Gelatine-Coated Carbonyl Iron Particles and Their Utilization in Magnetorheological Suspensions
Abstract
:1. Introduction
2. Experimental
2.1. Chemicals
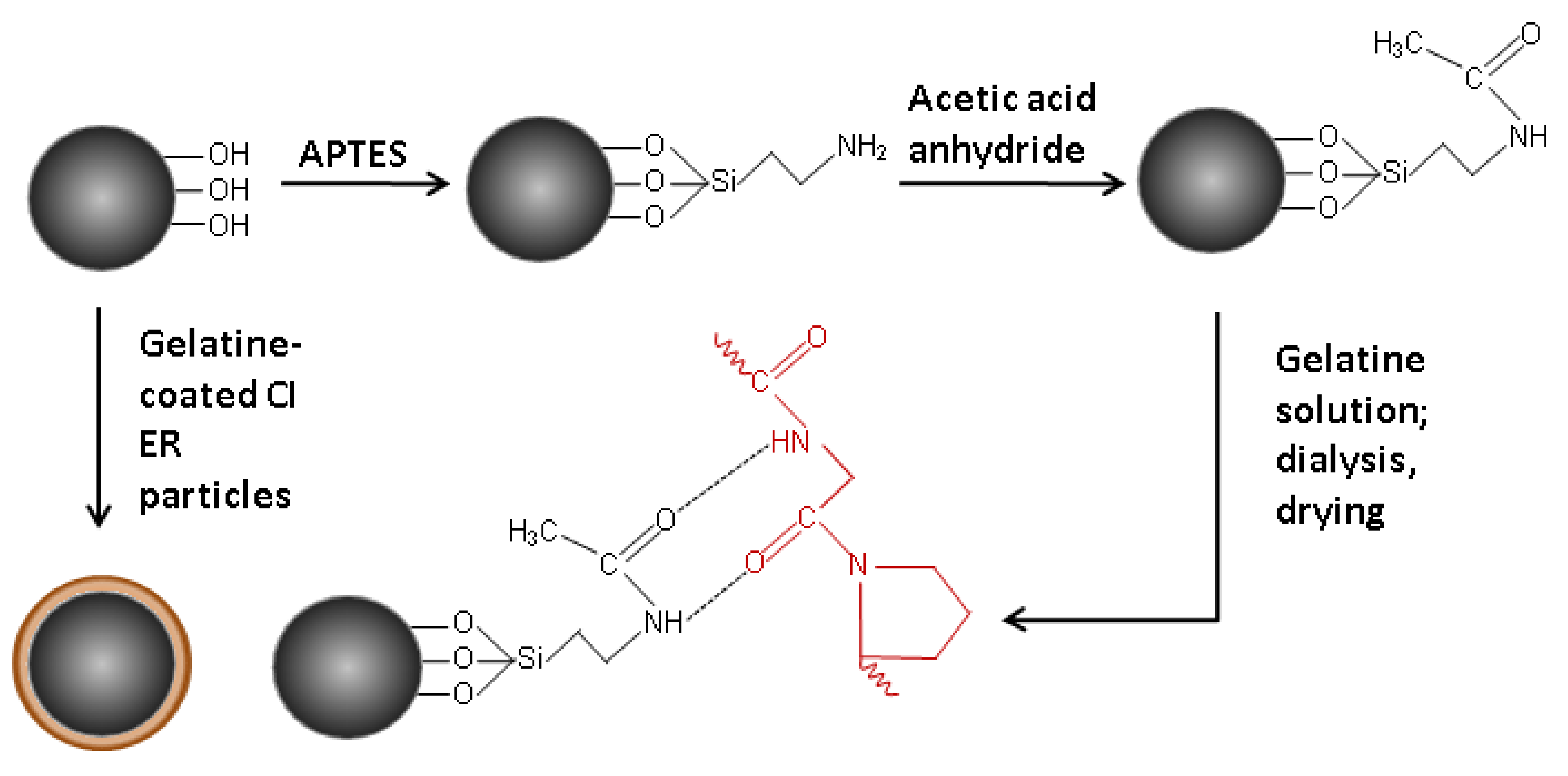
2.2. Coating of the Carbonyl Iron Particles
2.3. Characterization of the Particles
2.4. Preparation of MR Suspensions
2.5. Rheological Measurements
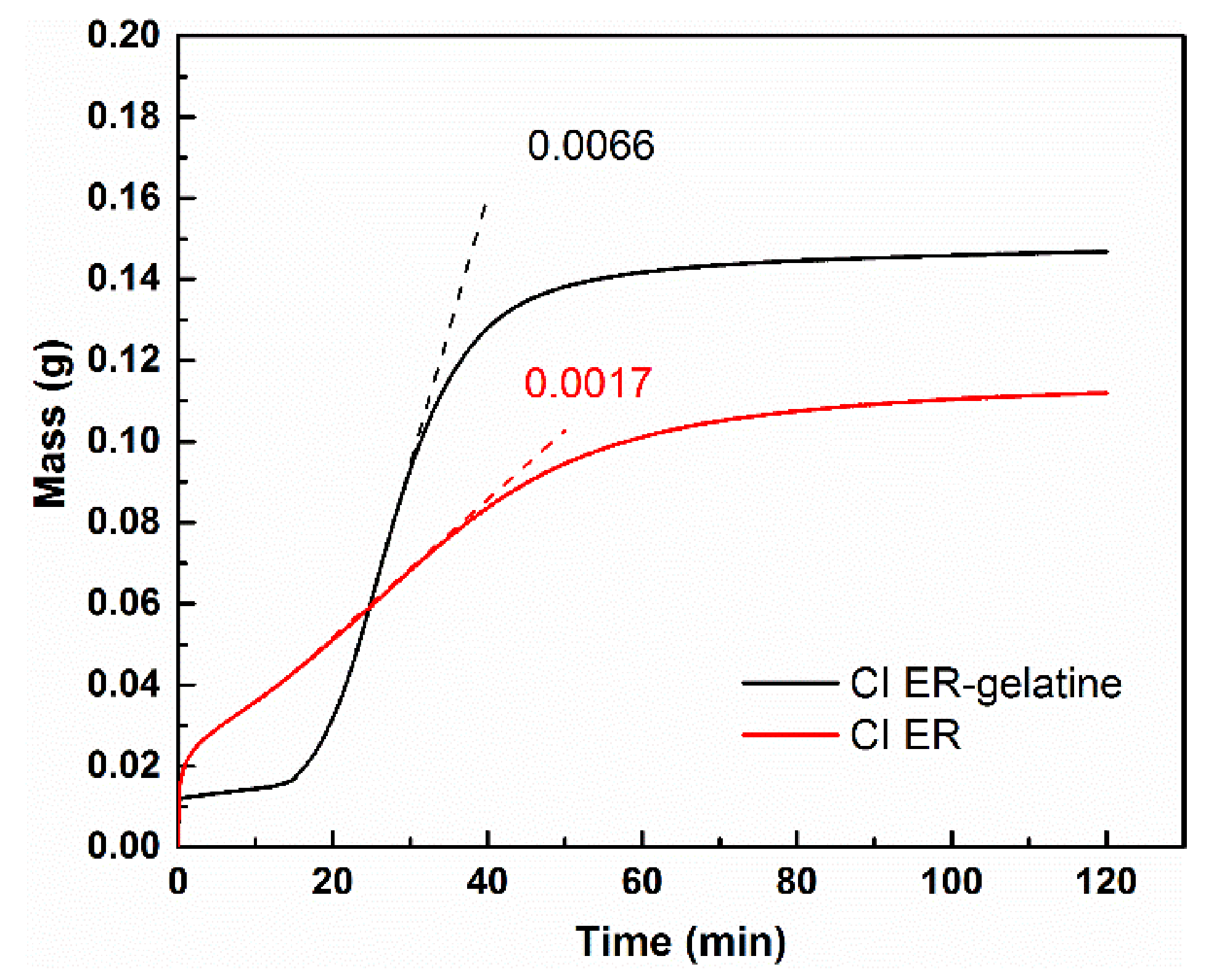
2.6. Sedimentation Test
2.7. Optical Microscopy
3. Results and Discussion
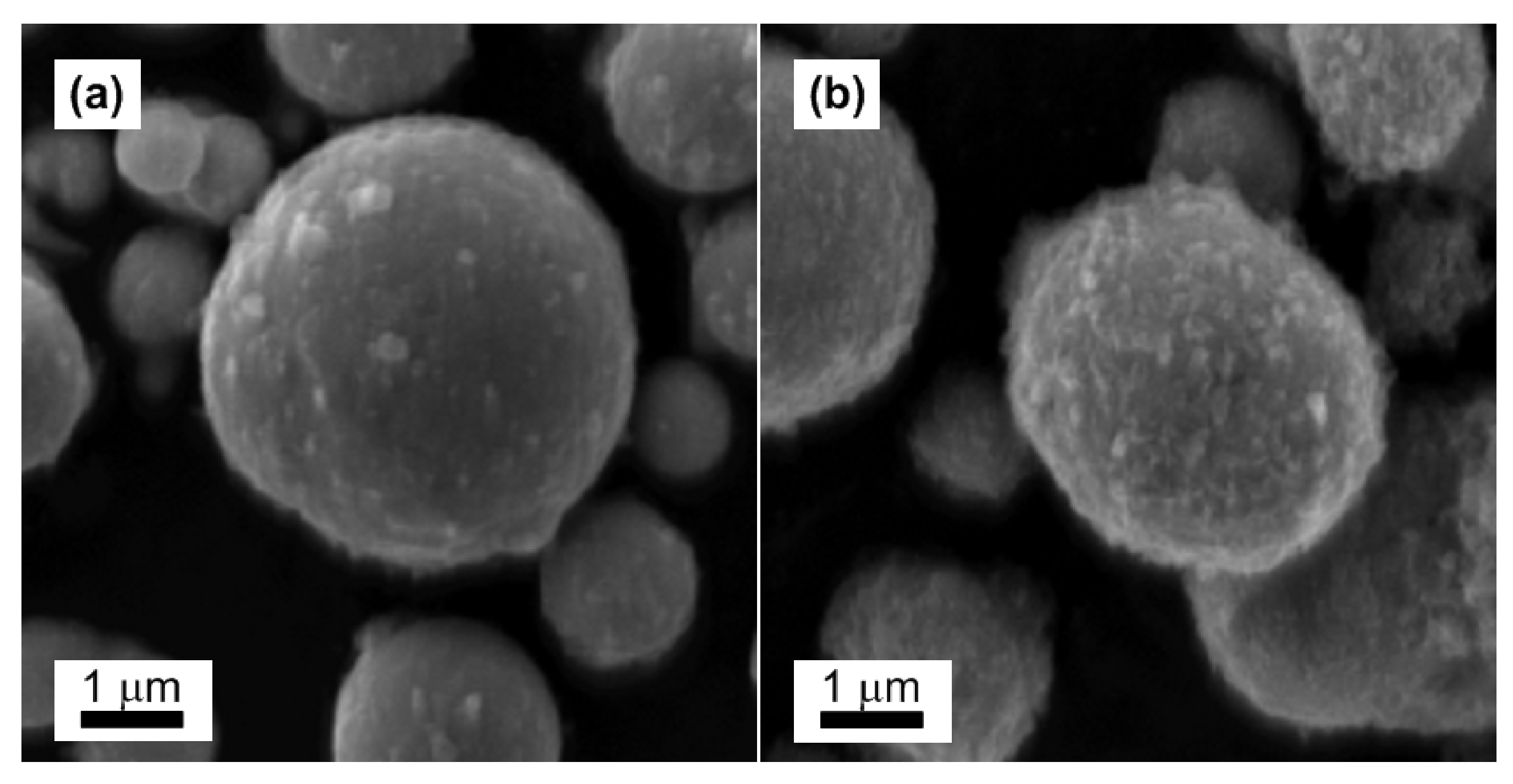
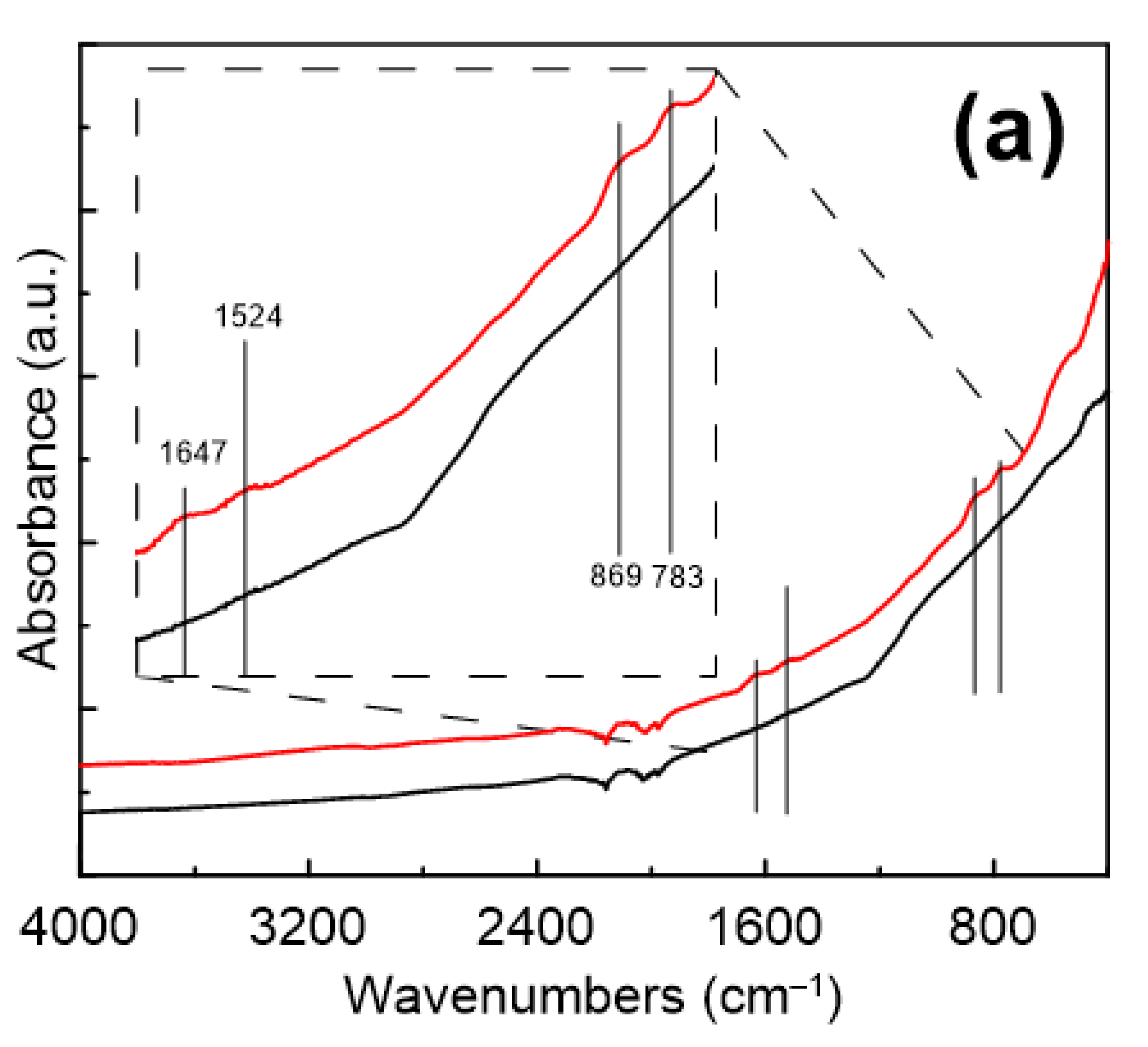
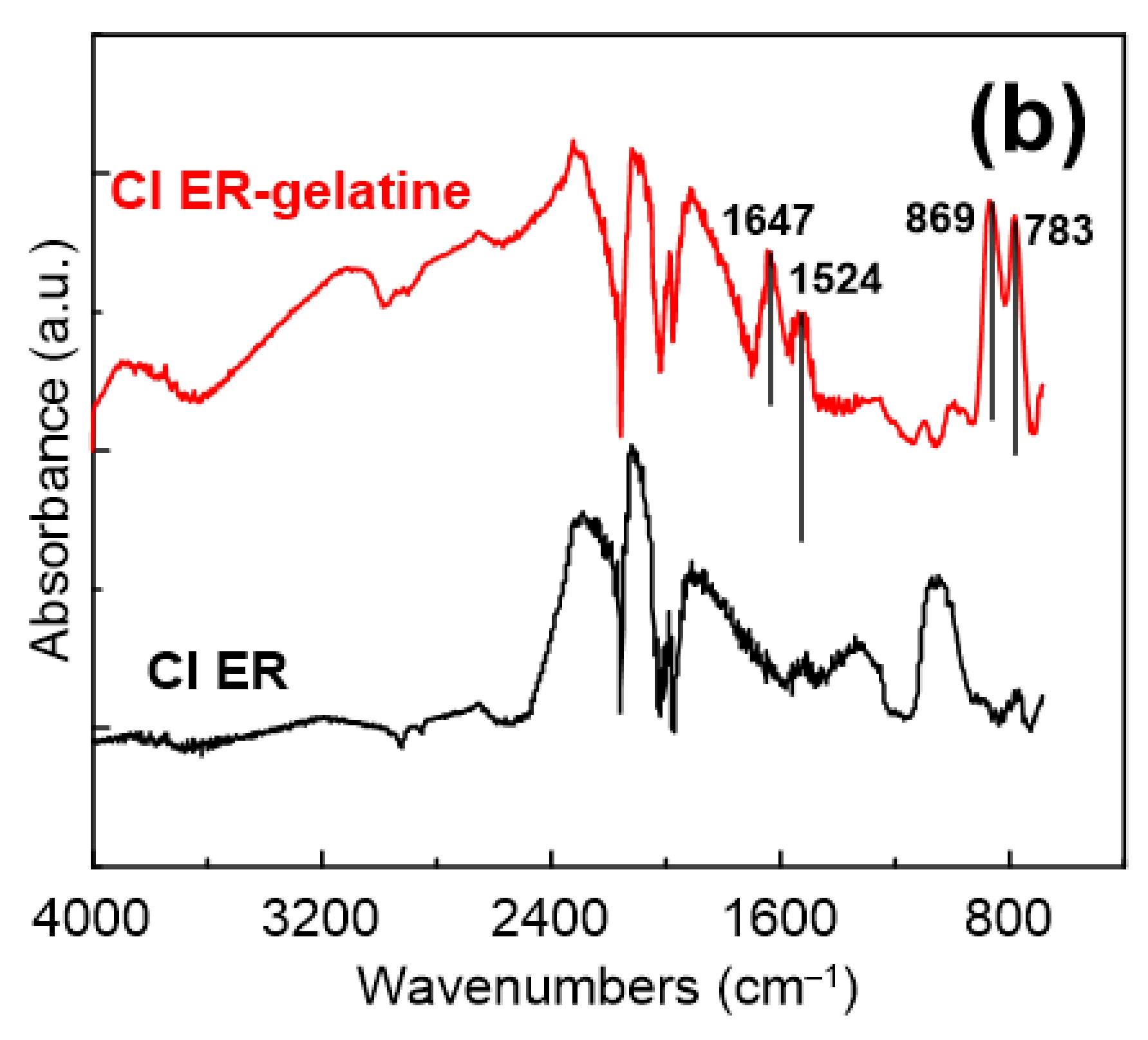
Particles Characterization
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Srinivas, M.; Boehm-Sturm, P.; Figdor, C.G.; de Vries, I.J.; Hoehn, M. Labeling cells for in vivo tracking using F-19 MRI. Biomaterials 2012, 33, 8830–8840. [Google Scholar] [CrossRef]
- Pankhurst, Q.A.; Connolly, J.; Jones, S.K.; Dobson, J. Applications of magnetic nanoparticles in biomedicine. J. Phys. D Appl. Phys. 2003, 36, 167–181. [Google Scholar] [CrossRef] [Green Version]
- Kashevsky, B.E.; Kashevsky, S.B.; Korenkov, V.S.; Istomin, Y.P.; Terpinskaya, T.I.; Ulashchik, V.S. Magnetic hyperthermia with hard-magnetic nanoparticles. J. Magn. Magn. Mater. 2015, 380, 335–340. [Google Scholar] [CrossRef]
