Reduction of Hysteresis in Hybrid Perovskite Transistors by Solvent-Controlled Growth
Abstract
1. Introduction
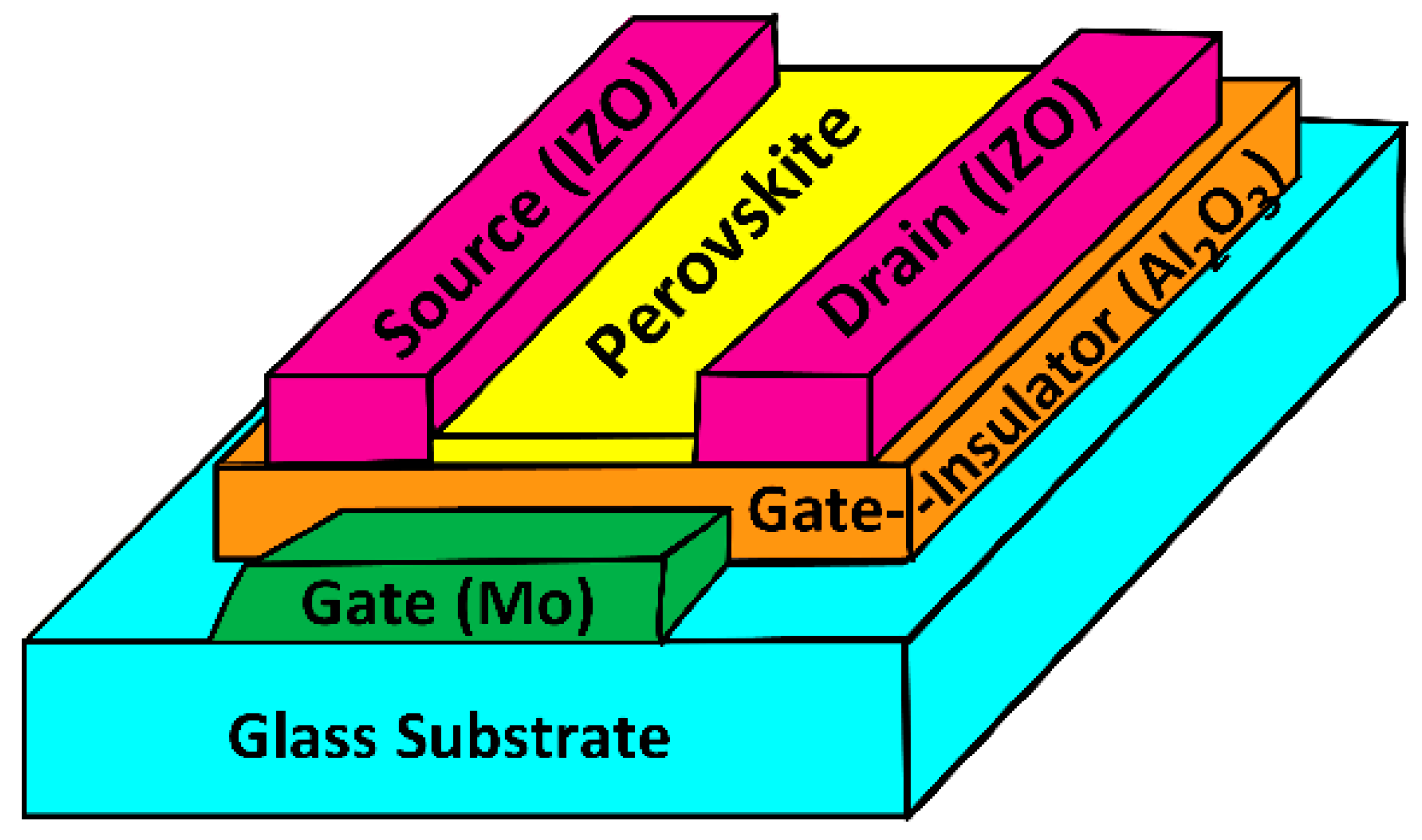
2. Materials and Methods
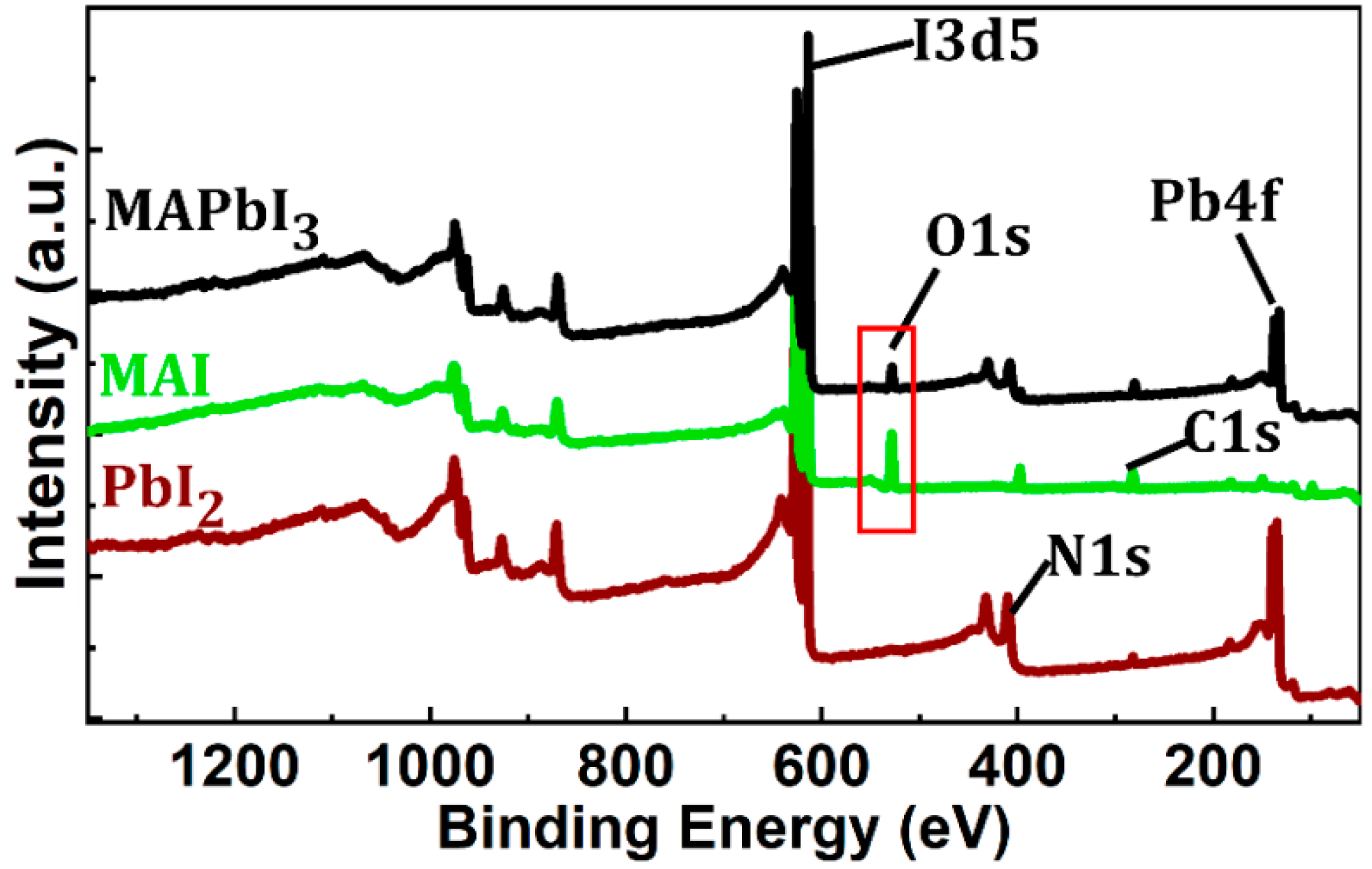
3. Results and Discussion
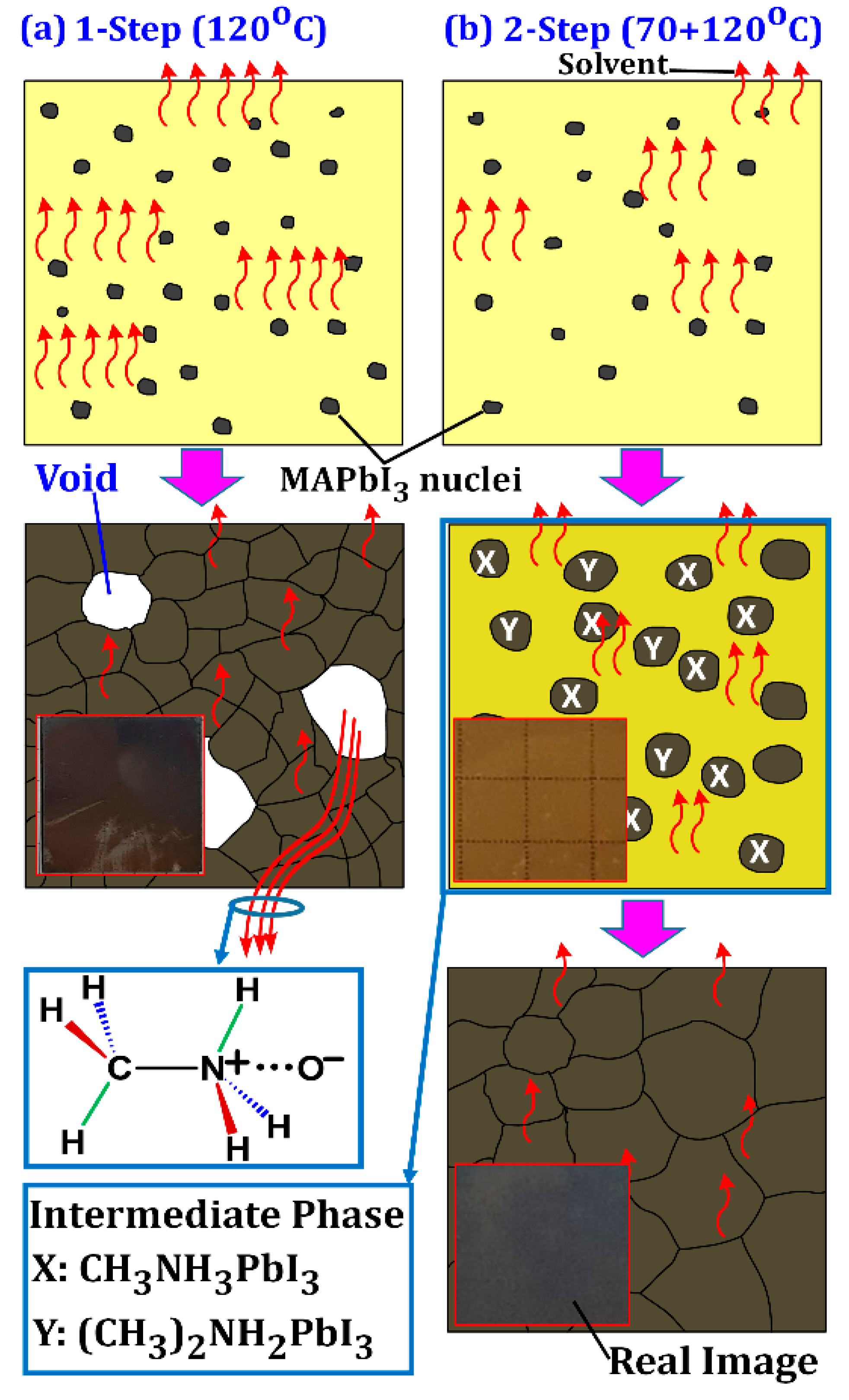
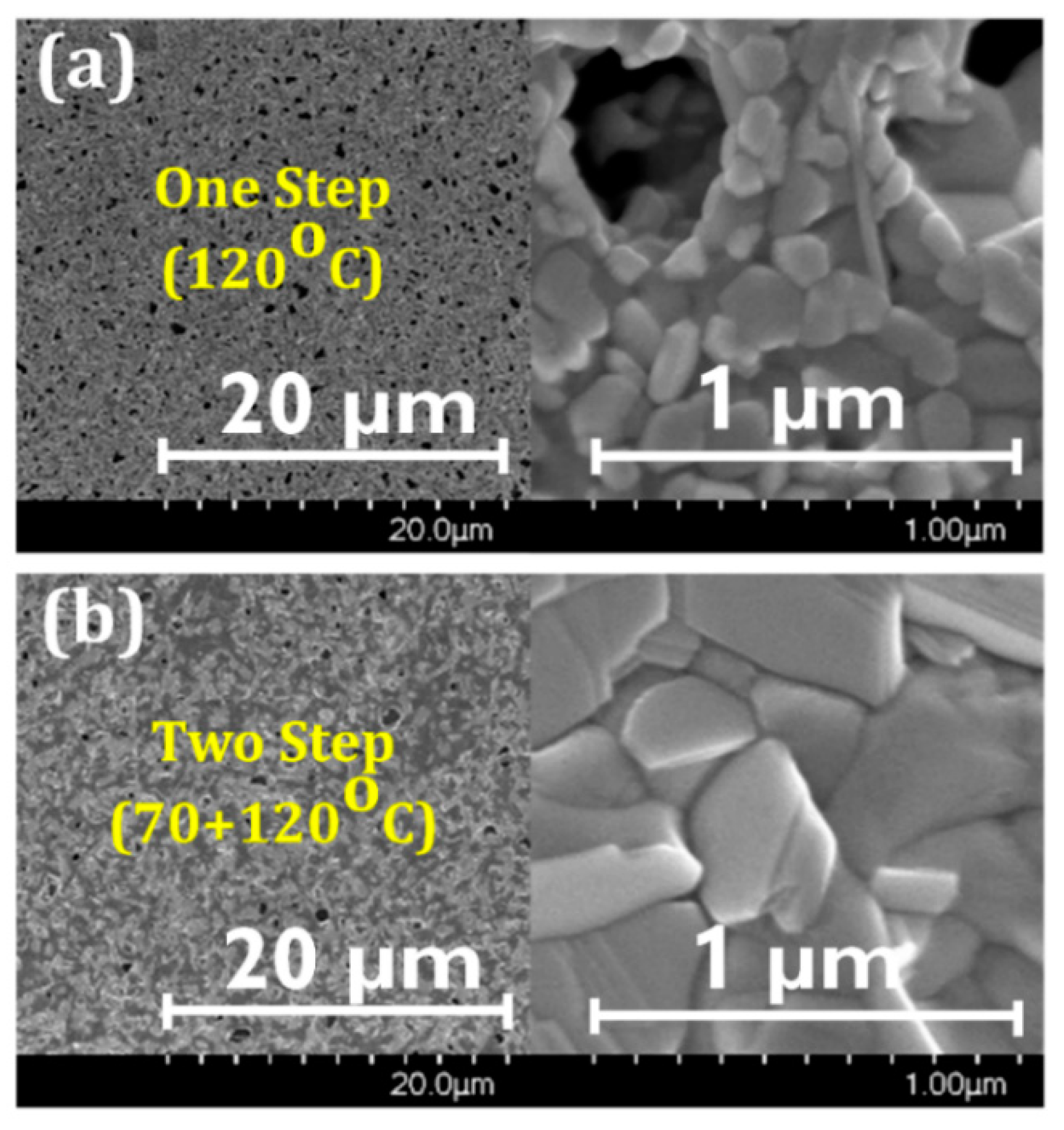
3.1. Film Morphology
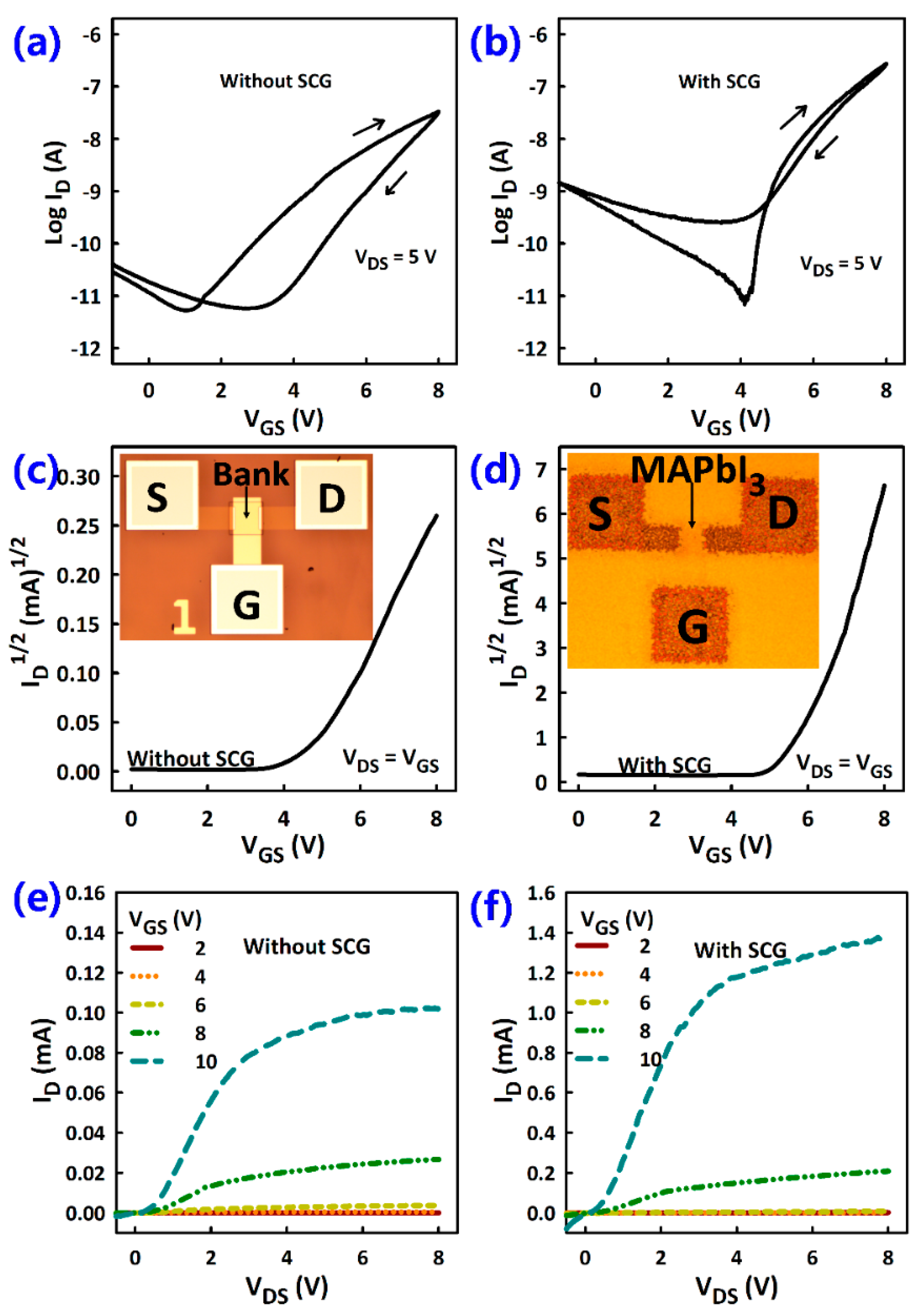
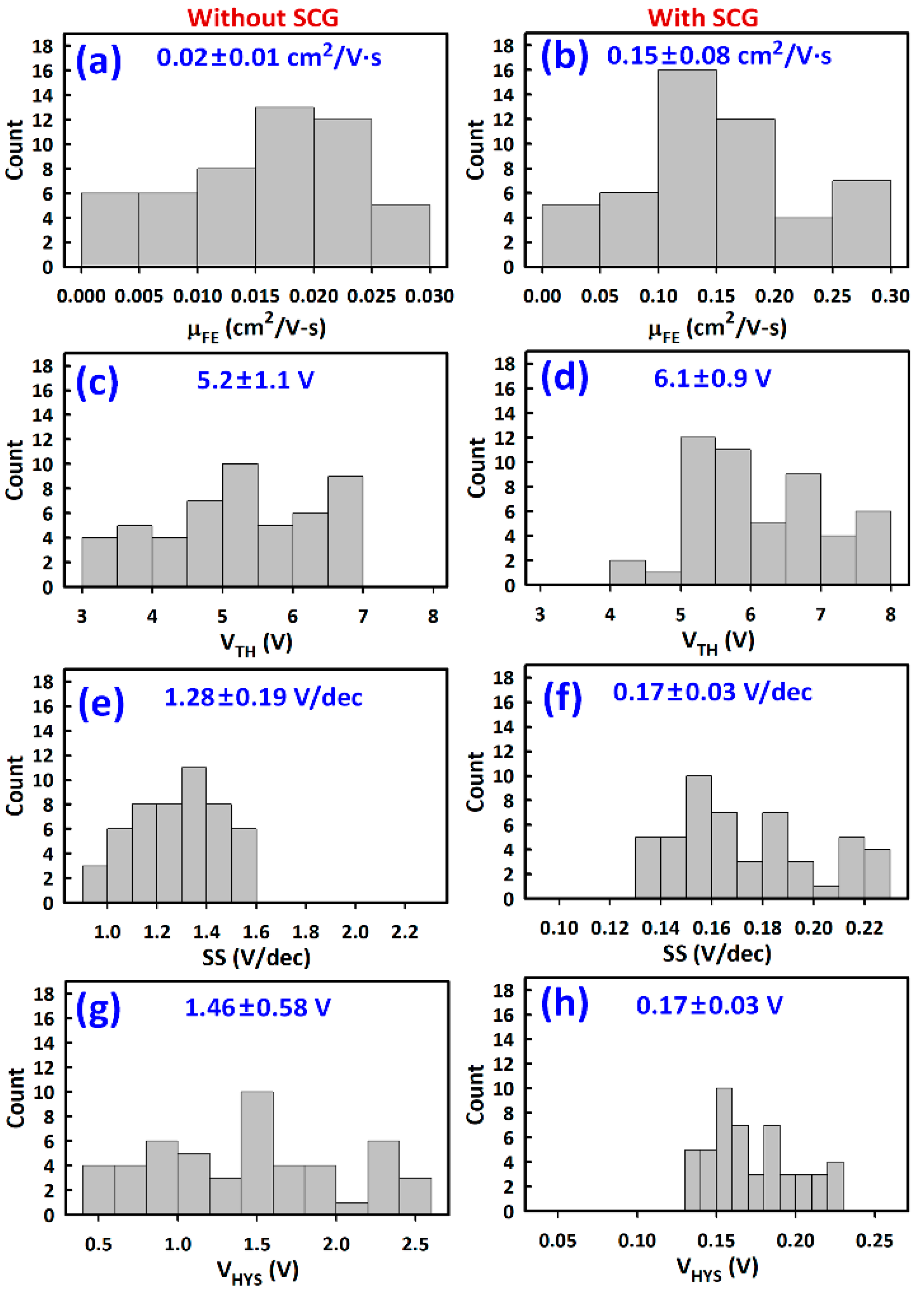
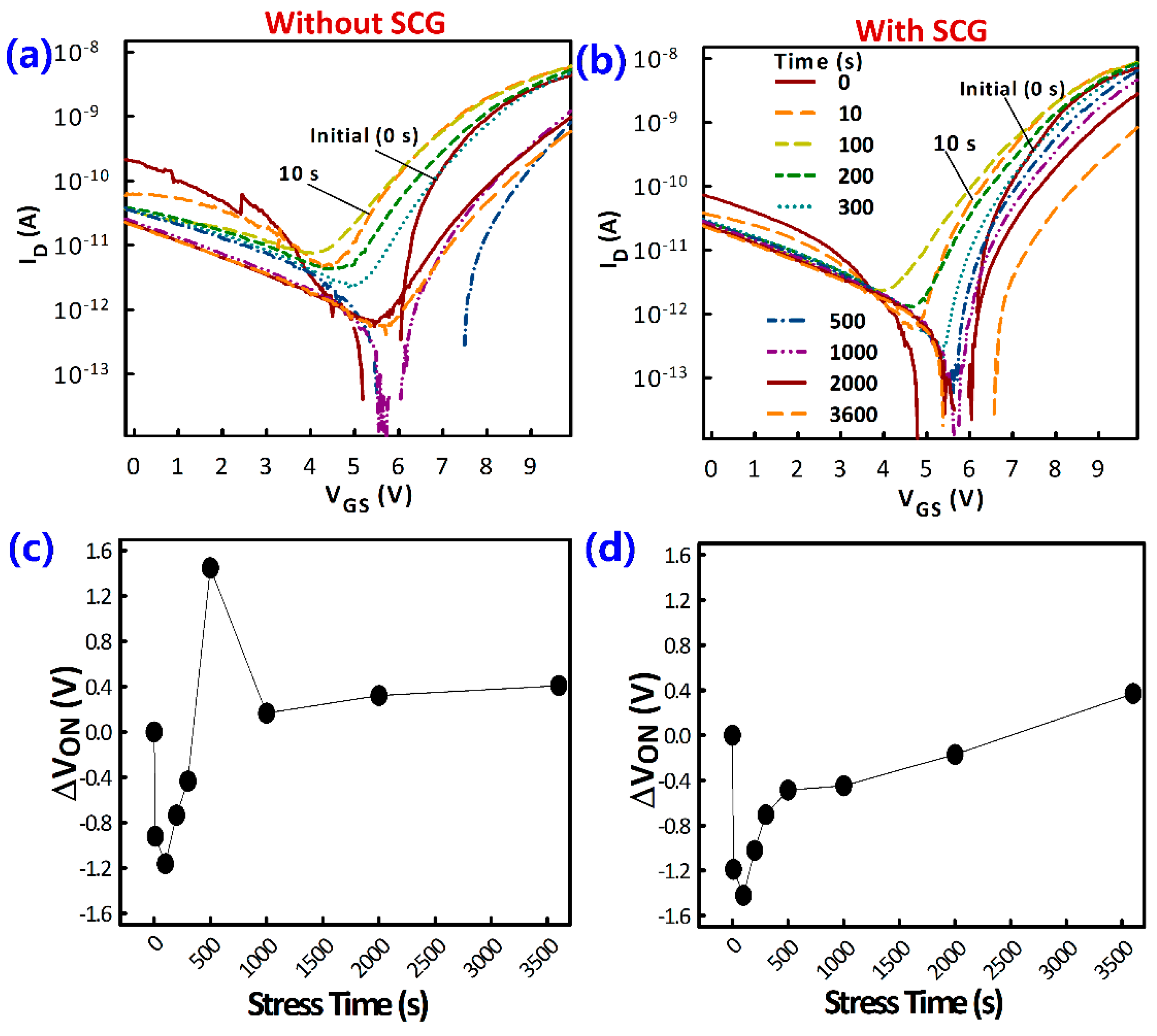
3.2. TFT Current-Voltage Characteristics
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Krishnamurthy, S.; Pandey, P.; Kaur, J.; Chakraborty, S.; Nayak, P.K.; Sadhanala, A.; Ogale1, S. Organic–inorganic hybrid and inorganic halide perovskites: Structural and chemical engineering, interfaces, and optoelectronic properties. J. Phys. D Appl. Phys. 2021, 54, 133002. [Google Scholar] [CrossRef]
- Chen, Q.; De Marco, N.; Yang, Y.M.; Song, T.B.; Chen, C.C.; Zhao, H.; Yang, Y. Under the spotlight: The organic–inorganic hybrid halide perovskite for optoelectronic applications. Nano Today 2015, 10, 355–396. [Google Scholar] [CrossRef]
- Green, M.; Dunlop, E.; Hohl-Ebinger, J.; Yoshita, M.; Kopidakis, N.; Hao, X. Solar cell efficiency tables (version 57). Prog. Photovolt. Res. 2021, 29, 3–15. [Google Scholar] [CrossRef]
- Stranks, S.D.; Snaith, H.J. Metal-halide perovskites for photovoltaic and light-emitting devices. Nat. Nanotechnol. 2015, 10, 391–402. [Google Scholar] [CrossRef] [PubMed]
- Sadhanala, A.; Deschler, F.; Thomas, T.H.; Dutton, S.E.; Goedel, K.C.; Hanusch, F.C.; Lai, M.L.; Steiner, U.; Bein, T.; Docampo, P.; et al. Preparation of single-phase films of CH3NH3Pb(I1–xBrx)3 with sharp optical band edges. J. Phys. Chem. Lett. 2014, 5, 2501–2505. [Google Scholar] [CrossRef]
