Hydrolytic Modification of SiO2 Microspheres with Na2SiO3 and the Performance of Supported Nano-TiO2 Composite Photocatalyst
Abstract
:1. Introduction
2. Experiment
2.1. Raw Materials and Reagents
2.2. Modification of SiO2 Microspheres
2.3. Preparation of SiO2-M/TiO2 Composite Photocatalyst
2.4. Preparation of Pure TiO2
2.5. Testing and Characterization
3. Results and Discussion
3.1. Characterization of SiO2 Microspheres
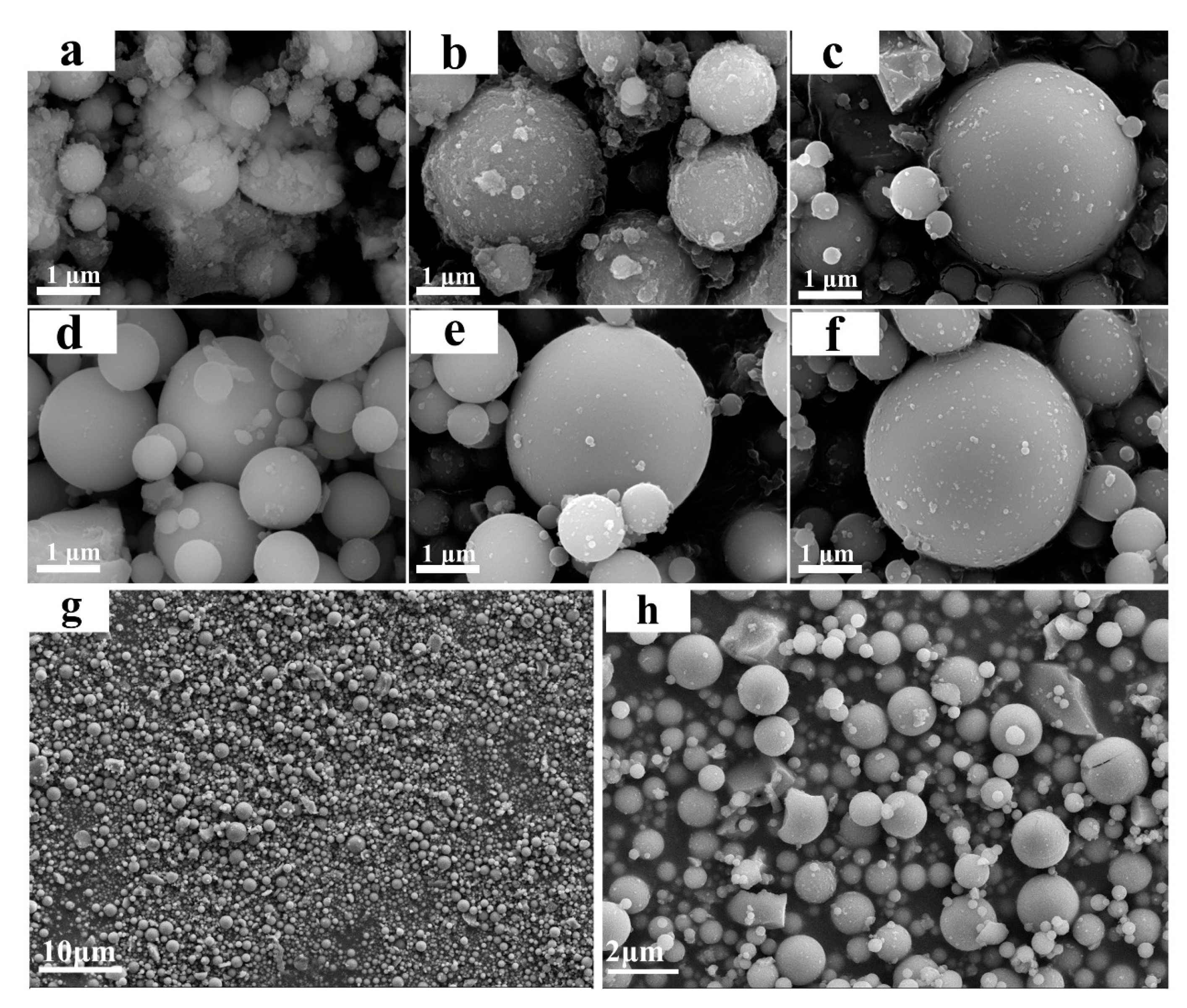
3.1.1. Morphology
3.1.2. Effect of Modified SiO2 Microspheres
3.1.3. TGA and DSC of SiO2-M
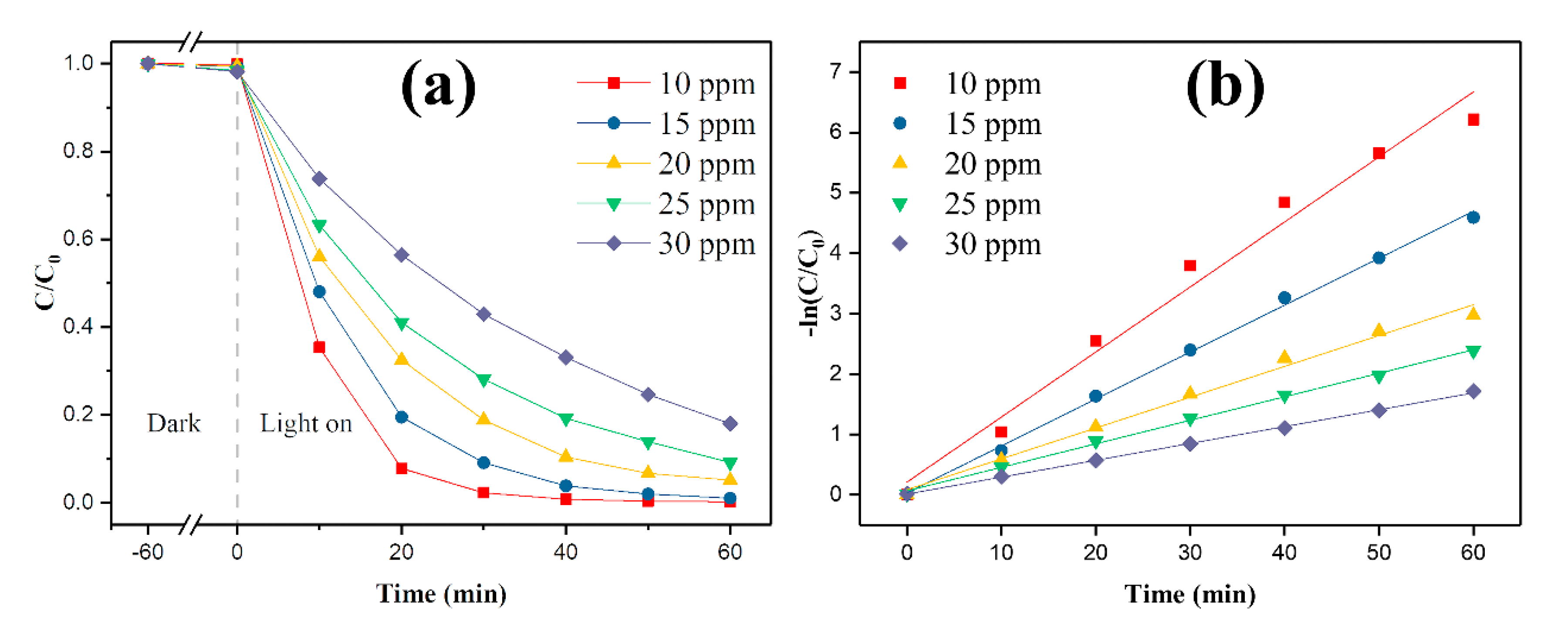
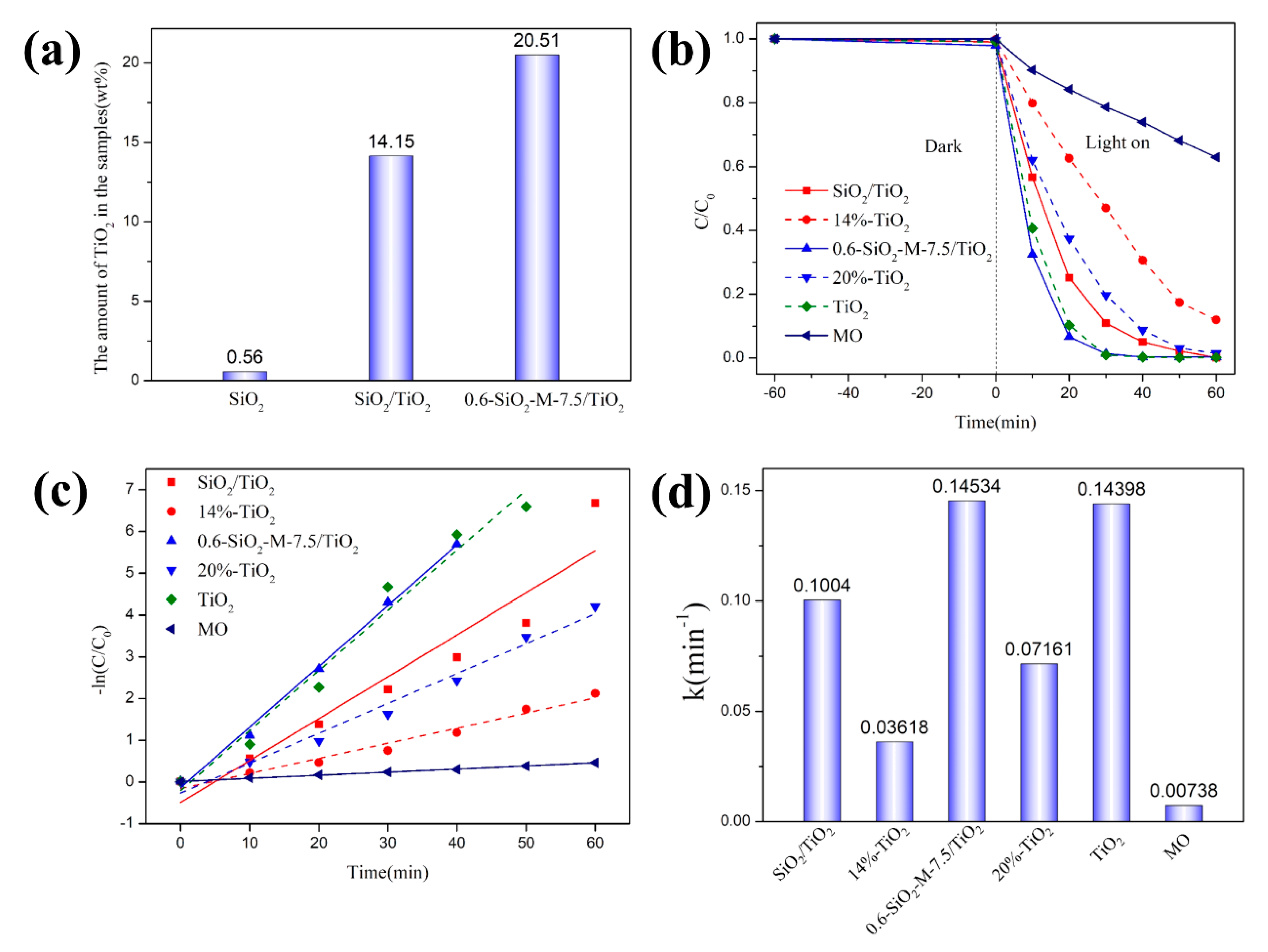
3.2. Photocatalytic Performance
3.3. Morphology and Structure of Composite Photocatalyst
3.3.1. Morphology
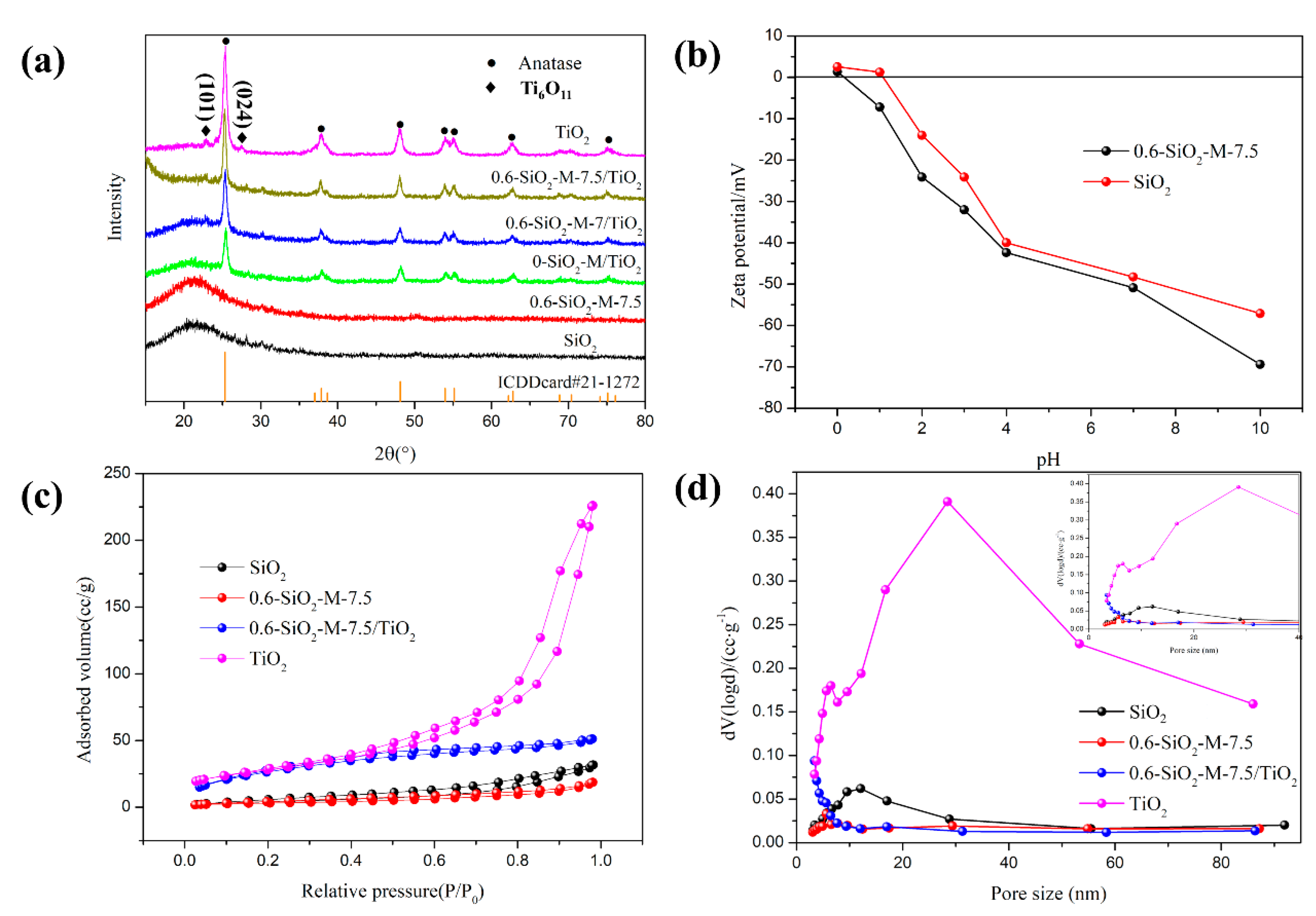
3.3.2. Crystalline and Structure
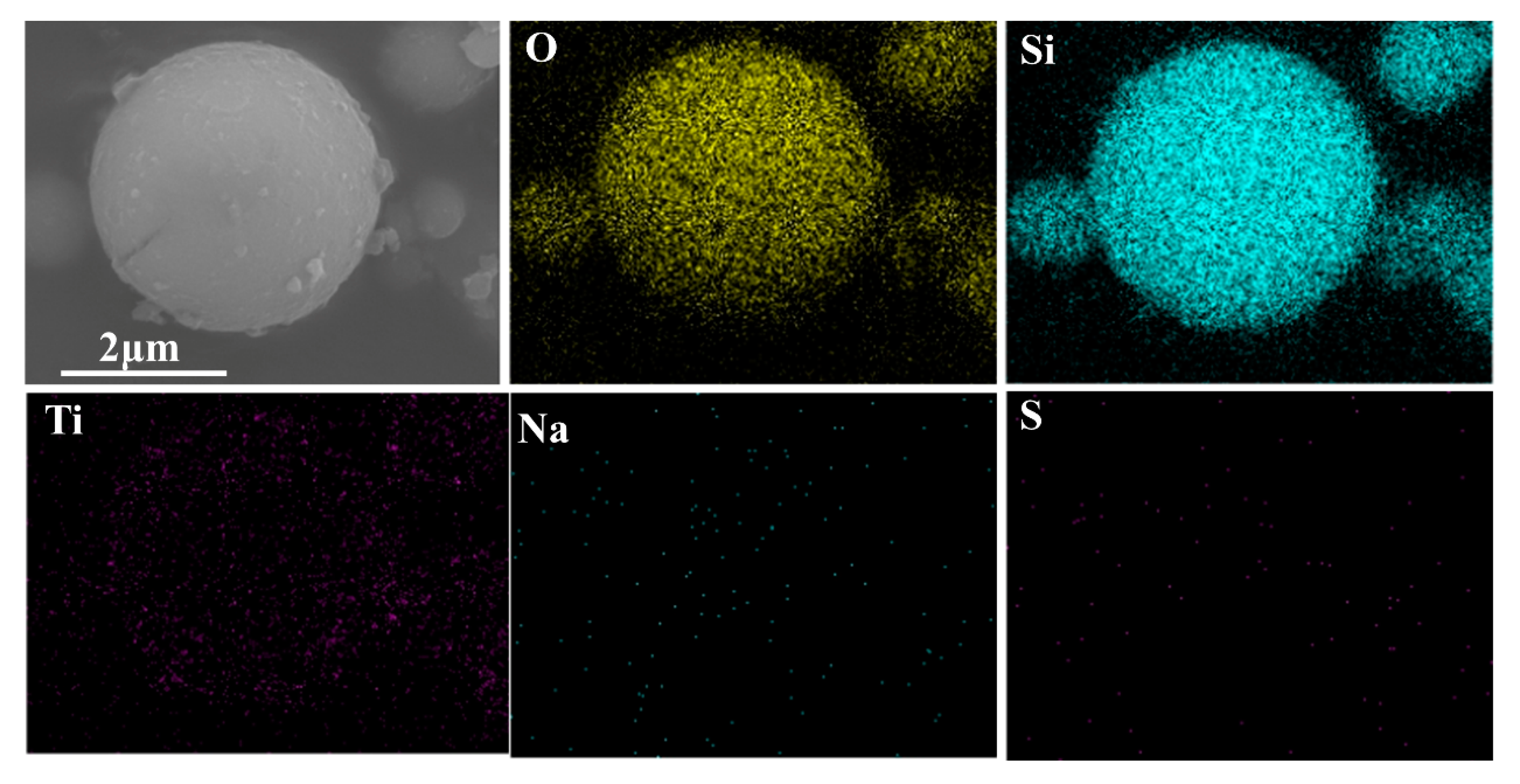
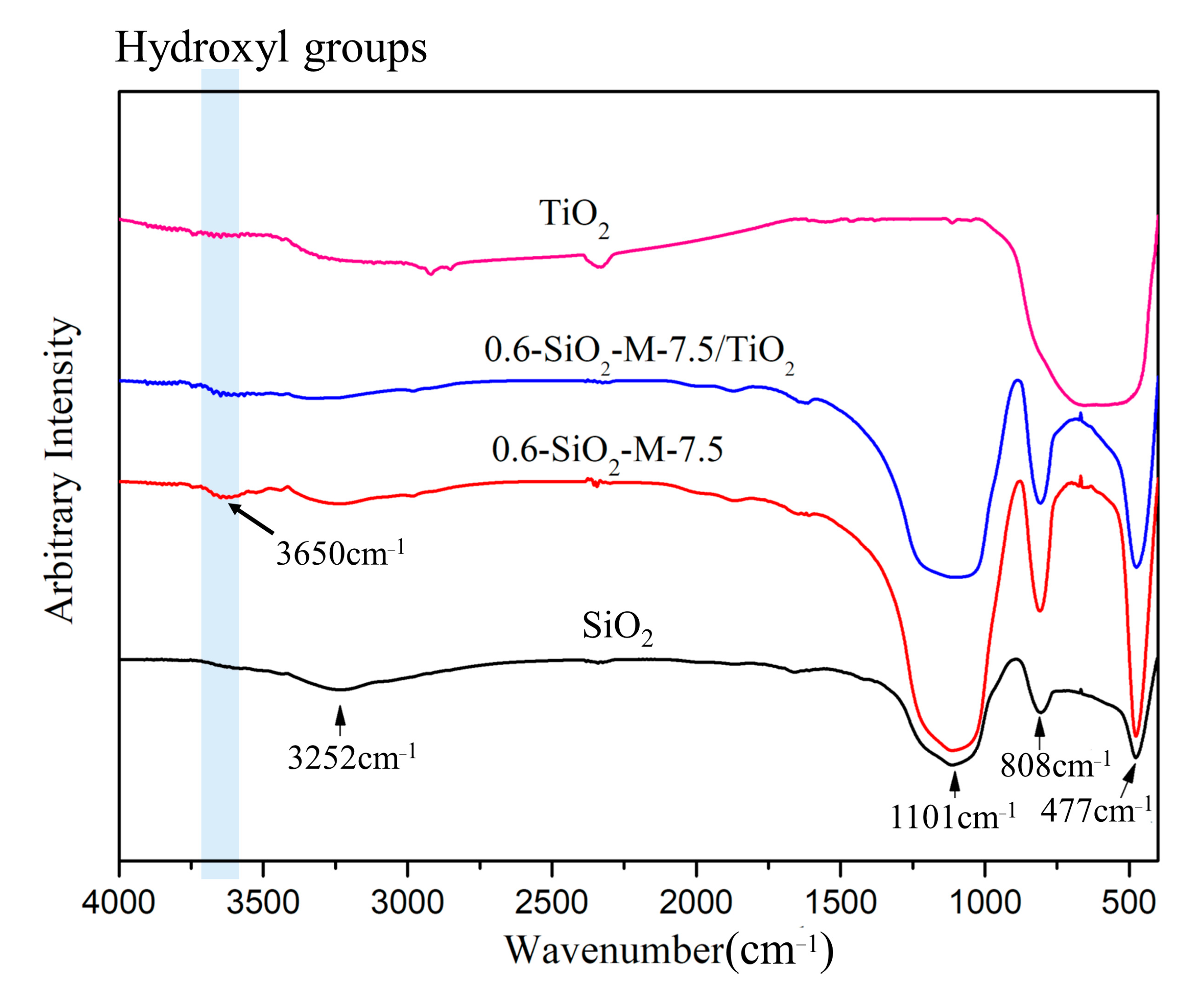
3.4. Interaction of SiO2-M with Nano-TiO2
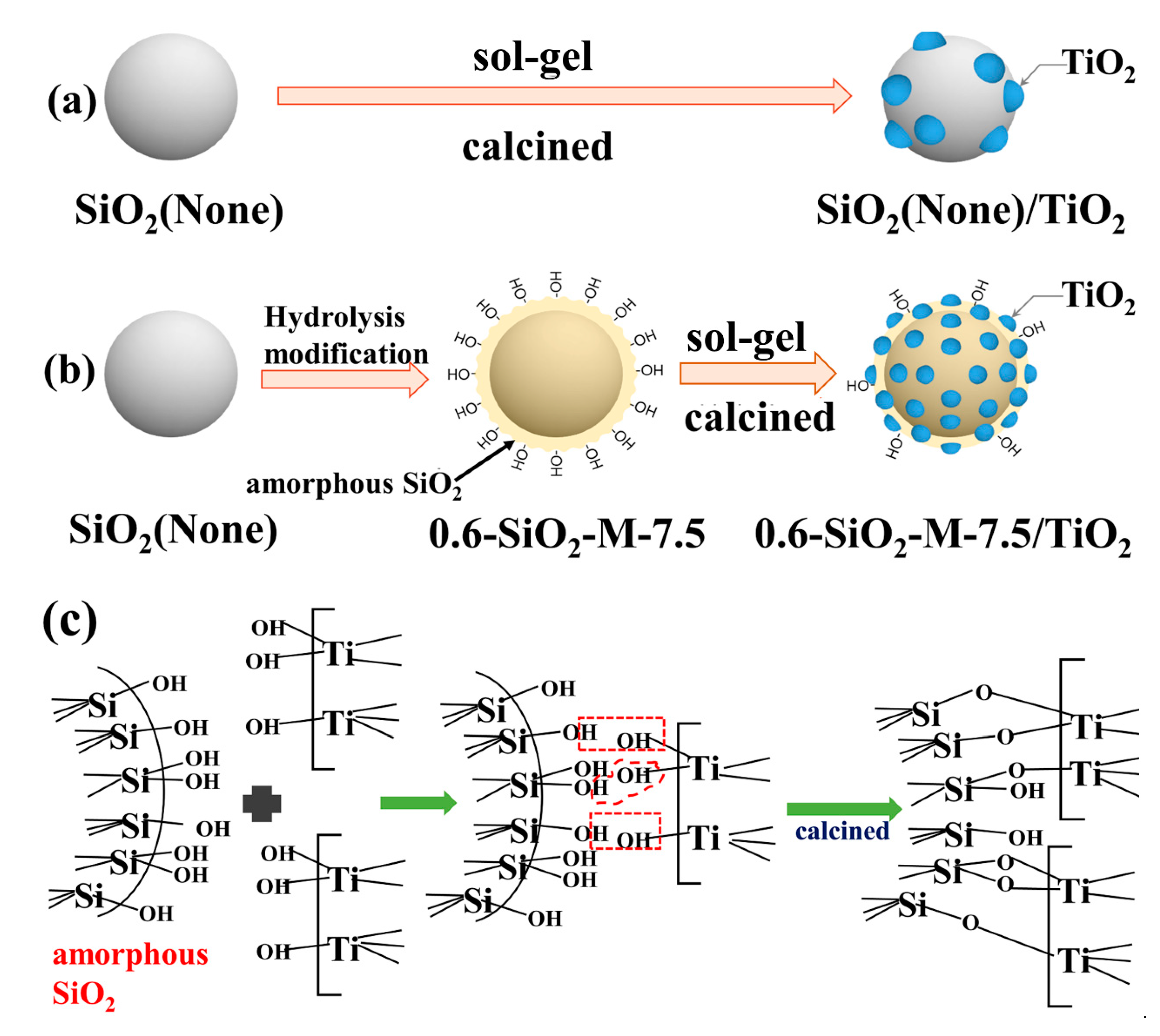
3.5. Mechanism of SiO2-M to Enhance the Performance of Nano-TiO2
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kong, W.; Wang, S.; Wu, D.; Chen, C.; Luo, Y.; Pei, Y.; Tian, B.; Zhang, J. Fabrication of 3D Sponge@AgBr-AgCl/Ag and Tubular Photoreactor for Continuous Wastewater Purification under Sunlight Irradiation. ACS Sustain. Chem. Eng. 2019, 7, 14051–14063. [Google Scholar] [CrossRef]
