The Impact of Contaminating Poly (Methyl Methacrylate) (PMMA) Bone Cements on Their Compressive Strength
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation
2.3. Mechanical Testing
2.4. Statistical Analysis
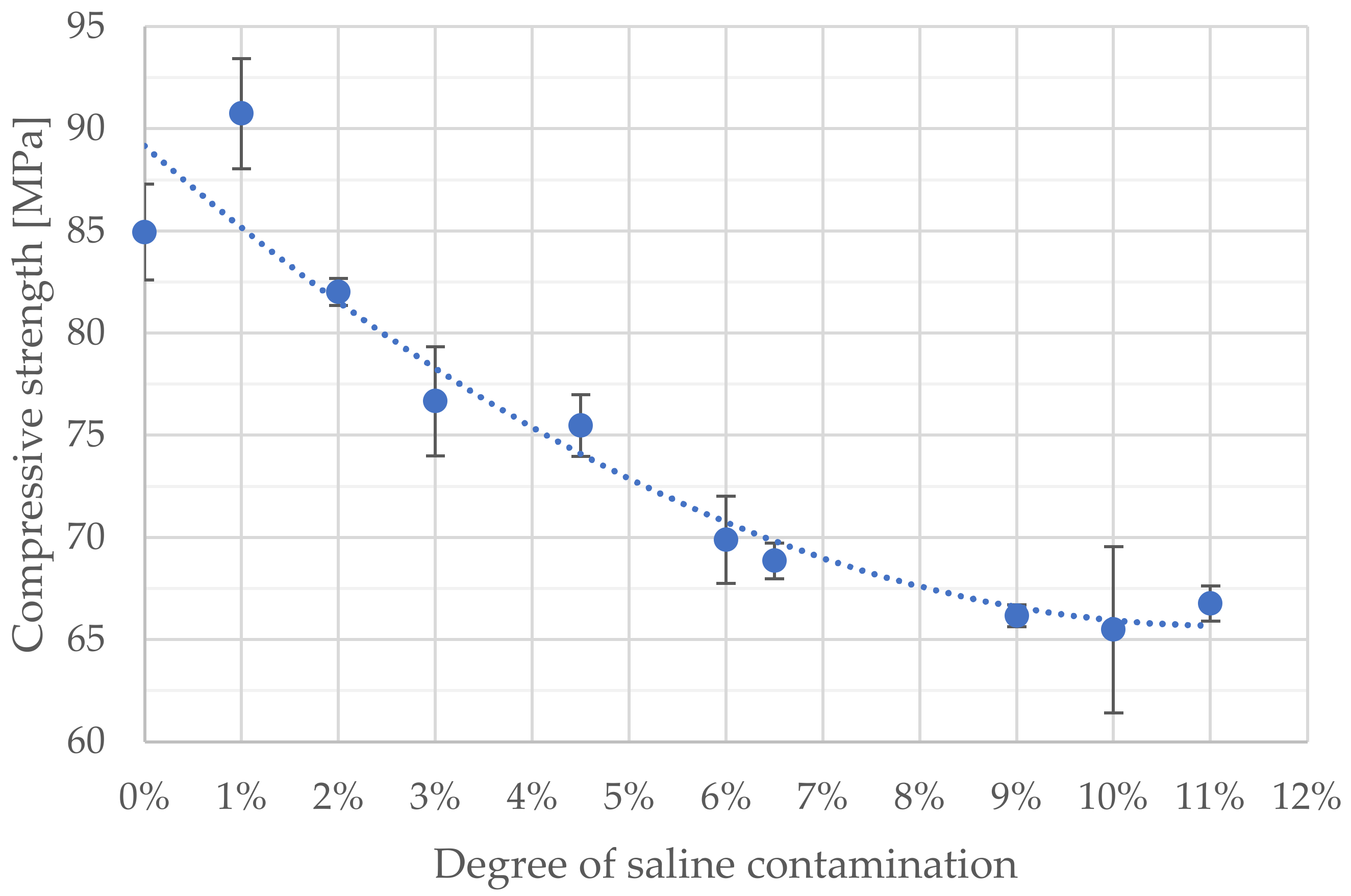
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Krakowski, P.; Gerkowicz, A.; Pietrzak, A.; Krasowska, D.; Jurkiewicz, A.; Gorzelak, M.; Schwartz, R.A. Psoriatic Arthritis—New Perspectives. AMS 2019, 15, 580–589. [Google Scholar] [CrossRef]
- Borkowski, L.; Lübek, T.; Jojczuk, M.; Nogalski, A.; Belcarz, A.; Palka, K.; Hajnos, M.; Ginalska, G. Behavior of New Hydroxyapatite/Glucan Composite in Human Serum: Behavior of New Hap/Glucan Composite. J. Biomed. Mater. Res. 2018, 106, 2653–2664. [Google Scholar] [CrossRef] [PubMed]
- Borkowski, L.; Przekora, A.; Belcarz, A.; Palka, K.; Jozefaciuk, G.; Lübek, T.; Jojczuk, M.; Nogalski, A.; Ginalska, G. Fluorapatite Ceramics for Bone Tissue Regeneration: Synthesis, Characterization and Assessment of Biomedical Potential. Mater. Sci. Eng. C 2020, 116, 111211. [Google Scholar] [CrossRef] [PubMed]
- Pei, X.; Wu, L.; Zhou, C.; Fan, H.; Gou, M.; Li, Z.; Zhang, B.; Lei, H.; Sun, H.; Liang, J.; et al. 3D Printed Titanium Scaffolds with Homogeneous Diamond-like Structures Mimicking That of the Osteocyte Microenvironment and Its Bone Regeneration Study. Biofabrication 2020, 13, 015008. [Google Scholar] [CrossRef]
- Monzón, R.A.; Coury, J.G.; Disse, G.D.; Lum, Z.C. Bone Cement in Total Hip and Knee Arthroplasty. JBJS Rev. 2019, 7, e6. [Google Scholar] [CrossRef]
