zm | - | 2.7 | 0.024 | - | 22 | - | - |
| - | 1.8Ni/Zm(ox-red) | - | - | - | - | 18 | 28 |
| 1.8Ni/Zm(red) | - | - | - | - | 25 | - | |
| 5.1NiO/Zm | - | 3.3 | 0.031 | 50 | 36 | - | - |
| - | 5.1Ni/Zm(ox-red) | - | - | - | - | 30 | 25 |
| 5.1Ni/Zm(red) | - | - | - | - | 35 | 37 | |
| 1.7NiO/Zhy | - | 5.4 | 0.040 | 32 | - | - | |
| - | 1.7Ni/Zhy(ox-red) | - | - | - | - | - | 20 |
| 1.7Ni/Zhy(red) | - | - | - | - | - | 25 | |
| 4.8NiO/Zhy | - | 4.4 | 0.044 | 52 | 26 | - | - |
| - | 4.8Ni/Zhy(ox-red) | - | - | - | - | 23 | - |
| 4.8Ni/Zhy(red) | - | - | - | - | 24 | 30 | |
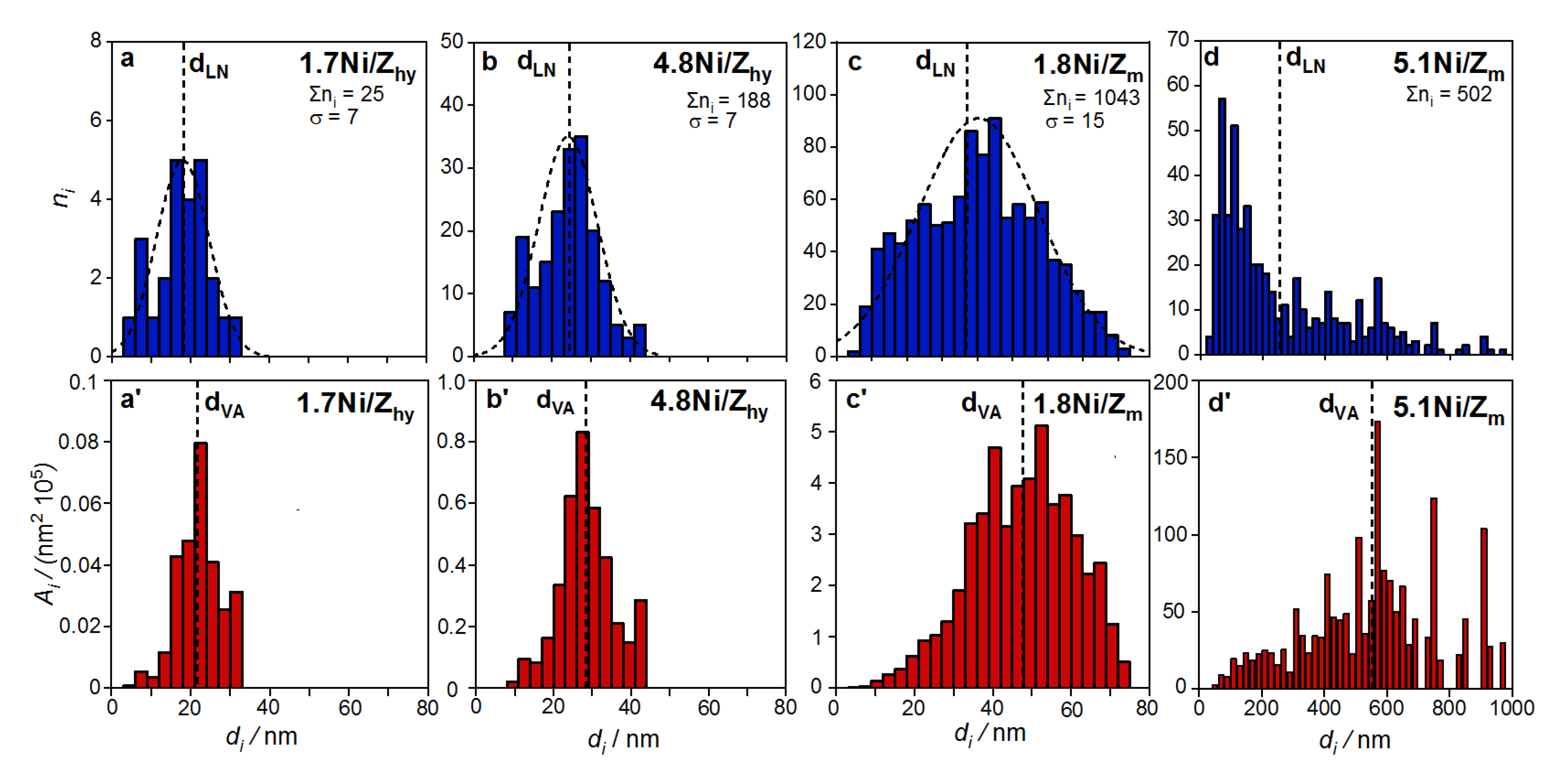
| Precursors | Catalysts | Particle Size, dFESEM (nm) | Ni Particle Number | Ni Particle Mean Diameter | Ni Dispersion | Exposed Ni Atoms | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| ZrO2 | NiO | Ni Activated Used | Σni 1 | dLN 2 (nm) | dVA 3 (nm) | D 4 (%) | NNi(exp) 5 (atoms/g) | |||
| - | Zm | 30–100 | - | - | - | - | - | - | - | - |
| NiO (unsupported) | - | - | 20–400 | - | - | - | - | - | - | - |
| - | Ni (unsupported) | 40–1500 | 50–2000 | - | - | - | - | - | ||
| 1.8NiO/Zm | - | - | 40–60 | - | - | - | - | - | - | - |
| - | 1.8Ni/Zm (ox-red) | - | - | 5–75 | 20–80 | 1043 | 36 ± 15 | 48 | 2.1 | 3.9·1018 |