- Schneider, J.; Matsuoka, M.; Takeuchi, M.; Zhang, J.; Horiuchi, Y.; Anpo, M.; Bahnemann, D.W. Understanding TiO2 Photocatalysis: Mechanisms and Materials. Chem. Rev. 2014, 114, 9919–9986. [Google Scholar] [CrossRef]
- Bian, Z.; Cao, F.; Zhu, J.; Li, H. Plant Uptake-Assisted Round-the-Clock Photocatalysis for Complete Purification of Aquaculture Wastewater Using Sunlight. Environ. Sci. Technol. 2015, 49, 2418–2424. [Google Scholar] [CrossRef]
- Chen, D.; Cheng, Y.; Zhou, N.; Chen, P.; Wang, Y.; Li, K.; Huo, S.; Cheng, P.; Peng, P.; Zhang, R.; et al. Photocatalytic degradation of organic pollutants using TiO2-based photocatalysts: A review. J. Clean. Prod. 2020, 268, 121725. [Google Scholar] [CrossRef]
- Xiao, H.; Liu, P.; Wang, W.; Ran, R.; Zhou, W.; Shao, Z. Ruddlesden-Popper Perovskite Oxides for Photocatalysis-Based Water Splitting and Wastewater Treatment. Energy Fuels 2020, 34, 9208–9221. [Google Scholar] [CrossRef]
- Yang, X.; Wang, D. Photocatalysis: From Fundamental Principles to Materials and Applications. ACS Appl. Energy Mater. 2018, 1, 6657–6693. [Google Scholar] [CrossRef]
- He, F.; Muliane, U.; Weon, S.; Choi, W. Substrate-specific mineralization and deactivation behaviors of TiO2 as an air-cleaning photocatalyst. Appl. Catal. B Environ. 2020, 275, 119145. [Google Scholar] [CrossRef]