- Jiang, H.-J.; Xu, J.; Qiu, Z.-Y.; Ma, X.-L.; Zhang, Z.-Q.; Tan, X.-X.; Cui, Y.; Cui, F.-Z. Mechanical Properties and Cytocompatibility Improvement of Vertebroplasty PMMA Bone Cements by Incorporating Mineralized Collagen. Materials 2015, 8, 2616–2634. [Google Scholar] [CrossRef]
- Jasper, L.E.; Deramond, H.; Mathis, J.M.; Belkoff, S.M. Material Properties of Various Cements for Use with Vertebroplasty. J. Mater. Sci. Mater. Med. 2002, 13, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Wu, Z.; He, D.; Shen, K.; Liu, X.; Li, H.; Jin, W. Bioactive Injectable Polymethylmethacrylate/Silicate Bioceramic Hybrid Cements for Percutaneous Vertebroplasty and Kyphoplasty. J. Mech. Behav. Biomed. Mater. 2019, 96, 125–135. [Google Scholar] [CrossRef]
- Lewis, G. Injectable Bone Cements for Use in Vertebroplasty and Kyphoplasty: State-of-the-Art Review. J. Biomed. Mater. Res. 2006, 76B, 456–468. [Google Scholar] [CrossRef] [PubMed]
- Karpiński, R.; Szabelski, J.; Maksymiuk, J. Effect of Physiological Fluids Contamination on Selected Mechanical Properties of Acrylate Bone Cement. Materials 2019, 12, 3963. [Google Scholar] [CrossRef] [PubMed]
- Karpiński; Szabelski. Maksymiuk Seasoning Polymethyl Methacrylate (PMMA) Bone Cements with Incorrect Mix Ratio. Materials 2019, 12, 3073. [Google Scholar] [CrossRef]
- Spierings, P.T.J. Testing and Performance of Bone Cements. In The Well-Cemented Total Hip Arthroplasty; Springer-Verlag: Berlin/Heidelberg, Germany, 2005; pp. 67–78. ISBN 978-3-540-24197-3. [Google Scholar]
- Schröder, C.; Nguyen, M.; Kraxenberger, M.; Chevalier, Y.; Melcher, C.; Wegener, B.; Birkenmaier, C. Modification of PMMA Vertebroplasty Cement for Reduced Stiffness by Addition of Normal Saline: A Material Properties Evaluation. Eur. Spine J. 2017, 26, 3209–3215. [Google Scholar] [CrossRef]