- Shi, D.; Adinolfi, V.; Comin, R.; Yuan, M.; Alarousu, E.; Buin, A.; Chen, Y.; Hoogland, S.; Rothenberger, A.; Katsiev, K.; et al. Low trap-state density and long carrier diffusion in organolead trihalide perovskite single crystals. Science 2015, 347, 519–522. [Google Scholar] [CrossRef]
- Sadhanala, A.; Ahmad, S.; Zhao, B.; Giesbrecht, N.; Pearce, P.M.; Deschler, F.; Hoye, R.L.Z.; Gödel, K.C.; Bein, T.; Docampo, P.; et al. Blue-green color tunable solution processable organolead chloride–bromide mixed halide perovskites for optoelectronic applications. Nano Lett. 2015, 15, 6095–6101. [Google Scholar] [CrossRef]
- Miaoa, J.; Zhang, F. Recent progress on highly sensitive perovskite photodetectors. J. Mater. Chem. C 2019, 7, 1741–1791. [Google Scholar] [CrossRef]
- Wu, T.; Pisula, W.; Rashid, M.Y.A.; Gao, P. Application of Perovskite-Structured Materials in Field-Effect Transistors. Adv. Electron. Mater. 2019, 5, 1900444. [Google Scholar] [CrossRef]
- Liu, X.K.; Xu, W.; Bai, S.; Jin, Y.; Wang, J.; Friend, R.H.; Gao, F. Metal halide perovskites for light-emitting diodes. Nat. Mater. 2021, 20, 10–21. [Google Scholar] [CrossRef]
- Dualeh, A.; Moehi, T.; Tetreault, N.; Teuscher, J.; Gao, P.; Nazeeruddin, M.K.; Gratzel, M. Temperature dependence of transport properties of spiro-MeOTAD as a hole transport material in solid-state dye-sensitized solar cells. ACS Nano 2013, 7, 2292–2301. [Google Scholar] [CrossRef]
- Snaith, H.J.; Abate, A.; Ball, J.M.; Eperon, G.E.; Leijtens, T.; Noel, N.K.; Stranks, S.D.; Wang, J.T.-W. Wojciechowski, K., Zhang, W. Anomalous hysteresis in perovskite solar cell. J. Phys. Chem. Lett. 2014, 5, 1511–1515. [Google Scholar] [CrossRef] [PubMed]
- Nayak, P.K.; Mahesh, S.; Snaith, H.J.; Cahen, D. Photovoltaic solar cell technologies: Analysing the state of the art. Nat. Rev. Mater. 2019, 4, 269–285. [Google Scholar] [CrossRef]