- Zhao, L.Y.; Liu, J.Y.; Ouyang, W.W.; Li, D.Y.; Li, L.; Li, L.Y.; Tang, J.T. Magnetic-mediated hyperthermia for cancer treatment: Research progress and clinical trials. Chin. Phys. B 2013, 22, 14. [Google Scholar] [CrossRef]
- Iacob, G.; Ciochina, A.D.; Bredetean, O.; Racuciu, M. Magnetite particle utilization for blood vessel embolization—A practical modeling. Optoelectron. Adv. Mater. Rapid Commun. 2008, 2, 446–449. [Google Scholar]
- Michaud, F.; Li, N.; Plantefeve, R.; Nosrati, Z.; Tremblay, C.; Saatchi, K.; Moran, G.; Bigot, A.; Hafeli, U.O.; Kadoury, S.; et al. Selective embolization with magnetized microbeads using magnetic resonance navigation in a controlled-flow liver model. Med. Phys. 2019, 46, 789–799. [Google Scholar] [CrossRef]
- Li, Z.X.; Kawashita, M.; Araki, N.; Mitsumori, M.; Hiraoka, M.; Doi, M. Magnetic SiO2 gel microspheres for arterial embolization hyperthermia. Biomed. Mater. 2010, 5, 9. [Google Scholar] [CrossRef]
- Adedoyin, A.A.; Ekenseair, A.K. Biomedical applications of magneto-responsive scaffolds. Nano Res. 2018, 11, 5049–5064. [Google Scholar] [CrossRef]
- Tartaj, P.; Morales, M.D.; Veintemillas-Verdaguer, S.; Gonzalez-Carreno, T.; Serna, C.J. The preparation of magnetic nanoparticles for applications in biomedicine. J. Phys. D Appl. Phys. 2003, 36, R182–R197. [Google Scholar] [CrossRef]