| 1.8Ni/Zm (red) | - | - | 20–75 | 20–80 | - | - | - | - | - | |
| 5.1NiO/Zm | - | - | 80–300 | - | - | - | - | - | - | - |
| - | 5.1Ni/Zm (ox-red) | - | - | 30–1000 | 50–700 | 502 | 2.6·102 | 5.5·102 | 0.18 | 9.4·1017 |
| 5.1Ni/Zm (red) | - | - | 30–700 | 35–700 | - | - | - | - | - | |
| 1.7NiO/Zhy | - | - | n.d. 6 | - | - | - | - | - | - | - |
| - | 1.7Ni/Zhy (ox-red) | - | - | 10–35 | 15–25 | 25 | 18 ± 7 | 22 | 4.6 | 7.1·1018 |
| 1.7Ni/Zhy (red) | - | - | 5–30 | 15–20 | - | - | - | - | - | |
| 4.8NiO/Zhy | - | - | n.d. 6 | - | - | - | - | - | - | - |
| - | 4.8Ni/Zhy (ox-red) | - | - | 10–45 | 15–40 | 188 | 24 ± 7 | 28 | 3.6 | 1.8·1019 |
| 4.8Ni/Zhy (red) | - | - | 8–35 | 15–40 | - | - | - | - | - | |
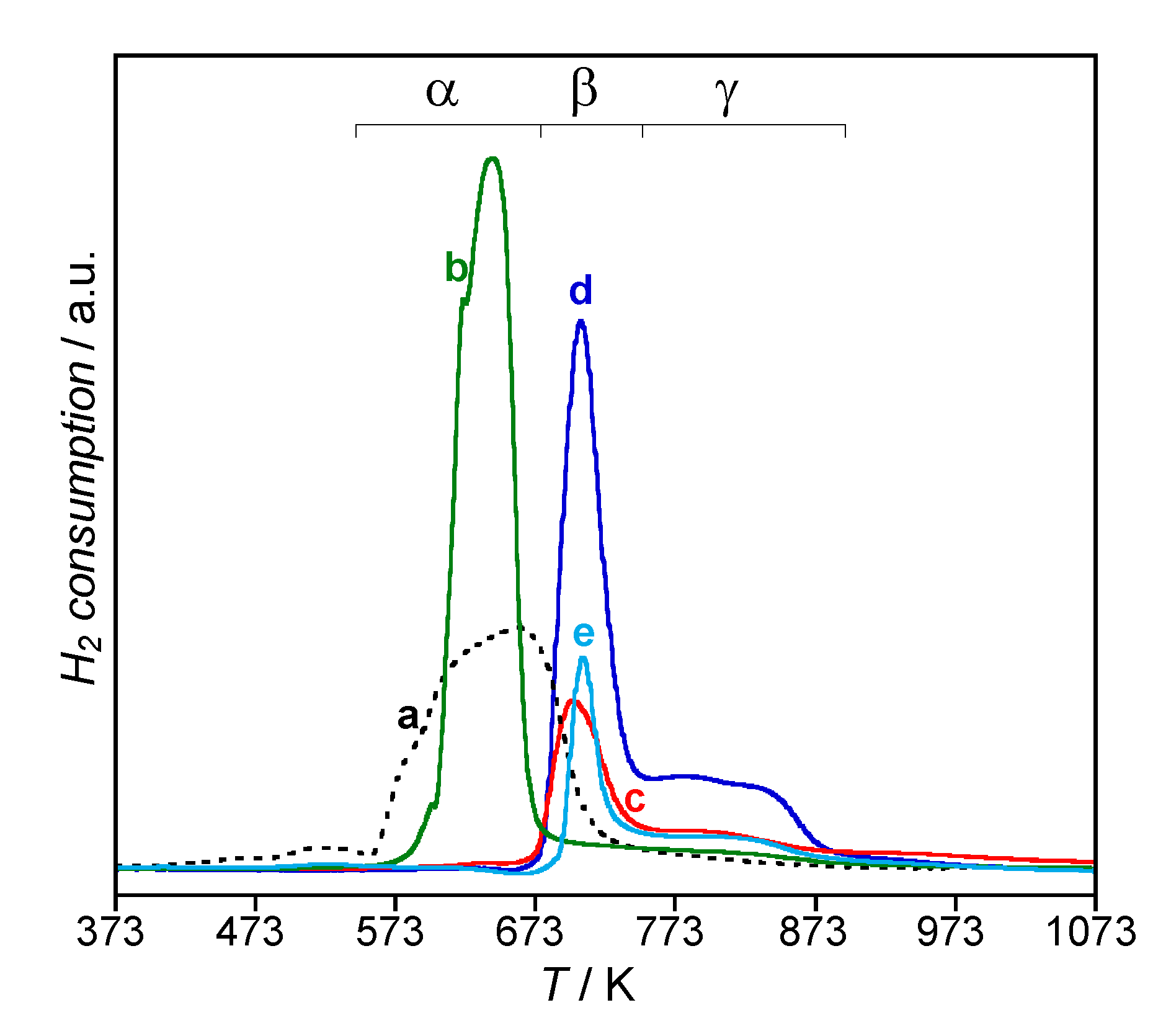
| Catalyst | H2 Consumption (μmoli g−1) | e/Ni 1 | % NiO Species | ||
|---|---|---|---|---|---|
| α | β | γ | |||
| NiO | 1330 | 2.00 | 100 | - | - |
| 1.8Ni/Zm | 31.04 | 2.02 | - | 60 | 40 |
| 5.1Ni/Zm | 97.07 | 2.03 | 89 | 4 | 7 |
| 1.7Ni/Zhy | 29.93 | 2.02 | - | 54 | 46 |
