Carboxin and Diuron Adsorption Mechanism on Sunflower Husks Biochar and Goethite in the Single/Mixed Pesticide Solutions
Abstract
1. Introduction
2. Materials and Methods
2.1. Pesticides
2.2. Adsorbent Preparation and Characteristics
2.3. Adsorption Kinetics
2.4. Adsorption Isotherms
2.5. Adsorption in the Mixed Systems
2.6. Statistical Analysis
3. Results and Discussion
3.1. Adsorbent Characterization
3.2. Adsorption Mechanism of Carboxin and Diuron on the Goethite and Biochar Surface
3.3. Carboxin and Diuron Adsorption on the Goethite and Biochar Surface in the Mixed Pesticide Solution
4. Conclusions
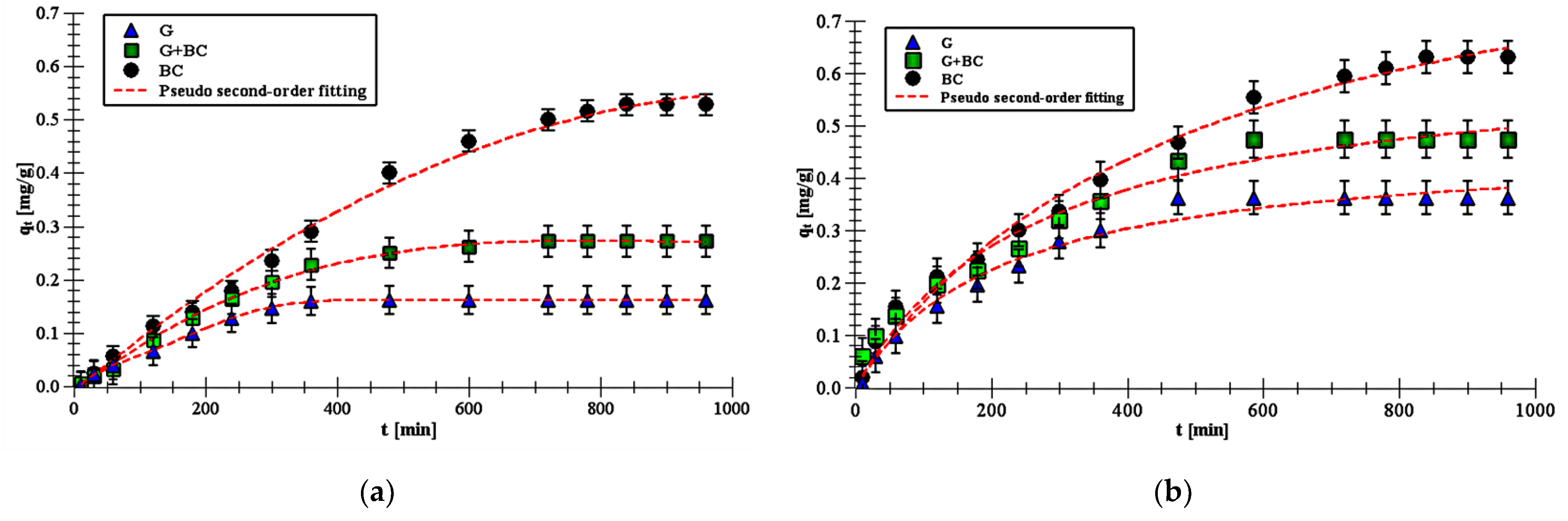
- The pseudo second-order model best fitted experimental data (R2 > 0.99). This suggests chemisorption process of carboxin and diuron on goethite, biochar and goethite–biochar mixture.
- The carboxin and diuron adsorption process was the fastest on goethite, whereas it was the slowest on biochar. This is probably related to the number of active sites on the adsorbents.