- Jozefczak, A.; Hornowski, T.; Rozynek, Z.; Skumiel, A.; Fossum, J.O. Rheological study of dextran-modified magnetite nanoparticle water suspension. Int. J. Thermophys. 2013, 34, 609–619. [Google Scholar] [CrossRef] [Green Version]
- Cvek, M.; Mrlik, M.; Ilcikova, M.; Mosnacek, J.; Babayan, V.; Kucekova, Z.; Humpolicek, P.; Pavlinek, V. The chemical stability and cytotoxicity of carbonyl iron particles grafted with poly(glycidyl methacrylate) and the magnetorheological activity of their suspensions. RSC Adv. 2015, 5, 72816–72824. [Google Scholar] [CrossRef] [Green Version]
- Vasiliev, V.G.; Sheremetyeva, N.A.; Buzin, M.I.; Turenko, D.V.; Papkov, V.S.; Klepikov, I.A.; Razumovskaya, I.V.; Muzafarov, A.M.; Kramarenko, E.Y. Magnetorheological fluids based on a hyperbranched polycarbosilane matrix and iron microparticles. Smart Mater. Struct. 2016, 25, 9. [Google Scholar] [CrossRef]
- Fu, Y.; Yao, J.J.; Zhao, H.H.; Zhao, G.; Wan, Z.S.; Qiu, Y. Bidisperse magnetic particles coated with gelatin and graphite oxide: Magnetorheology, dispersion stability, and the nanoparticle-enhancing effect. Nanomaterials 2018, 8, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parikh, N.; Parekh, K. Technique to optimize magnetic response of gelatin coated magnetic nanoparticles. J. Mater. Sci. Mater. Med. 2015, 26, 9. [Google Scholar] [CrossRef]