- Wu, H.; Inaba, T.; Wang, Z.-M.; Endo, T. Photocatalytic TiO2@CS-embedded cellulose nanofiber mixed matrix membrane. Appl. Catal. B Environ. 2020, 276, 119111. [Google Scholar] [CrossRef]
- Liu, M.; Zheng, L.; Bao, X.; Wang, Z.; Wang, P.; Liu, Y.; Cheng, H.; Dai, Y.; Huang, B.; Zheng, Z. Substrate-dependent ALD of Cux on TiO2 and its performance in photocatalytic CO2 reduction. Chem. Eng. J. 2021, 405, 126654. [Google Scholar] [CrossRef]
- Mukherjee, K.; Acharya, K.; Biswas, A.; Jana, N.R. TiO2 Nanoparticles Co-doped with Nitrogen and Fluorine as Visible-Light-Activated Antifungal Agents. ACS Appl. Nano Mater. 2020, 3, 2016–2025. [Google Scholar] [CrossRef]
- Wang, J.; Sun, S.; Ding, H.; Li, W.; Wang, X. Well-Designed CdS/TiO2/MS-SiO2 Z-Scheme Photocatalyst for Combating Poison with Poison. Ind. Eng. Chem. Res. 2020, 59, 7659–7669. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, K. Fabrication of Ce/N co-doped TiO2/diatomite granule catalyst and its improved visible-light-driven photoactivity. J. Hazard. Mater. 2017, 324, 139–150. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.; Peng, Z.; Jiang, R.; Jia, P.; Feng, J.; Yang, P.; Chi, Q.; Ye, W.; Xu, F.; Gao, P. Nanolayered Heterostructures of N-Doped TiO2 and N-Doped Carbon for Hydrogen Evolution. ACS Appl. Nano Mater. 2020, 3, 1373–1381. [Google Scholar] [CrossRef]