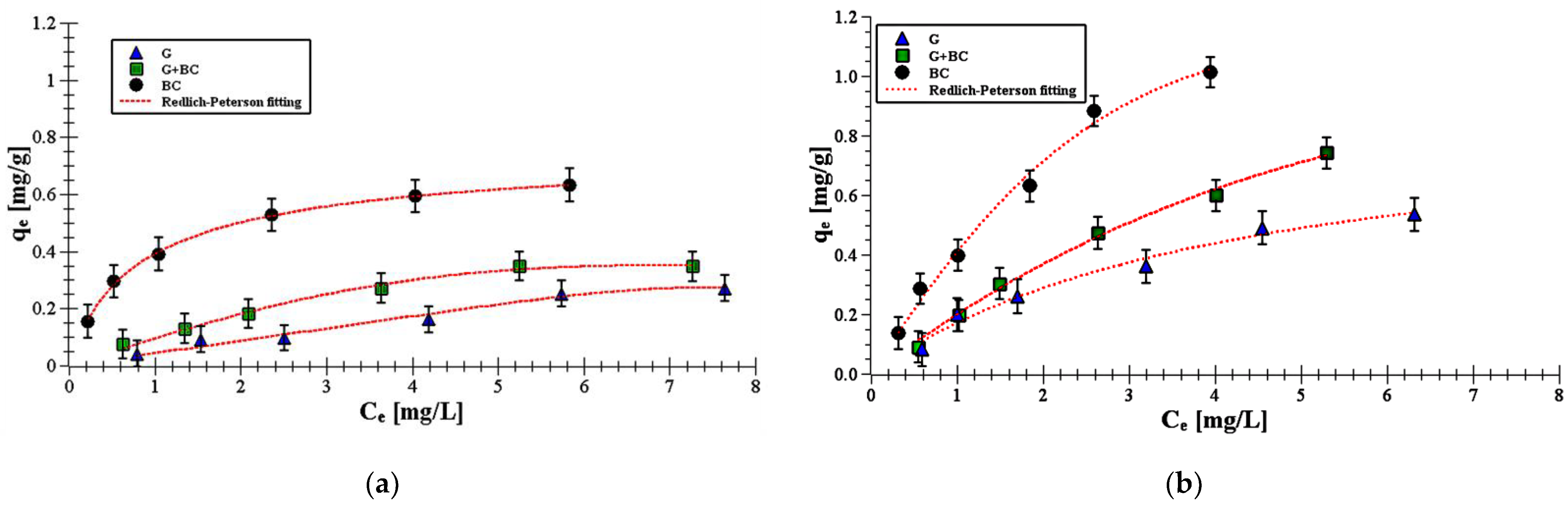
- Carboxin adsorbed on the solids in higher amounts than diuron. The qe value on biochar was 0.64 mg/g for carboxin and 0.53 mg/g for diuron. In turn, the qe value on goethite was 0.37 and 0.16 mg/g for carboxin and diuron, respectively.
- The Redlich–Peterson isotherm model best described experimental data of carboxin/diuron adsorption on biochar, goethite and goethite–biochar mixture.
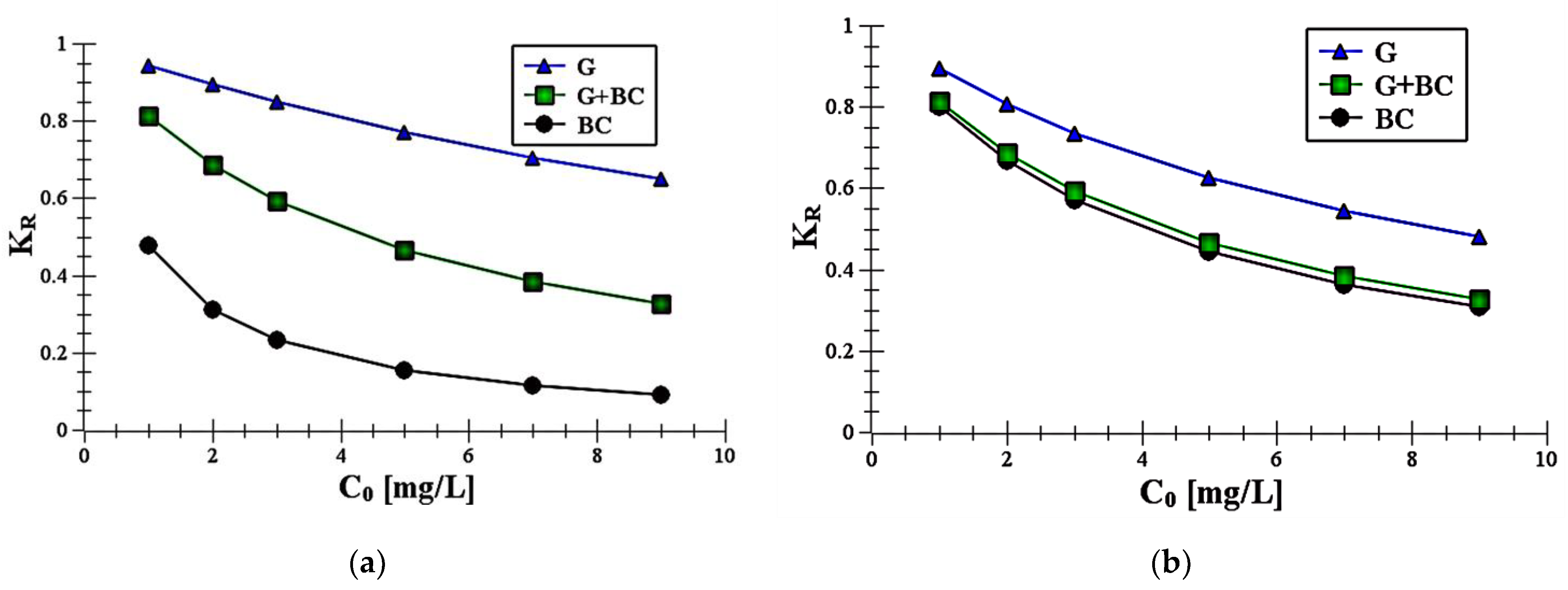
- The obtained KR parameters suggested that adsorption process of carboxin and diuron was the most favorable on biochar.