- Zhao, B.; Bai, S.; Kim, V.; Lamboll, R.; Shivanna, R.; Auras, F.; Richter, J.M.; Yang, L.; Dai, L.; Alsari, M.; et al. High-efficiency perovskite–polymer bulk heterostructure light-emitting diodes. Nat. Photonics 2018, 12, 783–789. [Google Scholar] [CrossRef]
- Seol, D.; Jeong, A.; Han, M.; Seo, S.; Yoo, T.; Choi, W.; Jung, H.; Shin, H.; Kim, Y. Origin of hysteresis in CH3NH3PbI3 perovskite thin films. Adv. Funct. Mat. 2017, 27, 1701924. [Google Scholar] [CrossRef]
- Tumen-Ulzii, G.; Matsushima, T.; Klotz, D.; Leyden, M.R.; Wang, P.; Qin, C.; Lee, J.; Lee, S.; Yang, Y.; Adachi, C. Hysteresis-less and stable perovskite solar cells with a self-assembled monolayer. Commun. Mater. 2020, 1, 31. [Google Scholar] [CrossRef]
- Shao, Y.; Fang, Y.; Li, T.; Wang, Q.; Dong, Q.; Deng, Y.; Yuan, Y.; Wei, H.; Wang, M.; Gruverman, A.; et al. Grain boundary dominated ion migration in polycrystalline organic–inorganic halide perovskite films. Energ. Environ. Sci. 2016, 9, 1752–1759. [Google Scholar] [CrossRef]
- Ma, W.; Zhang, X.; Xu, Z.; Guo, H.; Lu, G.; Meng, S. Reducing Anomalous Hysteresis in Perovskite Solar Cells by Suppressing the Interfacial Ferroelectric Order. ACS Appl. Mater. Inter. 2020, 12, 12275–12284. [Google Scholar] [CrossRef]
- Haque, F.; Hoang, N.T.T.; Ji, J.; Mativenga, M. Effect of Precursor Composition on Ion Migration in Hybrid Perovskite CH3NH3PbI3. IEEE Electron Devices Lett. 2019, 40, 1756–1759. [Google Scholar] [CrossRef]
- Kang, D.; Park, N. On the Current–Voltage Hysteresis in Perovskite Solar Cells: Dependence on Perovskite Composition and Methods to Remove Hysteresis. Adv. Mater. 2019, 31, 1805214. [Google Scholar] [CrossRef]