- Yu, Y.; Tang, Y.; Yuan, J.; Wu, Q.; Zheng, W.; Cao, Y. Fabrication of N-TiO2/InBO3 Heterostructures with Enhanced Visible Photocatalytic Performance. J. Phys. Chem. C 2014, 118, 13545–13551. [Google Scholar] [CrossRef]
- Veziroglu, S.; Obermann, A.-L.; Ullrich, M.; Hussain, M.; Kamp, M.; Kienle, L.; Leissner, T.; Rubahn, H.-G.; Polonskyi, O.; Strunskus, T.; et al. Photodeposition of Au Nanoclusters for Enhanced Photocatalytic Dye Degradation over TiO2 Thin Film. ACS Appl. Mater. Interfaces 2020, 12, 14983–14992. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Chen, W.; Zhang, C.; Liu, Y.; Zhao, C.; Dai, Y. Hydrothermal synthesis of porous TiO2 microspheres and their photocatalytic degradation of gaseous benzene. Chem. Eng. J. 2011, 170, 53–58. [Google Scholar] [CrossRef]
- Wang, J.; Sun, S.; Ding, H.; He, Z.; Wang, X.; Zhou, R.; Zhang, H. A semiconductor-insulator heterojunction induced by hydroxyl groups formed on the surface of SiO2 microspheres. Appl. Surf. Sci. 2020, 531. [Google Scholar] [CrossRef]
- Guesh, K.; Marquez-Alvarez, C.; Chebude, Y.; Diaz, I. Enhanced photocatalytic activity of supported TiO2 by selective surface modification of zeolite Y. Appl. Surf. Sci. 2016, 378, 473–478. [Google Scholar] [CrossRef]