- Carboxin/diuron adsorption is based on the creation of hydrogen bonds and donor-acceptor interactions between substituents on the solid surface and substituents of pesticide molecules. The π–π electron-donor–acceptor interactions may also occur between aromatic rings of pesticides and biochar.
- In the mixed solution, diuron was adsorbed on the selected solids in higher amounts, whereas carboxin in lower amounts than in the single systems. This is probably associated with different molecule area of used pesticides as well as formation of adsorption multilayer, within carboxin accelerates diuron bonding.
- Goethite and sunflower husks, as waste from the metallurgical industry and agriculture, respectively, can be used to prepare environmentally friendly adsorbents, capable of binding carboxin and diuron.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, J.; Yang, M.; Wang, Y.; Qu, L.; Zhong, G. Enhanced diuron remediation by microorganism-immobilized silkworm excre-ment composites and their impact on soil microbial communities. J. Hazard. Mater. 2019, 376, 29–36. [Google Scholar] [CrossRef]
- Hou, K.; Wang, G.; Zhu, Y.; Ezzatahmadi, N.; Fu, L.; Tang, A.; Yang, H.; Xi, Y. Sepiolite/Fe3O4 composite for effective degradation of diuron. Appl. Clay Sci. 2019, 181, 105–243. [Google Scholar] [CrossRef]
- Huang, B.; Yan, D.; Wang, X.; Wang, X.; Fang, W.; Zhang, D.; Ouyang, C.; Wang, Q.; Cao, A. Soil fumigation alters adsorption and degradation behavior of pesticides in soil. Environ. Pollut. 2019, 246, 264–273. [Google Scholar] [CrossRef] [PubMed]
- Souza, F.; Rocha, R.; Ferreira, N.; Rodrigo, M.; Lanza, M. Effects of coupling hybrid processes on the treatment of wastewater containing a commercial mixture of diuron and hexazinone herbicides. Electrochim. Acta 2019, 328, 135013. [Google Scholar] [CrossRef]
- European Union Pesticides Database. Available online: https://ec.europa.eu/food/plant/pesticides/max_residue_levels_en (accessed on 1 March 2021).
- Wei, D.; Wu, X.; Ji, M.; Xu, J.; Dong, F.; Liu, X.; Zheng, Y. Carboxin and its major metabolites residues in peanuts: Levels, dietary intake and chronic intake risk assessment. Food Chem. 2019, 275, 169–175. [Google Scholar] [CrossRef]
- Wydro, U.; Łapiński, D.; Ofman, P. Supporting the bioremediation processes of contaminated soils. Interdisciplinary issues in engineering and environmental protection (In Polish). Oficyna Wydawnicza Politech. Wrocławskiej 2015, 5, 503–515. [Google Scholar]
- Crini, G.; Lichtfouse, E.; Wilson, L.D.; Morin-Crini, N. Conventional and non-conventional adsorbents for wastewater treat-ment. Env. Chem. Lett. 2019, 17, 195–213. [Google Scholar] [CrossRef]
- Singh, A.K.; Singla, P. Biodegradation of diuron by endophytic Bacillus licheniformis strain SDS12 and its application in reducing diuron toxicity for green algae. Environ. Sci. Pollut. Res. 2019, 26, 26972–26981. [Google Scholar] [CrossRef]
- Quaglia, G.; Joris, I.; Broekx, S.; Desmet, N.; Koopmans, K.; Vandaele, K.; Seuntjens, P. A spatial approach to identify priority areas for pesticide pollution mitigation. J. Environ. Manag. 2019, 246, 583–593. [Google Scholar] [CrossRef]
- Fijałkowska, G.; Wiśniewska, M.; Szewczuk-Karpisz, K.; Jędruchniewicz, K.; Oleszczuk, O. Comparison of lead(II) ions accumulation and bioavailability on the montmorillonite and kaolinite surfaces in the presence of polyacrylamide soil flocculant. Chemosphere 2021, 276, 130088. [Google Scholar] [CrossRef] [PubMed]