| 4.8Ni/Zhy | 83.75 | 1.97 | - | 68 | 32 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pietrogiacomi, D.; Campa, M.C.; Pettiti, I.; Tuti, S.; Luccisano, G.; Ardemani, L.; Luisetto, I.; Gazzoli, D. Oscillatory Behaviour of Ni Supported on ZrO2 in the Catalytic Partial Oxidation of Methane as Determined by Activation Procedure. Materials 2021, 14, 2495. https://doi.org/10.3390/ma14102495
Pietrogiacomi D, Campa MC, Pettiti I, Tuti S, Luccisano G, Ardemani L, Luisetto I, Gazzoli D. Oscillatory Behaviour of Ni Supported on ZrO2 in the Catalytic Partial Oxidation of Methane as Determined by Activation Procedure. Materials. 2021; 14(10):2495. https://doi.org/10.3390/ma14102495
Chicago/Turabian StylePietrogiacomi, Daniela, Maria Cristina Campa, Ida Pettiti, Simonetta Tuti, Giulia Luccisano, Leandro Ardemani, Igor Luisetto, and Delia Gazzoli. 2021. "Oscillatory Behaviour of Ni Supported on ZrO2 in the Catalytic Partial Oxidation of Methane as Determined by Activation Procedure" Materials 14, no. 10: 2495. https://doi.org/10.3390/ma14102495
APA StylePietrogiacomi, D., Campa, M. C., Pettiti, I., Tuti, S., Luccisano, G., Ardemani, L., Luisetto, I., & Gazzoli, D. (2021). Oscillatory Behaviour of Ni Supported on ZrO2 in the Catalytic Partial Oxidation of Methane as Determined by Activation Procedure. Materials, 14(10), 2495. https://doi.org/10.3390/ma14102495