- Huang, Q.; Tian, S.; Zeng, D.; Wang, X.; Song, W.; Li, Y.; Xiao, W.; Xie, C. Enhanced Photocatalytic Activity of Chemically Bonded TiO2/Graphene Composites Based on the Effective Interfacial Charge Transfer through the C-Ti Bond. ACS Catal. 2013, 3, 1477–1485. [Google Scholar] [CrossRef]
- Zuo, R.; Du, G.; Zhang, W.; Liu, L.; Liu, Y.; Mei, L.; Li, Z. Photocatalytic Degradation of Methylene Blue Using TiO2 Impregnated Diatomite. Adv. Mater. Sci. Eng. 2014, 2014. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; Elzatahry, A.; Aldhayan, D.; Zhao, D. Core-shell structured titanium dioxide nanomaterials for solar energy utilization. Chem. Soc. Rev. 2018, 47, 8203–8237. [Google Scholar] [CrossRef]
- Byrne, J.A.; Eggins, B.R.; Brown, N.M.D.; McKinney, B.; Rouse, M. Immobilisation of TiO2 powder for the treatment of polluted water. Appl. Catal. B Environ. 1998, 17, 25–36. [Google Scholar] [CrossRef]
- Sun, S.; Ding, H.; Mei, L.; Chen, Y.; Hao, Q.; Chen, W.; Xu, Z.; Chen, D. Construction of SiO2-TiO2/g-C3N4 composite photocatalyst for hydrogen production and pollutantdegradation: Insight into the effect of SiO2. Chin. Chem. Lett. 2020, 31, 2287–2294. [Google Scholar] [CrossRef]
- Zhang, M.; Lei, E.; Zhang, R.; Liu, Z. The effect of SiO2 on TiO2-SiO2 composite film for self-cleaning application. Surf. Interfaces 2019, 16, 194–198. [Google Scholar] [CrossRef]