- Wiśniewska, M.; Sternik, D.; Nowicki, P.; Chibowski, S.; Medykowska, M.; Gęca, M.; Szewczuk-Karpisz, K. Adsorption, viscosity and thermal behaviour of nanosized proteins with different internal stability immobilised on the surface of mesoporous activated biocarbon obtained from the horsetail herb precursor. Appl. Nanosci. 2021, 1–14. [Google Scholar] [CrossRef]
- Premarathna, K.; Rajapaksha, A.U.; Adassoriya, N.; Sarkar, B.; Sirimuthu, N.M.; Cooray, A.; Ok, Y.S.; Vithanage, M. Clay-biochar composites for sorptive removal of tetracycline antibiotic in aqueous media. J. Environ. Manag. 2019, 238, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Albadarin, A.B.; Mo, J.; Glocheux, Y.; Allen, S.; Walker, G.; Mangwandi, C. Preliminary investigation of mixed adsorbents for the removal of copper and methylene blue from aqueous solutions. Chem. Eng. J. 2014, 255, 525–534. [Google Scholar] [CrossRef]
- Bai, Y.; Hong, J. Preparation of a Novel Millet Straw Biochar-Bentonite Composite and Its Adsorption Property of Hg2+ in Aqueous Solution. Materials 2021, 14, 1117. [Google Scholar] [CrossRef] [PubMed]
- El-Naggar, A.; Lee, S.S.; Rinklebe, J.; Farooq, M.; Song, H.; Sarmah, A.K.; Zimmerman, A.R.; Ahmad, M.; Shaheen, S.M.; Ok, Y.S. Biochar application to low fertility soils: A review of current status, and future prospects. Geoderma 2019, 337, 536–554. [Google Scholar] [CrossRef]
- Tomczyk, A.; Szewczuk-Karpisz, K.; Sokołowska, Z.; Kercheva, M.; Dimitrov, E. Purification of Aqueous Media by Biochars: Feedstock Type Effect on Silver Nanoparticles Removal. Molecules 2020, 25, 2930. [Google Scholar] [CrossRef]
- Szewczuk-Karpisz, K.; Nowicki, P.; Sokołowska, Z.; Pietrzak, R. Hay-based activated biochars obtained using two different heating methods as effective low-cost sorbents: Solid surface characteristics, adsorptive properties and aggregation in the mixed Cu(II)/PAM system. Chemosphere 2020, 250, 126312. [Google Scholar] [CrossRef]
- Szewczuk-Karpisz, K.; Bogatyrov, V.M.; Galaburda, M.; Sokołowska, Z. Study on Adsorption and Aggregation in the Mixed System of Polyacrylamide, Cu(II) Ions and Innovative Carbon–Silica Composite. Polymers 2020, 12, 961. [Google Scholar] [CrossRef]
- Szewczuk-Karpisz, K.; Wiśniewska, M.; Medykowska, M.; Galaburda, M.V.; Bogatyrov, V.M.; Oranska, O.I.; Błachnio, M.; Oleszczuk, P. Simultaneous adsorption of Cu(II) ions and poly(acrylic acid) on the hybrid carbon-mineral nanocomposites with metallic elements. J. Hazard. Mater. 2021, 412, 125138. [Google Scholar] [CrossRef]
- Boehm, H.P. Some aspects of the surface chemistry of carbon blocks and another carbons. Carbon 1994, 32, 759–769. [Google Scholar] [CrossRef]
- Schönherr, J.; Buchheim, J.R.; Scholz, P.; Adelhelm, P. Boehm Titration Revisited (Part I): Practical Aspects for Achieving a High Precision in Quantifying Oxygen-Containing Surface Groups on Carbon Materials. Carbon 2018, 4, 21. [Google Scholar] [CrossRef]
- Lagergren, S. Zur theorie der sogenannten adsorption geloster stoffe. Kunglinga Svenska Vetenskapsakade-miens. Handlingar 1898, 24, 1–39. [Google Scholar]
- Ho, Y.; McKay, G. Pseudo-second order model for sorption processes. Process Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Sips, R. On the Structure of a Catalyst Surface. J. Chem. Phys. 1948, 16, 490–495. [Google Scholar] [CrossRef]
- Langmuir, I. The constitution and fundamental properties of solids and liquids. J. Am. Chem. Soc. 1916, 38, 2221–2295. [Google Scholar] [CrossRef]
- Ho, Y.; Huang, C.; Huang, H. Equilibrium sorption isotherm for metal ions on tree fern. Process Biochem. 2002, 37, 1421–1430. [Google Scholar] [CrossRef]