- ISO 5833:2002. Implants for Surgery—Acrylic Resin Cements; International Organization for Standardization: Geneva, Switzerland, 2002. [Google Scholar]
- Rabiej, M. Grupa Wydawnicza Helion Analizy statystyczne z programami Statistica i Excel; Wydawnictwo Helion: Gliwice, Poland, 2018; ISBN 978-83-283-3922-4. [Google Scholar]
- Salkind, N. Tukey’s Honestly Significant Difference (HSD). In Encyclopedia of Research Design; SAGE Publications, Inc.: Thousand Oaks, CA, USA, 2010. [Google Scholar]
- Abdi, H.; Williams, L.J. Newman–Keuls Test and Tukey Test. In Encyclopedia of Research Design; SAGE Publications, Inc.: Thousand Oaks, CA, USA, 2010. [Google Scholar]
- Ahmed, S.S.; Begum, F.; Kayani, B.; Haddad, F.S. Risk Factors, Diagnosis and Management of Prosthetic Joint Infection after Total Hip Arthroplasty. Expert Rev. Med. Devices 2019, 16, 1063–1070. [Google Scholar] [CrossRef]
- Dunne, N.; Hill, J.; Mcafee, P.; Todd, K.; Kirkpatrick, R.; Tunney, M.; Patrick, S. In Vitro Study of the Efficacy of Acrylic Bone Cement Loaded with Supplementary Amounts of Gentamicin: Effect on Mechanical Properties, Antibiotic Release, and Biofilm Formation. Acta Orthop. 2007, 78, 774–785. [Google Scholar] [CrossRef] [PubMed]
- Pelletier, M.H.; Malisano, L.; Smitham, P.J.; Okamoto, K.; Walsh, W.R. The Compressive Properties of Bone Cements Containing Large Doses of Antibiotics. J. Arthroplast. 2009, 24, 454–460. [Google Scholar] [CrossRef] [PubMed]
- Wolf-Brandstetter, C.; Roessler, S.; Storch, S.; Hempel, U.; Gbureck, U.; Nies, B.; Bierbaum, S.; Scharnweber, D. Physicochemical and Cell Biological Characterization of PMMA Bone Cements Modified with Additives to Increase Bioactivity. J. Biomed. Mater. Res. 2013, 101B, 599–609. [Google Scholar] [CrossRef]