- Wang, D.; Geng, Z.; Hou, P.; Yang, P.; Cheng, X.; Huang, S. Rhodamine B Removal of TiO2@SiO2 Core-Shell Nanocomposites Coated to Buildings. Crystals 2020, 10, 80. [Google Scholar] [CrossRef] [Green Version]
- Cizmar, T.; Stangar, U.L.; Fanetti, M.; Arcon, I. Effects of Different Copper Loadings on the Photocatalytic Activity of TiO2-SiO2 Prepared at a Low Temperature for the Oxidation of Organic Pollutants in Water. Chemcatchem 2018, 10, 2982–2993. [Google Scholar] [CrossRef]
- Wang, J.; Sun, S.; Ding, H.; Chen, W.; Liang, Y. Preparation of a composite photocatalyst with enhanced photocatalytic activity: Smaller TiO2 carried on SiO2 microsphere. Appl. Surf. Sci. 2019, 493, 146–156. [Google Scholar] [CrossRef]
- Zhang, H.; Sun, S.; Ding, H.; Deng, T.; Wang, J. Effect of calcination temperature on the structure and properties of SiO2 microspheres/nano-TiO2 composites. Mater. Sci. Semicond. Process. 2020, 115, 105099. [Google Scholar] [CrossRef]
- Mueller, R.; Kammler, H.K.; Wegner, K.; Pratsinis, S.E. OH surface density of SiO2 and TiO2 by thermogravimetric analysis. Langmuir 2003, 19, 160–165. [Google Scholar] [CrossRef]
- Wu, X.; Fan, M.; Shen, X.; Cui, S.; Tan, G. Silica aerogels formed from soluble silicates and methyl trimethoxysilane (MTMS) using CO2 gas as a gelation agent. Ceram. Int. 2018, 44, 821–829. [Google Scholar] [CrossRef]