- Peereboom, L. Adsorption of Bio-Renewable Substrates on Supported Metal Catalyst in Water. Ph.D. Thesis, Department of Chemical Engineering, Michigan State University, Lansing, MI, USA, 2007. [Google Scholar]
- Kumara, N.T.R.N.; Hamdan, N.; Petra, M.I.; Tennakoon, K.U.; Ekanayake, P. Equilibrium Isotherm Studies of Adsorption of Pigments Extracted from Kuduk-kuduk (Melastoma malabathricum L.) Pulp onto TiO2Nanoparticles. J. Chem. 2014, 2014, 1–6. [Google Scholar] [CrossRef]
- Szewczuk-Karpisz, K.; Tomczyk, A.; Komaniecka, I.; Choma, A.; Adamczuk, A.; Sofińska-Chmiel, W. Impact of Sinorhizobium meliloti Exopolysaccharide on Adsorption and Aggregation in the Copper(II) Ions/Supporting Electrolyte/Kaolinite Sys-tem. Materials 2021, 14, 1950. [Google Scholar] [CrossRef] [PubMed]
- Tadanier, C.J.; Eick, M.J. Formulating the Charge-distribution Multisite Surface Complexation Model Using FITEQL. Soil Sci. Soc. Am. J. 2002, 66, 1505–1517. [Google Scholar] [CrossRef]
- Pigna, M.; Krishnamurti, G.S.R.; Violante, A. Kinetics of Arsenate Sorption-Desorption from Metal Oxides. Soil Sci. Soc. Am. J. 2006, 70, 2017–2027. [Google Scholar] [CrossRef]
- Oh, Y.J.; Yoo, J.J.; Kim, Y.I.; Yoon, J.K.; Yoon, H.N.; Kim, J.H.; Park, S.B. Oxygen functional groups and electrochemical ca-pacitive behavior of incompletely reduced grapheme oxides as a thin-film electrode of supercapacitor. Electrochim. Acta 2014, 116, 118–128. [Google Scholar] [CrossRef]
- Stobinski, L.; Lesiak, B.; Malolepszy, A.; Mazurkiewicz, M.; Mierzwa, B.; Zemek, J.; Jiricek, P.; Bieloshapka, I. Graphene oxide and reduced graphene oxide studied by the XRD, TEM and electron spectroscopy methods. J. Electron. Spectrosc. Relat. Phenom. 2014, 195, 145–154. [Google Scholar] [CrossRef]
- Barinov, A.; Malciglu, O.B.; Fabris, S.; Sun, T.; Gregoratti, L.; Dalmiglio, M.; Kiskinova, M. Initial stages of oxidation on gra-phitic surfaces: Photoemission study and density functional theory calculations. J. Phys. Chem. C 2009, 113, 9009–9013. [Google Scholar] [CrossRef]
- Moulder, J.F.; Stickle, W.F.; Sobol, P.E.; Bomben, K.D. Handbook of X-ray Photoelectron Spectroscopy; Perkin-Elmer Corporation: Waltham, MA, USA, 1993. [Google Scholar]
- McCafferty, E.; Wightman, J.P. Determination of the concentration of surface hydroxyl groups on metal oxide films by a quantative XPS method. Surf. Interface Anal. 1998, 23, 549–564. [Google Scholar] [CrossRef]
- Shchukarev, A.; Korolkov, D. XPS Study of group IA carbonates. Open Chem. 2004, 2, 347–362. [Google Scholar] [CrossRef]
- Rheinheimer, V.; Unluer, C.; Liu, J.; Ruan, S.; Pan, J.; Monteiro, P.J.M. XPS study on the stability and transformation of hy-drate and carbonate phases within MgO systems. Materials 2016, 10, 75. [Google Scholar] [CrossRef]
- Brouers, F.; Al-Musawi, T.J. On the optimal use of isotherm models for the characterization of biosorption of lead onto al-gae. J. Mol. Liq. 2015, 212, 46–51. [Google Scholar] [CrossRef]
- Ayawei, N.; Ebelegi, A.N.; Wankasi, D. Modelling and Interpretation of Adsorption Isotherms. J. Chem. 2017, 2017, 1–11. [Google Scholar] [CrossRef]
- Correa-Navarro, Y.M.; Giraldo, L.; Moreno-Pirajan, J.C. Biochar from figue bagasse for removal of caffeine and diclofenac from aqueous solution. Molecules 2020, 25, 1849. [Google Scholar] [CrossRef]
- PubChem [Internet]. Bethesda (MD): National Library of Medicine (US), National Center for Biotechnology Information. Available online: https://pubchem.ncbi.nlm.nih.gov/ (accessed on 1 March 2021).