- Endogan, T.; Serbetci, K.; Hasirci, N. Effects of Ingredients on Thermal and Mechanical Properties of Acrylic Bone Cements. J. Appl. Polym. Sci. 2009, 113, 4077–4084. [Google Scholar] [CrossRef]
- Harper, E.J.; Braden, M.; Bonfield*, W. Mechanical Properties of Hydroxyapatite Reinforced Poly(Ethylmethacrylate) Bone Cement after Immersion in a Physiological Solution: Influence of a Silane Coupling Agent. J. Mater. Sci. Mater. Med. 2000, 11, 491–497. [Google Scholar] [CrossRef] [PubMed]
- Matuszewski, Ł.; Olchowik, G.; Mazurkiewicz, T.; Kowalczyk, B.; Zdrojewska, A.; Matuszewska, A.; Ciszewski, A.; Gospodarek, M.; Morawik, I. Biomechanical Parameters of the BP-Enriched Bone Cement. Eur. J. Orthop. Surg. Traumatol. 2014, 24, 435–441. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Karpiński, R.; Szabelski, J.; Krakowski, P.; Jonak, J. Effect of Physiological Saline Solution Contamination on Selected Mechanical Properties of Seasoned Acrylic Bone Cements of Medium and High Viscosity. Materials 2021, 14, 110. [Google Scholar] [CrossRef] [PubMed]
- Machrowska, A.; Szabelski, J.; Karpiński, R.; Krakowski, P.; Jonak, J.; Jonak, K. Use of Deep Learning Networks and Statistical Modeling to Predict Changes in Mechanical Parameters of Contaminated Bone Cements. Materials 2020, 13, 5419. [Google Scholar] [CrossRef] [PubMed]
- Machrowska, A.; Karpiński, R.; Jonak, J.; Szabelski, J.; Krakowski, P. Numerical Prediction of the Component-Ratio-Dependent Compressive Strength of Bone Cement. Appl. Comput. Sci. 2020, 87–101. [Google Scholar] [CrossRef]