- Intorasoot, S.; Techateerawat, J.; Intorasoot, A. Genomic DNA isolation from dried blood using gelatin-coated magnetic particles. Curr. Sci. 2013, 105, 81–84. [Google Scholar]
- Intorasoot, S.; Srirung, R.; Intorasoot, A.; Ngamratanapaiboon, S. Application of gelatin-coated magnetic particles for isolation of genomic DNA from bacterial cells. Anal. Biochem. 2009, 386, 291–292. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.S.; Ma, Y.Y.; Tong, Y.; Dong, X.F. Development of manganese ferrite/graphene oxide nanocomposites for magnetorheological fluid with enhanced sedimentation stability. J. Ind. Eng. Chem. 2017, 48, 142–150. [Google Scholar] [CrossRef]
- Rendos, A.; Li, R.; Woodman, S.; Ling, X.; Brown, K.A. Reinforcing magnetorheological fluids with highly anisotropic 2d materials. ChemPhysChem 2021, 22, 431–432. [Google Scholar] [CrossRef] [PubMed]
- Jamari, S.K.M.; Nordin, N.A.; Ubaidillah Aziz, S.A.A.; Nazmi, N.; Mazlan, S.A. Systematic Review on the Effects, Roles and Methods of Magnetic Particle Coatings in Magnetorheological Materials. Materials 2020, 13, 21. [Google Scholar] [CrossRef] [PubMed]
- De Vicente, J.; Klingenberg, D.J.; Hidalgo-Alvarez, R. Magnetorheological fluids: A review. Soft Matter 2011, 7, 3701–3710. [Google Scholar] [CrossRef]