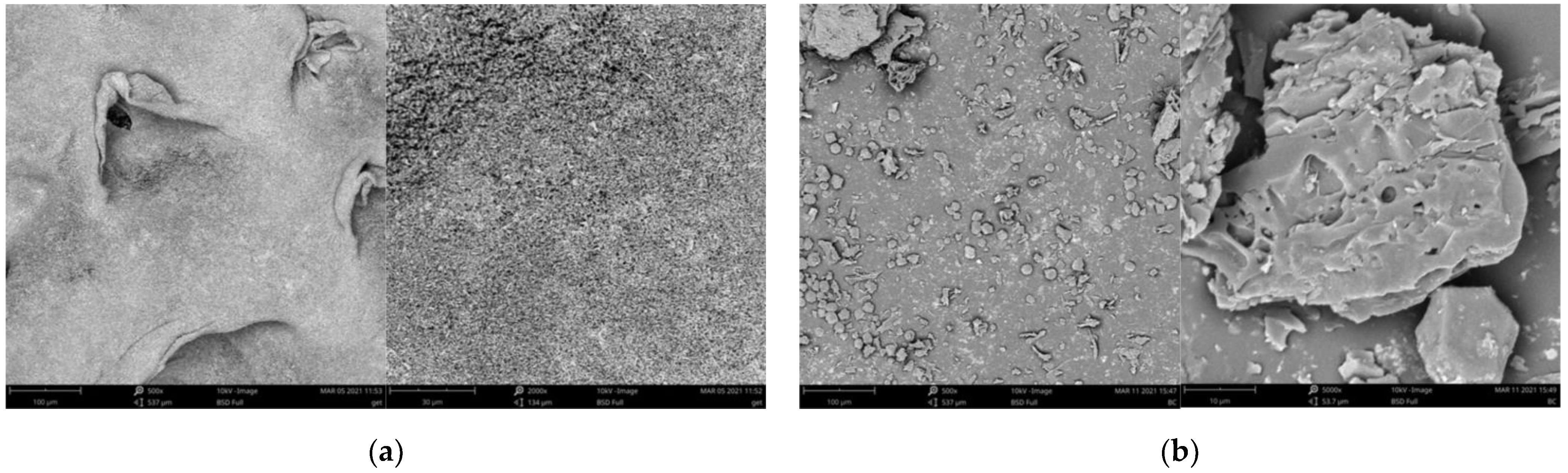
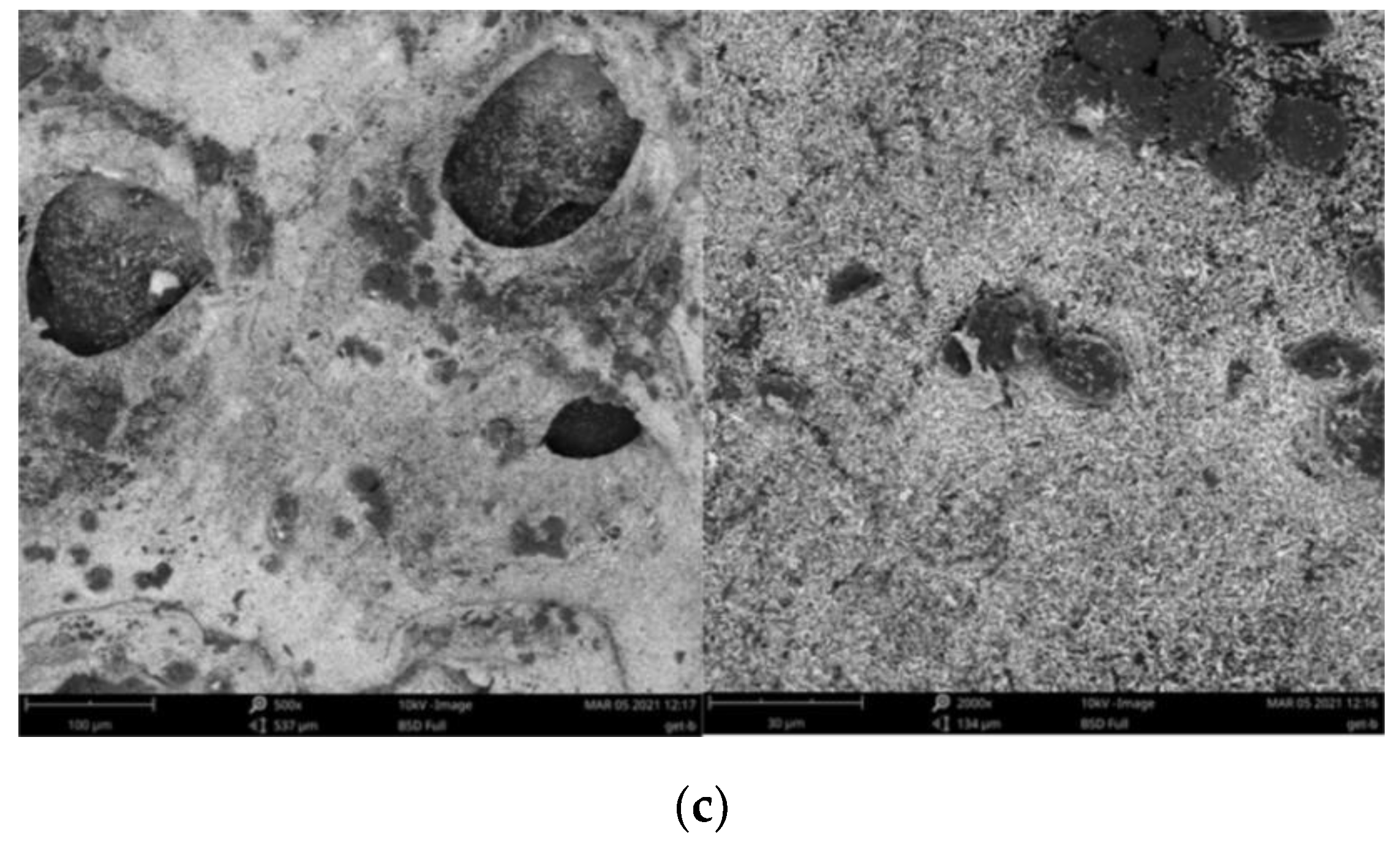
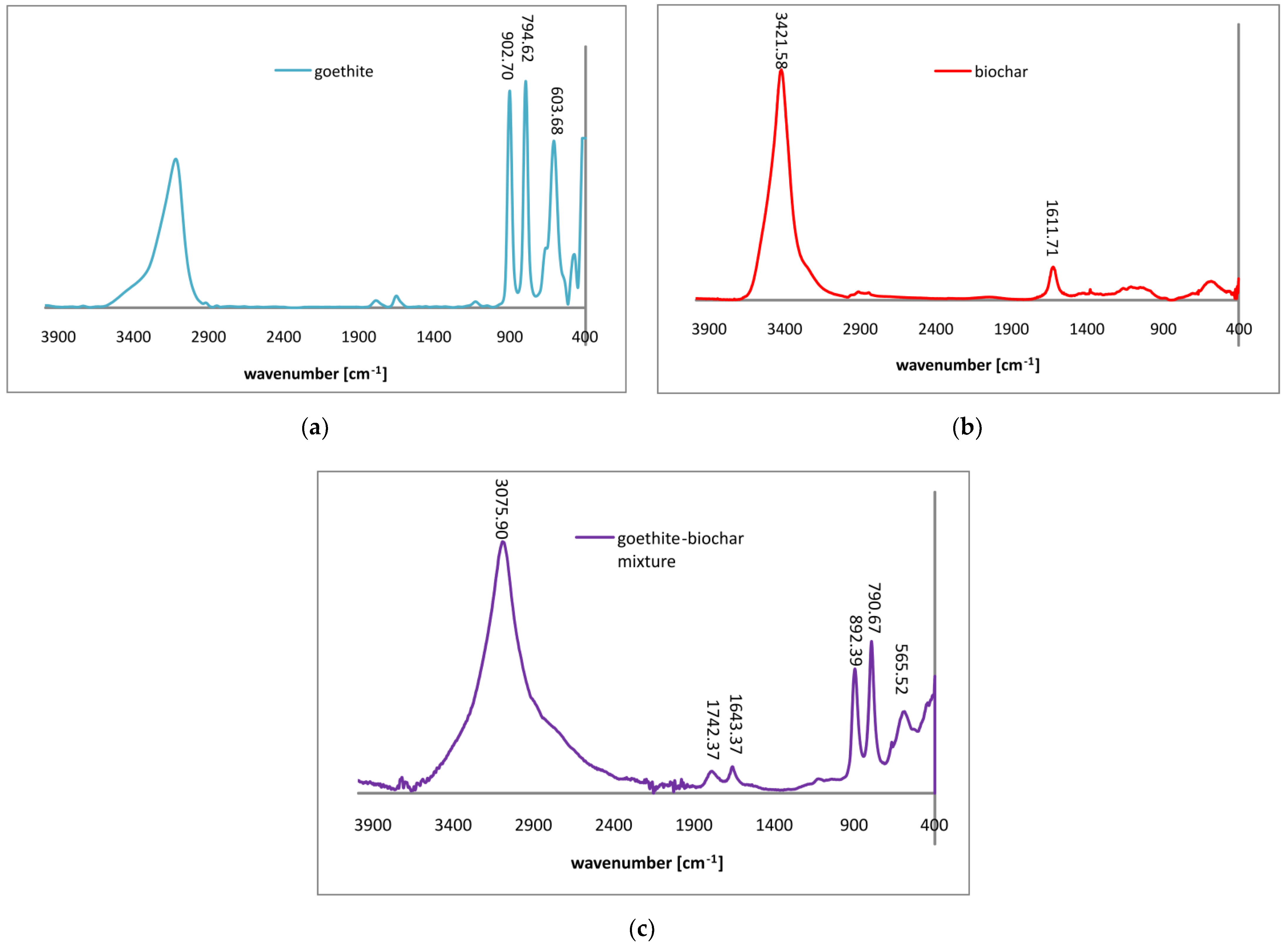


| Parameter | Goethite | Biochar | Goethite–Biochar Mixture |
|---|---|---|---|
| SBET (m2/g) 1 | 11.10 | 7.02 | 48.48 |
| Vt (cm3/g) 1 | 0.028 | 0.0024 | 0.029 |
| D (nm) 1 | 12.14 | 49.45 | 9.51 |
| Acidic group content 2 (mmol/g) | - | 2.9 | 0.4 |
| Basic group content 2 (mmol/g) | - | 3.2 | 0.55 |
| Material | Position | Concentration (%) | Species | Description |
|---|---|---|---|---|
| Goethite | 530.44 | 40.9 | Fe–O–Fe | Iron oxide |
| 531.79 | 54.8 | Fe–OH | Iron hydroxide | |
| 533.6 | 4.3 | H2O | Water | |
| Biochar | 528.1 | 6.5 | O−2 | Oxide anion/metal oxides |
| 529.6 | 12.0 | quin O−2 Me–O–C | Quinones Oxide anion/metal oxides Carbonates | |
| 531.7 | 43.8 | O=C–O− O=C | Carboxyl groups Carboxyl groups | |
| 533.4 | 36.3 | Al–O Al–OH Si–O–Si Mg–O | Alumina Aluminum hydroxyl groups Silica/silicates Magnesium-oxygen bond | |