- Beránek, L. An Examination of the Langmuir-Hinshelwood Model Using Ion Exchange Catalysts. Catal. Rev. 1977, 16, 1–35. [Google Scholar] [CrossRef]
- Qin, L.; Wang, Y.; Vivar, M.; Huang, Q.; Zhu, L.; Fuentes, M.; Wang, Z. Comparison of photovoltaic and photocatalytic performance of non-concentrating and V-trough SOLWAT (solar water purification and renewable electricity generation) systems for water purification. Energy 2015, 85, 251–260. [Google Scholar] [CrossRef]
- Lei, Y.; Li, J.; Wang, Z.; Sun, J.; Chen, F.; Liu, H.; Ma, X.; Liu, Z. Atomic-scale investigation of a new phase transformation process in TiO2 nanofibers. Nanoscale 2017, 9, 4601–4609. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mortazavi-Derazkola, S.; Salavati-Niasari, M.; Amiri, O.; Abbasi, A. Fabrication and characterization of Fe3O4@SiO2@TiO2@Ho nanostructures as a novel and highly efficient photocatalyst for degradation of organic pollution. J. Energy Chem. 2017, 26, 17–23. [Google Scholar] [CrossRef] [Green Version]
- Resende, S.F.; Nunes, E.H.M.; Houmard, M.; Vasconcelos, W.L. Simple sol-gel process to obtain silica-coated anatase particles with enhanced TiO2-SiO2 interfacial area. J. Colloid Interface Sci. 2014, 433, 211–217. [Google Scholar] [CrossRef] [Green Version]