- Zhang, J.T.; Song, W.L.; Peng, Z.; Gao, J.W.; Wang, N.; Choi, S.B.; Kim, G.W. Microstructure simulation and constitutive modelling of magnetorheological fluids based on the hexagonal close-packed structure. Materials 2020, 13, 20. [Google Scholar] [CrossRef] [Green Version]
- Fu, Y.; Yao, J.J.; Zhao, H.H.; Zhao, G.; Wan, Z.S.; Qiu, Y. Fabrication and magnetorheology of bidisperse magnetic microspheres coated with gelatin and multi-walled carbon nanotubes. Smart Mater. Struct. 2018, 27, 8. [Google Scholar] [CrossRef]
- Belyavskii, S.G.; Mingalyov, P.G.; Giulieri, F.; Combarrieau, R.; Lisichkin, G.V. Chemical modification of the surface of a carbonyl iron powder. Protect. Met. 2006, 42, 244–252. [Google Scholar] [CrossRef]
- Cvek, M.; Mrlik, M.; Ilcikova, M.; Plachy, T.; Sedlacik, M.; Mosnacek, J.; Pavlinek, V. A facile controllable coating of carbonyl iron particles with poly(glycidyl methacrylate): A tool for adjusting MR response and stability properties. J. Mater. Chem. C 2015, 3, 4646–4656. [Google Scholar] [CrossRef]
- Xiao, S.J.; Textor, M.; Spencer, N.D.; Sigrist, H. Covalent attachment of cell-adhesive, (Arg-Gly-Asp)-containing peptides to titanium surfaces. Langmuir 1998, 14, 5507–5516. [Google Scholar] [CrossRef]
- Saw, M.M.; Chandler, B.; Ho, K.M. Benefits and risks of using gelatin solution as a plasma expander for perioperative and critically ill patients: A meta-analysis. Anaesth. Intensive Care 2012, 40, 17–32. [Google Scholar] [CrossRef] [Green Version]