- Zhong, Y.; Hufnagel, M.; Thelakkat, M.; Li, C.; Huettner, S. Role of PCBM in the Suppression of Hysteresis in Perovskite Solar Cells. Adv. Funct. Mater. 2020, 30, 1908920. [Google Scholar] [CrossRef]
- Zhang, C.; Sun, D. Valy Vardeny, Multifunctional optoelectronic–spintronic device based on hybrid organometal trihalide perovskites. Z. Adv. Electron. Mater. 2017, 3, 1600426. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, M.; Eperon, G.E.; Leijtens, T.C.; McMeekin, D.; Saliba, M.; Zhang, W.; Bastiani, M.; Pertrozza, A.; Herz, L.M.; et al. Charge selective contacts, mobile ions and anomalous hysteresis in organic–inorganic perovskite solar cells. Mater. Horiz. 2015, 2, 315. [Google Scholar] [CrossRef]
- Mativenga, M.; Ji, J.; Hoang, N.T.T.; Haque, F. Ambient air stability of hybrid perovskite thin-film transistors by ambient air processing. Adv. Mater. Interfaces 2020, 7, 1901777. [Google Scholar] [CrossRef]
- Luo, P.; Xia, W.; Zhou, S.; Sun, L.; Cheng, J.; Xu, C.; Lu, Y. Solvent engineering for ambient-air-processed, phase-stable CsPbI3 in perovskite solar cells. J. Phys. Chem. Lett. 2016, 7, 3603–3608. [Google Scholar] [CrossRef]
- Jeon, N.; Noh, J.; Kim, Y.; Yang, W.; Ryu, S.; Seok, S. Solvent engineering for high-performance inorganic–organic hybrid perovskite solar cells. Nat. Mater. 2014, 13, 897–903. [Google Scholar] [CrossRef]
- Gong, J.; Guo, P.; Benjamin, S.E.; Van Patten, P.G.; Schaller, R.D.; Xu, T. Cation engineering on lead iodide perovskites for stable and high-performance photovoltaic applications. J. Energy Chem. 2018, 27, 1017–1039. [Google Scholar] [CrossRef]
- Yang, C.; Chueh, C.; Liang, P.W.; Crump, M.; Lin, F.; Zhu, Z.; Jen, A.K.Y. Effects of formamidinium and bromide ion substitution in methylammonium lead triiodide toward high-performance perovskite solar cells. Nano Energy 2016, 22, 328–337. [Google Scholar] [CrossRef]