| 534.7 | 1.4 | Si–OH C–OH O=C–O− H2O/O2 | Silica hydroxyl groups Hydroxyl groups (aromatic) Carboxyl groups Water/adsorbed oxygen |
| Kinetic Equation | Parameter | Carboxin | Diuron | ||||
|---|---|---|---|---|---|---|---|
| G | G + BC | BC | G | G + BC | BC | ||
| Pseudo-first order | k1 × 10−2 (1/min) | 0.48 ± 0.07 | 0.34 ± 0.04 | 0.25 ± 0.01 | 0.75 ± 0.01 | 0.49 ± 0.04 | 0.21 ± 0.05 |
| qe (mg/g) | 0.37 ± 0.07 | 0.43 ± 0.21 | 0.59 ± 0.21 | 0.19 ± 0.07 | 0.31 ± 0.01 | 0.54 ± 0.01 | |
| R2 | 0.990 | 0.982 | 0.981 | 0.863 | 0.971 | 0.987 | |
| Pseudo-second order | k2 × 10−2 (g/mg·min) | 0.85 ± 0.07 | 0.69 ± 0.07 | 0.44 ± 0.03 | 0.79 ± 0.07 | 0.36 ± 0.07 | 0.19 ± 0.03 |
| qe (mg/g) | 0.37 ± 0.04 | 0.49 ± 0.03 | 0.64 ± 0.04 | 0.16 ± 0.04 | 0.27 ± 0.03 | 0.53 ± 0.04 | |
| R2 | 0.998 | 0.998 | 0.996 | 0.998 | 0.996 | 0.999 | |
| Intra-particle diffusion model | kD × 10−2 (g/mg·min1/2) | 2.31 ± 0.04 | 1.63 ± 0.06 | 1.29 ± 0.12 | 1.92 ± 0.04 | 0.88 ± 0.06 | 0.58 ± 0.09 |
| R2 | 0.921 | 0.963 | 0.925 | 0.847 | 0.885 | 0.922 | |
| Isotherms | Parameter | Carboxin | Diuron | ||||