- Sun, S.; Li, C.; Sun, Z.; Wang, J.; Ding, H. In-situ design of efficient hydroxylated SiO2/g-C3N4 composite photocatalyst: Synergistic effect of compounding and surface hydroxylation. Chem. Eng. J. 2021, 416, 129107. [Google Scholar] [CrossRef]




| Sample | Number of Surface Hydroxyl Groups (/nm2) |
|---|---|
| SiO2 microspheres | 0.38 |
| 0.6-SiO2-M-7.5 | 1.64 |
| Sample | SBET/(m2/g) | VTotal/(cm3/g) | DAver/nm |
|---|---|---|---|
| SiO2 | 23.58 | 0.05 | 3.84 |
| 0.6-SiO2-M-7.5 | 10.49 | 0.03 | 5.61 |
| 0.6-SiO2-M-7.5/TiO2 | 21.12 | 0.04 | 3.39 |
| TiO2 | 90.45 | 0.34 | 5.61 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tu, Y.; Ao, W.; Wang, C.; Ren, T.; Zhang, L.; Zhong, J.; Li, W.; Ding, H. Hydrolytic Modification of SiO2 Microspheres with Na2SiO3 and the Performance of Supported Nano-TiO2 Composite Photocatalyst. Materials 2021, 14, 2553. https://doi.org/10.3390/ma14102553
Tu Y, Ao W, Wang C, Ren T, Zhang L, Zhong J, Li W, Ding H. Hydrolytic Modification of SiO2 Microspheres with Na2SiO3 and the Performance of Supported Nano-TiO2 Composite Photocatalyst. Materials. 2021; 14(10):2553. https://doi.org/10.3390/ma14102553
Chicago/Turabian StyleTu, Yu, Weihua Ao, Chunhong Wang, Tianyu Ren, Lijuan Zhang, Jiaxin Zhong, Wei Li, and Hao Ding. 2021. "Hydrolytic Modification of SiO2 Microspheres with Na2SiO3 and the Performance of Supported Nano-TiO2 Composite Photocatalyst" Materials 14, no. 10: 2553. https://doi.org/10.3390/ma14102553