- Ji, J.; Haque, F.; Hoang, N.T.T.; Mativenga, M. Ambipolar transport in methylammonium lead iodide thin film transistors. Crystals 2019, 9, 539. [Google Scholar] [CrossRef]
- Huang, L.; Hu, Z.; Xu, J.; Zhang, K.; Zhang, J.; Zhu, Y. Multi-step slow annealing perovskite films for high performance planar perovskite solar cells. Sol. Energy Mater. Sol. 2015, 141, 377–382. [Google Scholar] [CrossRef]
- Kim, Y.G.; Kim, T.Y.; Oh, J.H.; Choi, K.S.; Kim, Y.J.; Kim, S.Y. Cesium lead iodide solar cells controlled by annealing temperature. Phys. Chem. Chem. Phys. 2017, 19, 6257–6263. [Google Scholar] [CrossRef] [PubMed]
- Foley, B.J.; Girard, J.; Sorenson, B.A.; Chen, A.Z.; Niezgoda, J.S.; Alpert, M.R.; Harper, A.F.; Smilgies, D.M.; Clancy, P.; Saidi, W.A.; et al. Controlling nucleation, growth, and orientation of metal halide perovskite thin films with rationally selected additives. J. Mater. Chem. A 2017, 5, 113–123. [Google Scholar] [CrossRef]
- Bukke, R.N.; Avis, C.; Chowdhury, M.D.H.; Kim, T.; Lin, T.; Jang, J. Improvement in performance of solution-processed indium-zinc-tin oxide thin-film transistors by UV/O3 treatment on zirconium oxide gate insulator. Jpn. J. Appl. Phys. 2016, 55, 03CC02–03CC08. [Google Scholar]
- Mude, N.N.; Bukke, R.N.; Jang, J. High Performance of Solution-Processed Amorphous p-Channel Copper-Tin-Sulphur-Gallium Oxide Thin-Film Transistor by UV/O3 Photocuring. ACS Appl. Mater. Interfaces 2021, 13, 20277–20287. [Google Scholar] [CrossRef]
- Oluwabi, A.T.; Gaspar, D.; Katerski, A.; Mere, A.; Krunks, M.; Pereira, L.; Oja Acik, I. Influence of Post-UV/Ozone Treatment of Ultrasonic-Sprayed Zirconium Oxide Dielectric Films for a Low-Temperature Oxide Thin Film Transistor. Materials 2020, 13, 6. [Google Scholar] [CrossRef]