- Phillips, G.O.; Williams, P.A. Handbook of Hydrocolloids; Woodhead Publishing: Cambridge, UK, 2009. [Google Scholar]
- Gaihre, B.; Khil, M.S.; Lee, D.R.; Kim, H.Y. Gelatin-coated magnetic iron oxide nanoparticles as carrier system: Drug loading and in vitro drug release study. Int. J. Pharm. 2009, 365, 180–189. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.C.; Tanaka, J. FT-IR study for hydroxyapatite/collagen nanocomposite cross-linked by glutaraldehyde. Biomaterials 2002, 23, 4811–4818. [Google Scholar] [CrossRef]
- Machovsky, M.; Mrlik, M.; Kuritka, I.; Pavlinek, V.; Babayan, V. Novel synthesis of core-shell urchin-like ZnO coated carbonyl iron microparticles and their magnetorheological activity. RSC Adv. 2014, 4, 996–1003. [Google Scholar] [CrossRef] [Green Version]
- Mrlik, M.; Sedlacik, M.; Pavlinek, V.; Bazant, P.; Saha, P.; Peer, P.; Filip, P. Synthesis and magnetorheological characteristics of ribbon-like, polypyrrole-coated carbonyl iron suspensions under oscillatory shear. J. Appl. Polym. Sci. 2013, 128, 2977–2982. [Google Scholar] [CrossRef]
- Konig, R.; Muller, S.; Dinnebier, R.E.; Hinrichsen, B.; Muller, P.; Ribbens, A.; Hwang, J.; Liebscher, R.; Etter, M.; Pistidda, C. The crystal structures of carbonyl iron powde—Revised using in situ synchrotron XRPD. Z. Krist. Cryst. Mater. 2017, 232, 835–842. [Google Scholar]