- Karpinski, R.; Szabelski, J.; Maksymiuk, J. Analysis of the Properties of Bone Cement with Respect to Its Manufacturing and Typical Service Lifetime Conditions. MATEC Web Conf. 2018, 244, 01004. [Google Scholar] [CrossRef]
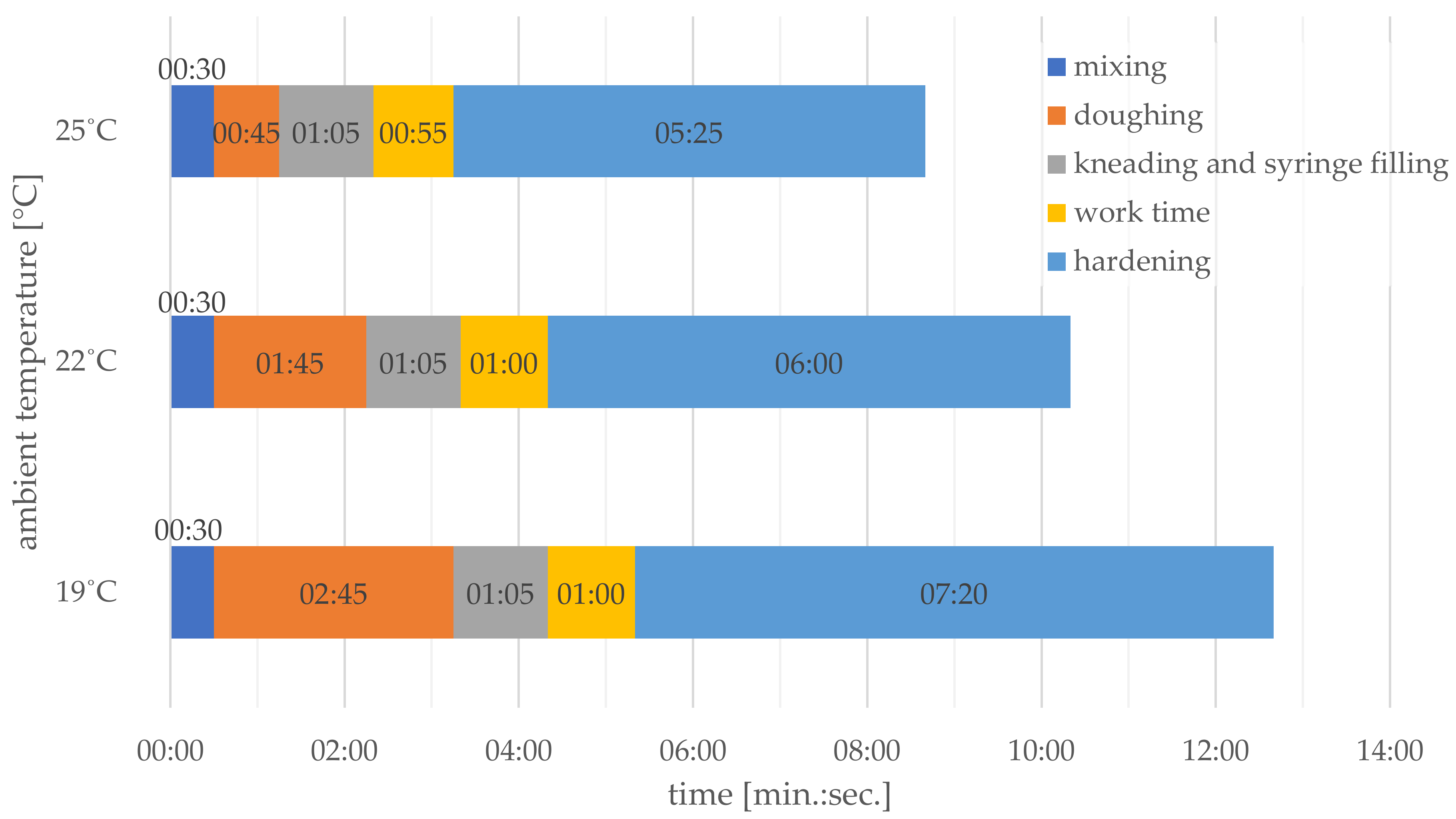
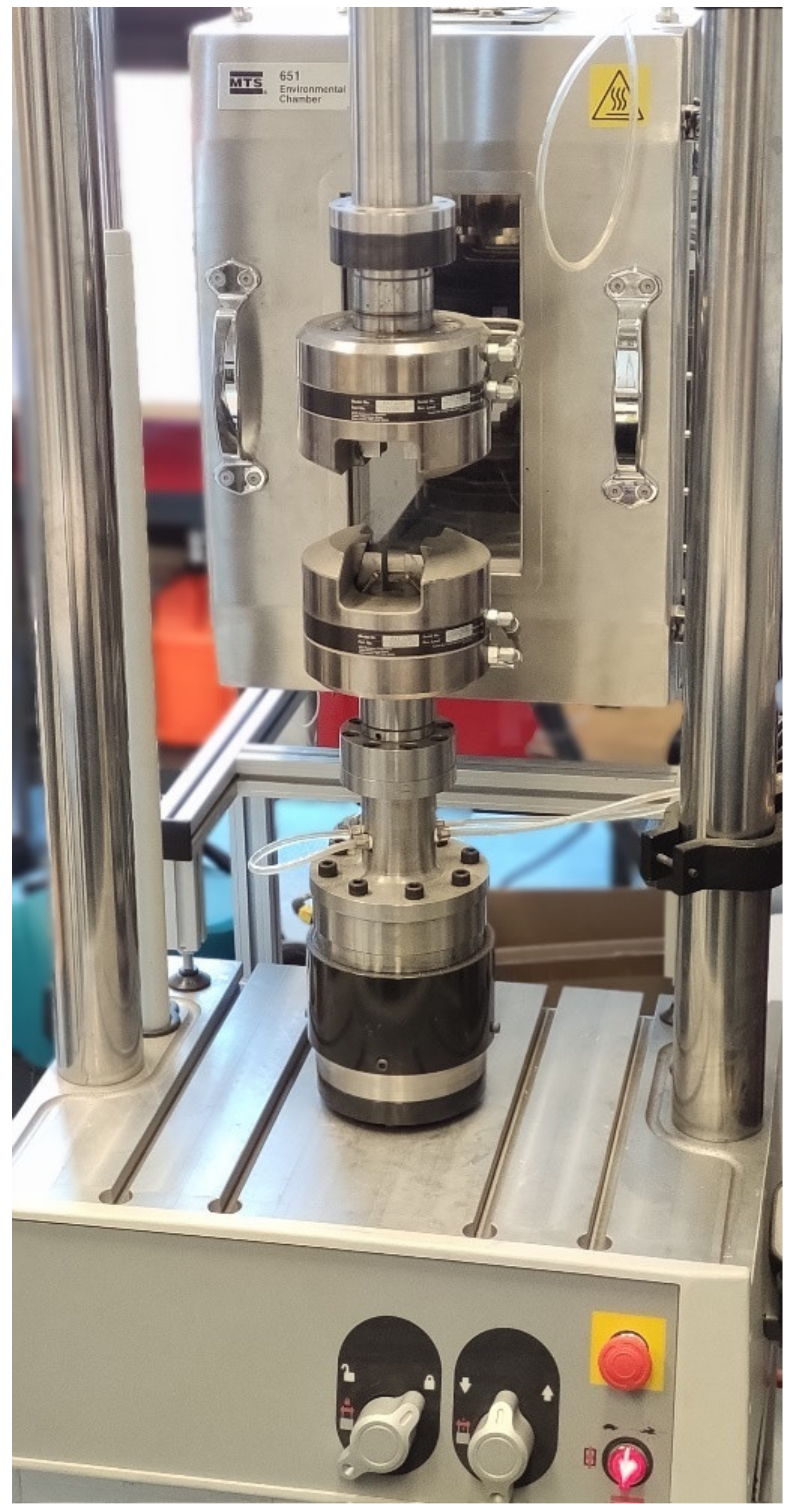
| Cement Type | Maximum Cross-Linking Temperature | Cross-Linking Time [min:s] |
|---|---|---|
| Boneloc | 36 °C | 11:00 |
| Cemex RX | 44 °C | 13:20 |
| Sulfix-6 | 48 °C | 10:50 |
| Zimmer LVC | 52 °C | 11:50 |
| Palacos R Genta | 56 °C | 10:40 |
| SamrtSet Genta | 56 °C | 09:50 |
| Osteopal | 58 °C | 12:10 |
| CMW Endurance | 63 °C | 12:10 |
| CMW 3 | 65 °C | 10:50 |
| CMW 1 Genta | 67 °C | 09:10 |
| Surgical Simplex Ro | 69 °C | 11:50 |
| Compound Name | CMW 3 |
|---|---|
| Bone Cement Powder Component | |
| Polymethyl methacrylate | 83.88 |
| Benzoyl peroxide | 2.00 |
| Barium sulphate | 10.00 |
| Bone Cement Liquid Component | |
| Methyl methacrylate | 97.5 |
| N,N-dimethyl-p-toluidine | <2.50 |
| Hydroquinone (ppm) | 75 |
| Degree of Doping | Average Compressive Strength [MPa] | Standard Deviation |
|---|---|---|
| non-doped | 84.95 | 2.35 |
| 1.0% | 90.75 | 2.69 |
| 2.0% | 82.02 | 0.66 |
| 3.0% | 76.67 | 2.67 |
| 4.5% | 75.48 | 1.50 |
| 6.0% | 69.90 | 2.14 |
| 6.5% | 68.86 | 0.88 |
| 9.0% | 66.17 | 0.53 |
| 10.0% | 65.50 | 4.07 |
| 11.0% | 66.77 | 0.87 |
| Contamination with Saline | Average Compressive Strength [MPa] | Homogeneous Groups | ||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | ||
| 0% | 84.95 | x | ||||
| 1% | 90.75 | x | ||||
| 2% | 82.02 | x | ||||
| 3% | 76.67 | x | ||||
| 4.5% | 75.48 | x | ||||
| 6% | 69.90 | x | ||||
| 6.5% | 68.86 | x | ||||
| 9% | 66.17 | x | x | |||
| 10% | 63.25 | x | ||||