- Pylnev, M.; Barbisan, A.M.; Wei, T.-C. Effect of wettability of substrate on metal halide perovskite growth. Appl. Surf. Sci. 2021, 541, 148559. [Google Scholar] [CrossRef]
- Jingbi, Y.; Yang, Y.; Hong, Z.; Song, T.-B.; Meng, L.; Liu, Y.; Jiang, C.; Zhou, H.; Chang, W.-H.; Li, G.; et al. Moisture assisted perovskite film growth for high performance solar cells.Y. Appl. Phys. Lett. 2014, 105, 183902. [Google Scholar]
- Ren, Z.; Ng, A.; Shen, Q.; Gokkaya, H.C.; Wang, J.; Yang, L.; Yiu, W.; Bai, G.; Djurišić, A.B.; Leung, W.W.; et al. Thermal assisted oxygen annealing for high efficiency planar CH3NH3PbI3 perovskite solar cells. Sci. Rep. 2014, 4, 6752. [Google Scholar] [CrossRef]
- Brenes, R.; Guo, D.; Osherov, A.; Noel, N.K.; Eames, C.; Hutter, E.M.; Pathak, S.K.; Niroui, F.; Friend, R.H.; SaifulIslam, M.; et al. Metal halide perovskite polycrystalline films exhibiting properties of single crystals. Joule 2017, 1, 155–167. [Google Scholar] [CrossRef]
- John, M.; Nima, Y.; Chris, P.; Joop, H.; Jan, S. Nucleation and Crystal Growth in Continuous Crystallization. In The Handbook of Continuous Crystallization; Royal Society of Chemistry: London, UK, 2020. [Google Scholar]
- Wang, P.; Zhang, X.; Zhou, Y.; Jiang, Q.; Ye, Q.; Chu, Z.; Li, X.; Yang, X.; Yin, Z.; You, J. Solvent-controlled growth of inorganic perovskite films in dry environment for efficient and stable solar cells. Nat. Commun. 2018, 9, 2225. [Google Scholar] [CrossRef]
- Rolston, N.; Bush, K.A.; Printz, A.D.; Gold-Parke, A.; Ding, Y.; Toney, M.F.; McGehee, M.D.; Dauskardt, R.H. Engineering stress in perovskite solar cells to improve stability. Adv. Energy Mater. 2018, 15, 1802139. [Google Scholar] [CrossRef]