- Plachy, T.; Cvek, M.; Munster, L.; Hanulikova, B.; Suly, P.; Vesel, A.; Qilin, C. Enhanced magnetorheological effect of suspensions based on carbonyl iron particles coated with poly(amidoamine) dendrons. Rheol. Acta 2021, 60, 263–276. [Google Scholar] [CrossRef]
- Cvek, M.; Mrlik, M.; Pavlinek, V. A rheological evaluation of steady shear magnetorheological flow behavior using three-parameter viscoplastic models. J. Rheol. 2016, 60, 687. [Google Scholar] [CrossRef]
- Plachy, T.; Kutalkova, E.; Sedlacik, M.; Vesel, A.; Masar, M.; Kuritka, I. Impact of corrosion process of carbonyl iron particles on magnetorheological behavior of their suspensions. J. Ind. Eng. Chem. 2018, 66, 362–369. [Google Scholar] [CrossRef]
- Ginder, J.M.; Davis, L.C. Shear stresses in magnetorheological fluids—Role of magnetic saturation. Appl. Phys. Lett. 1994, 65, 3410–3412. [Google Scholar] [CrossRef]
- De Sousa, S.R.G.; dos Santos, M.P.; Bombard, A.J.F. Magnetorheological gel based on mineral oil and polystyrene-b-poly(ethene-co-butadiene)-b-polystyrene. Smart Mater. Struct. 2019, 28, 9. [Google Scholar]
- Marins, J.A.; Plachy, T.; Kuzhir, P. Iron-sepiolite magnetorheological fluids with improved performances. J. Rheol. 2019, 63, 125–139. [Google Scholar] [CrossRef] [Green Version]
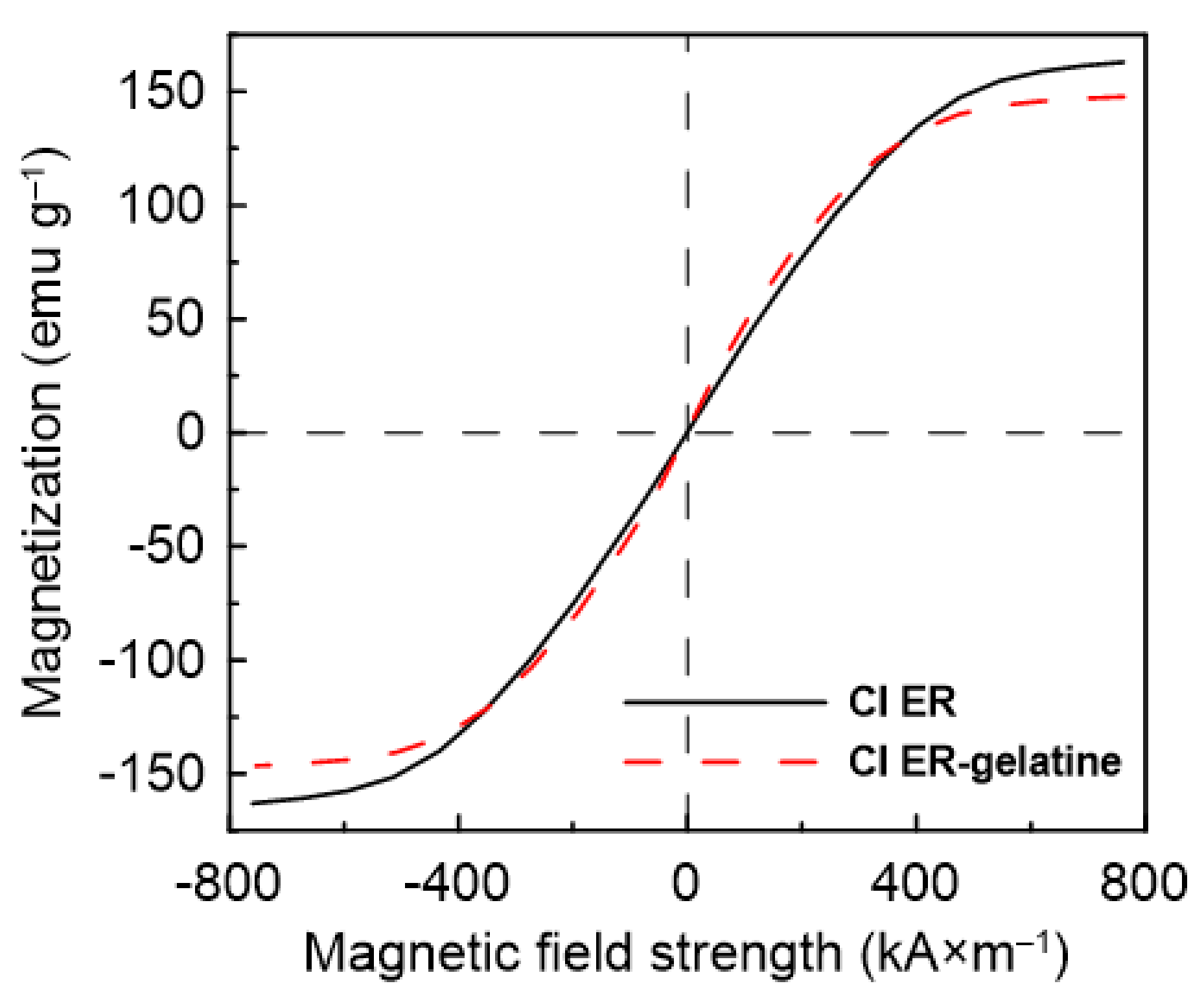