| Amount of Contamination | 0% 84.95 | 1% 90.75 | 2% 82.02 | 3% 76.67 | 4.5% 75.48 | 6% 69.90 | 6.5% 68.86 | 9% 66.17 | 10% 63.25 | 11% 66.77 |
|---|---|---|---|---|---|---|---|---|---|---|
| 0% | 0.00 | 0.27 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | |
| 1% | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | ||
| 2% | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | |||
| 3% | 0.99 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | ||||
| 4.5% | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | |||||
| 6% | 1.00 | 0.06 | 0.00 | 0.19 | ||||||
| 6.5% | 0.38 | 0.01 | 0.72 | |||||||
| 9% | 0.55 | 1.00 | ||||||||
| 10% | 0.29 | |||||||||
| 11% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szabelski, J.; Karpiński, R.; Krakowski, P.; Jonak, J. The Impact of Contaminating Poly (Methyl Methacrylate) (PMMA) Bone Cements on Their Compressive Strength. Materials 2021, 14, 2555. https://doi.org/10.3390/ma14102555
Szabelski J, Karpiński R, Krakowski P, Jonak J. The Impact of Contaminating Poly (Methyl Methacrylate) (PMMA) Bone Cements on Their Compressive Strength. Materials. 2021; 14(10):2555. https://doi.org/10.3390/ma14102555
Chicago/Turabian StyleSzabelski, Jakub, Robert Karpiński, Przemysław Krakowski, and Józef Jonak. 2021. "The Impact of Contaminating Poly (Methyl Methacrylate) (PMMA) Bone Cements on Their Compressive Strength" Materials 14, no. 10: 2555. https://doi.org/10.3390/ma14102555
APA StyleSzabelski, J., Karpiński, R., Krakowski, P., & Jonak, J. (2021). The Impact of Contaminating Poly (Methyl Methacrylate) (PMMA) Bone Cements on Their Compressive Strength. Materials, 14(10), 2555. https://doi.org/10.3390/ma14102555