- Ramirez, C.; Yadavalli, S.K.; Garces, H.F.; Zhou, Y.; Padture, N.P. Thermo-mechanical behavior of organic-inorganic halide perovskites for solar cells. Scr. Mater. 2018, 150, 36–41. [Google Scholar] [CrossRef]
- Bansal, N.P.; Doremus, R.H. Handbook of Glass Properties; Elsevier: Amsterdam, The Netherlands, 1986. [Google Scholar]
- Osterwald, C.R.; McMahon, T.J. History of accelerated and qualification testing of terrestrial photovoltaic modules: A literature review. Prog. Photovolt. Res. Appl. 2009, 17, 11–33. [Google Scholar] [CrossRef]
- McMahon, T.J. Accelerated testing and failure of thin-film PV modules. Prog. Photovolt. Res. Appl. 2004, 12, 235–248. [Google Scholar] [CrossRef]
- Yin, W.-J.; Shi, T.; Yan, Y. Unusual defect physics in CH3NH3PbI3 perovskite solar cell absorber. Appl. Phys. Lett. 2014, 104, 063903. [Google Scholar] [CrossRef]
- Eames, G.C.; Frost, J.M.; Barnes, P.R.; O’regan, B.C.; Walsh, A.; Islam, M.S. Ionic transport in hybrid lead iodide perovskite solar cells. Nat. Commun. 2015, 6, 7497. [Google Scholar] [CrossRef]
- Weller, H.M.T.; Weber, O.J.; Henry, P.F.; Pumpo, A.M.D.; Hansen, T.C. Complete structure and cation orientation in the perovskite photovoltaic methylammonium lead iodide between 100 and 352 K. Chem. Commun. 2015, 51, 4180–4183. [Google Scholar] [CrossRef]
- Zhu, H.; Liu, A.; Shim, K.I.; Hong, J.; Han, J.W.; Noh, Y.Y. High-Performance and Reliable Lead-Free Layered-Perovskite Transistors. Adv. Mater. 2020, 32, 2002717. [Google Scholar] [CrossRef]
- Su, B.-Y.; Chu, S.-Y.; Juang, Y.-D.; Chen., H.-C. High-performance low-temperature solution-processed InGaZnO thin-film transistors via ultraviolet-ozone photo-annealing. Appl. Phys. Lett. 2013, 102, 192101. [Google Scholar] [CrossRef]
- Bukke, R.N.; Saha, J.K.; Mude, N.N.; Kim, Y.G.; Lee, S.; Jang, J. Lanthanum Doping in Zinc Oxide for Highly Reliable Thin-Film Transistors on Flexible Substrates by Spray Pyrolysis. ACS Appl. Mater. Interfaces 2020, 31, 35164–35174. [Google Scholar]