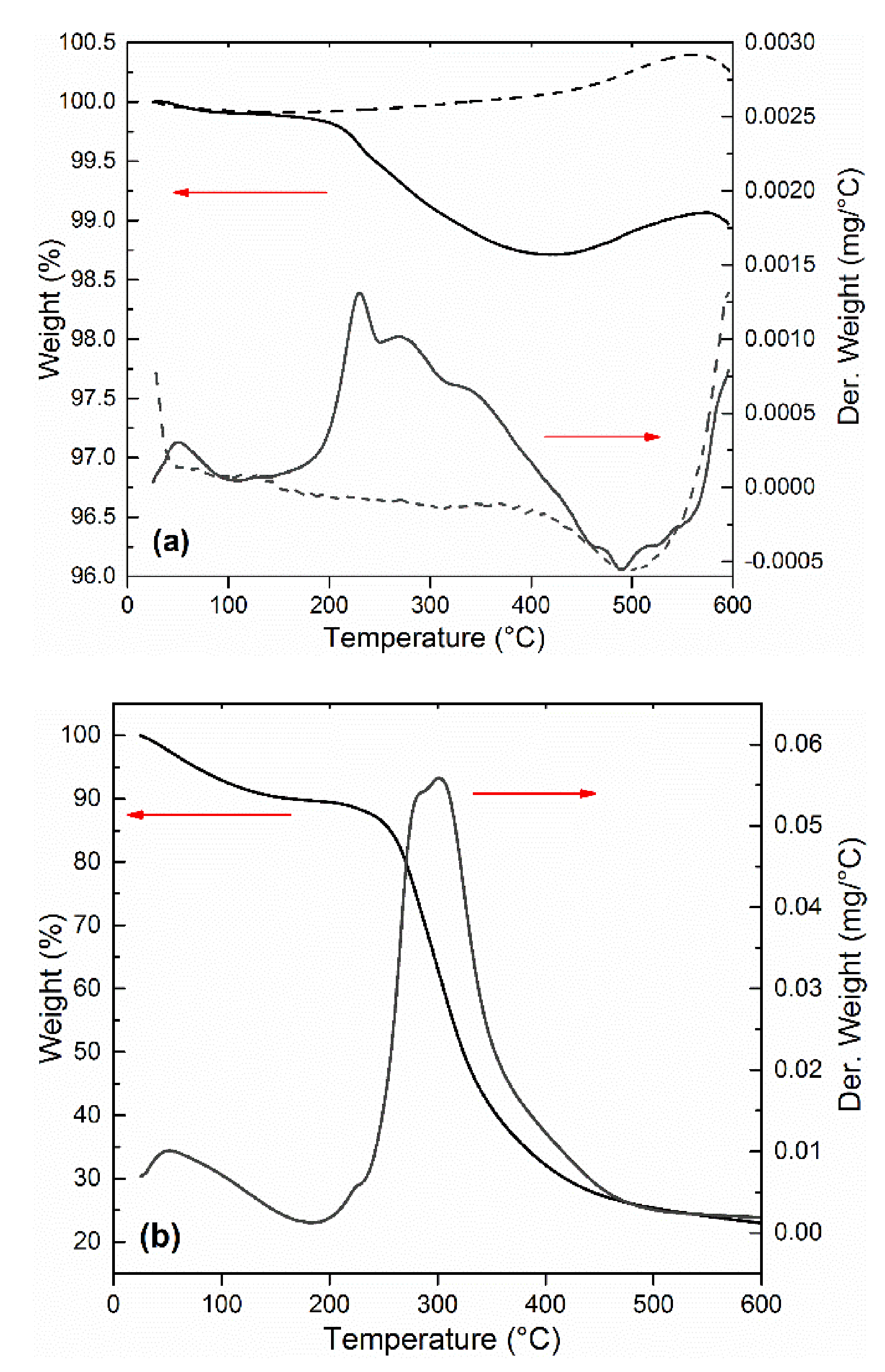
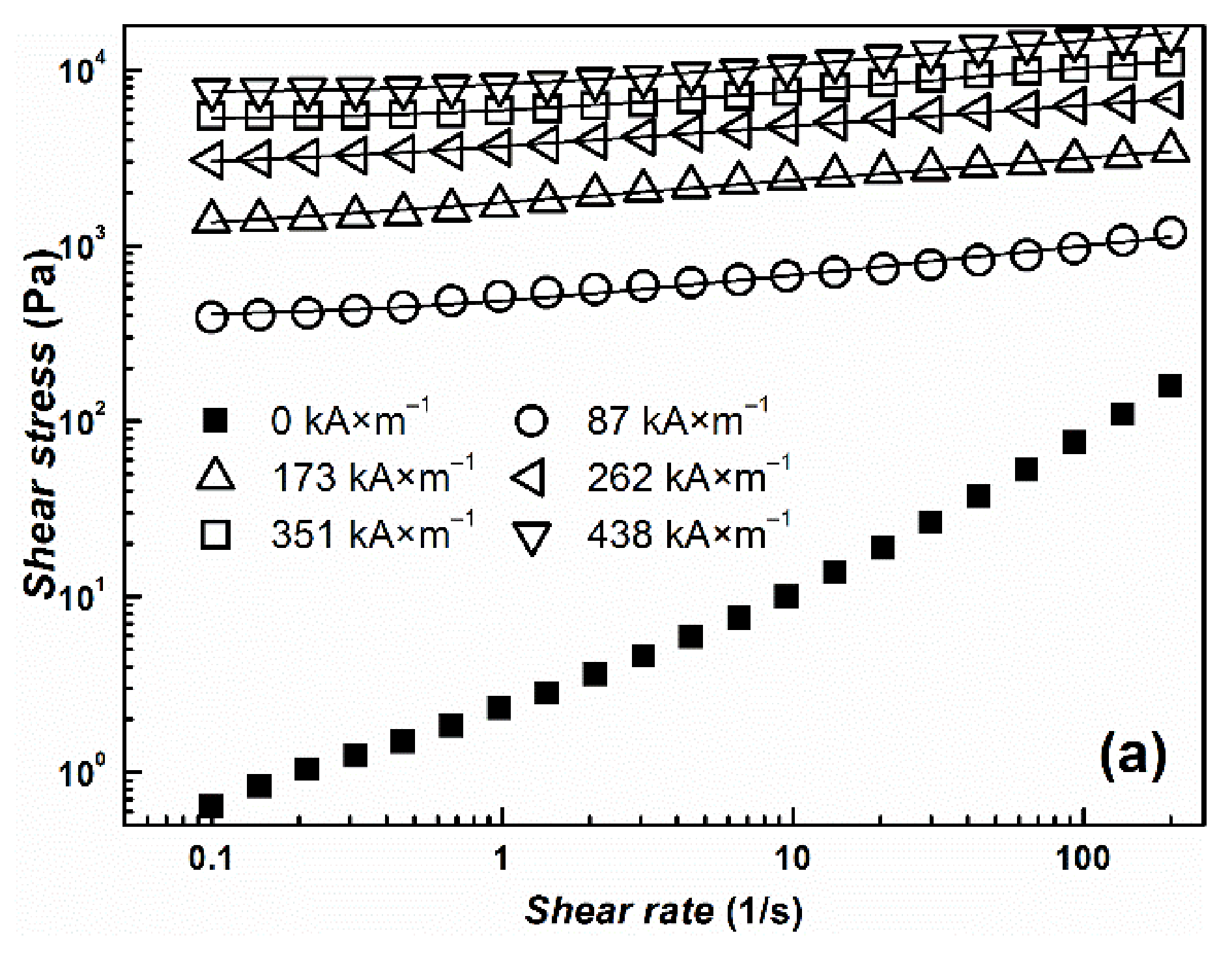
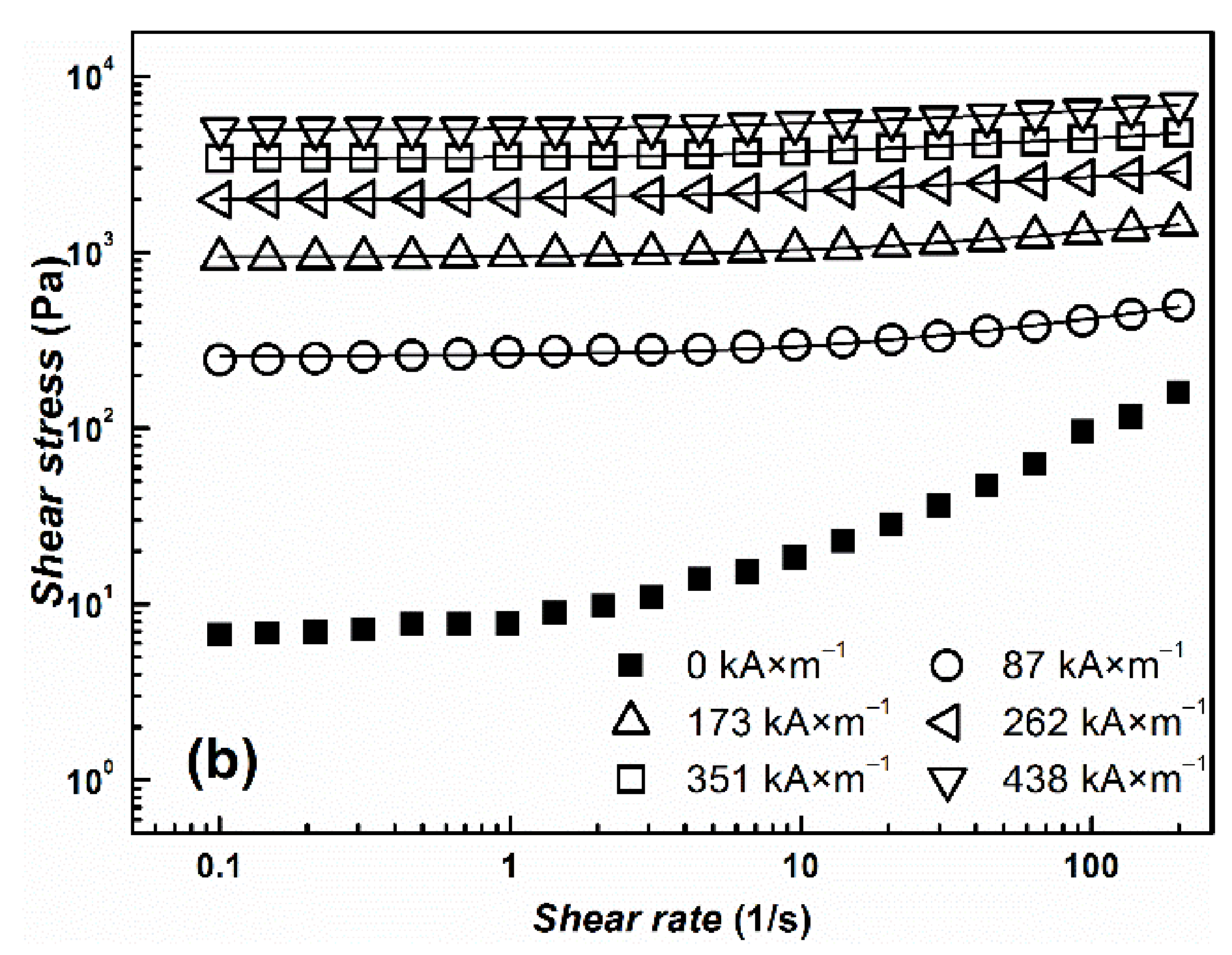
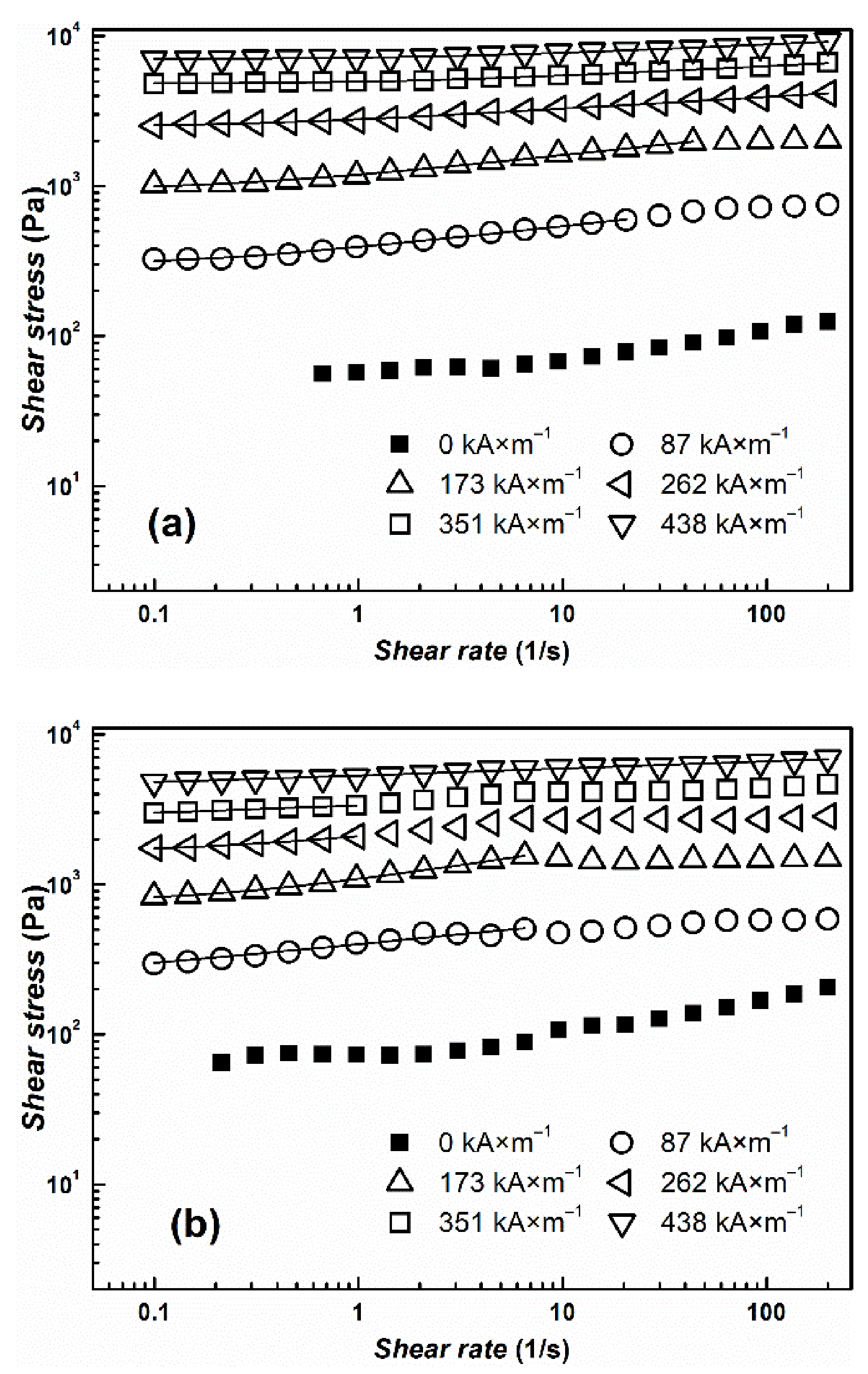

| Yield Stress (Pa) | ||||
|---|---|---|---|---|
| Magnetic Field Intensity (kA×m−1) | Silicone-Oil | 6% Gelatine Solution | ||
| CI ER | CI ER-Gelatine | CI ER | CI ER-Gelatine | |
| 87 | 370.0 ± 30 | 270.0 ± 15 | 300.0 ± 10 | 220.0 ± 10 |
| 173 | 1100 ± 60 | 940.0 ± 10 | 970.0 ± 20 | 800.0 ± 80 |
| 262 | 2800 ± 50 | 2000 ± 30 | 2600 ± 100 | 1700 ± 90 |
| 351 | 5100 ± 70 | 3500 ± 20 | 4900 ± 100 | 2800 ± 70 |
| 438 | 7400 ± 80 | 5000 ± 10 | 7200 ± 100 | 4400 ± 80 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Plachy, T.; Rohrer, P.; Holcapkova, P. Gelatine-Coated Carbonyl Iron Particles and Their Utilization in Magnetorheological Suspensions. Materials 2021, 14, 2503. https://doi.org/10.3390/ma14102503
Plachy T, Rohrer P, Holcapkova P. Gelatine-Coated Carbonyl Iron Particles and Their Utilization in Magnetorheological Suspensions. Materials. 2021; 14(10):2503. https://doi.org/10.3390/ma14102503
Chicago/Turabian StylePlachy, Tomas, Patrik Rohrer, and Pavlina Holcapkova. 2021. "Gelatine-Coated Carbonyl Iron Particles and Their Utilization in Magnetorheological Suspensions" Materials 14, no. 10: 2503. https://doi.org/10.3390/ma14102503