- Zhu, X.; Lee, J.; Lu, W.D. Iodine Vacancy Redistricution in Organic Inorganic Halide Perovskite Films and Resistive Switching Effects. Adv. Mater. 2017, 29, 1700527. [Google Scholar] [CrossRef] [PubMed]
- Saliba, M.; Matsui, T.; Seo, J.-Y.; Domanski, K.; Correa-Baena, J.-P.; Nazeeruddin, M.K.; Zakeeruddin, S.M.; Tress, W.; Abate, A.; Hagfeldt, A.; et al. Cesium-containing triple cation perovskite solar cells: Improved stability, reproducibility and high efficiency. Energ. Environ. Sci. 2016, 9, 1989. [Google Scholar] [CrossRef] [PubMed]
- Nikolka, M.; Schweicher, G.; Armitage, J.; Nasrallah, I.; Jellett, C.; Guo, Z.; Hurhangee, M.; Sadhanala, A.; McCulloch, I.; Nielsen, C.B.; et al. Performance Improvements in Conjugated Polymer Devices by Removal of Water-Induced Traps. Adv. Mater. 2018, 30, 1801874. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haque, F.; Bukke, R.N.; Mativenga, M. Reduction of Hysteresis in Hybrid Perovskite Transistors by Solvent-Controlled Growth. Materials 2021, 14, 2573. https://doi.org/10.3390/ma14102573
Haque F, Bukke RN, Mativenga M. Reduction of Hysteresis in Hybrid Perovskite Transistors by Solvent-Controlled Growth. Materials. 2021; 14(10):2573. https://doi.org/10.3390/ma14102573
Chicago/Turabian StyleHaque, Farjana, Ravindra Naik Bukke, and Mallory Mativenga. 2021. "Reduction of Hysteresis in Hybrid Perovskite Transistors by Solvent-Controlled Growth" Materials 14, no. 10: 2573. https://doi.org/10.3390/ma14102573
APA StyleHaque, F., Bukke, R. N., & Mativenga, M. (2021). Reduction of Hysteresis in Hybrid Perovskite Transistors by Solvent-Controlled Growth. Materials, 14(10), 2573. https://doi.org/10.3390/ma14102573