|---|---|---|---|---|---|---|---|
| G | G + BC | BC | G | G + BC | BC | ||
| Freundlich | KF (mg/g(L/mg)1/n) | 0.18 ± 0.02 | 0.21 ± 0.02 | 0.41 ± 0.03 | 0.05 ± 0.01 | 0.12 ± 0.02 | 0.36 ± 0.02 |
| 1/n | 0.78 ± 0.18 | 0.69 ± 0.09 | 0.62 ± 0.14 | 0.81 ± 0.16 | 0.59 ± 0.24 | 0.35 ± 0.05 | |
| R2 | 0.971 | 0.988 | 0.979 | 0.962 | 0.953 | 0.959 | |
| Langmuir | KL (L/mg) | 0.12 ± 0.06 | 0.23 ± 0.03 | 0.25 ± 0.06 | 0.06 ± 0.04 | 0.23 ± 0.06 | 1.10 ± 0.02 |
| Qm (mg/g) | 0.91 ± 0.12 | 1.93 ± 0.29 | 2.08 ± 0.31 | 0.59 ± 0.13 | 0.73 ± 0.07 | 0.94 ± 0.01 | |
| R2 | 0.982 | 0.994 | 0.988 | 0.962 | 0.979 | 0.999 | |
| Langmuir–Freundlich | KLF (L/mg) | 0.32 ± 0.15 | 0.49 ± 0.11 | 0.65 ± 0.06 | 0.03 ± 0.01 | 0.24 ± 0.08 | 0.25 ± 0.07 |
| Am (mg/g) | 0.49 ± 0.05 | 0.68 ± 0.07 | 1.77 ± 0.27 | 0.26 ± 0.12 | 0.55 ± 0.13 | 1.83 ± 0.44 | |
| m | 1.39 ± 0.58 | 1.29 ± 0.55 | 1.06 ± 0.05 | 1.02 ± 0.52 | 0.89 ± 0.10 | 0.52 ± 0.08 | |
| R2 | 0.983 | 0.997 | 0.989 | 0.963 | 0.979 | 0.998 | |
| Redlich–Peterson | KRP (L/g) | 0.95 ± 0.22 | 3.75 ± 0.62 | 5.61 ± 0.72 | 0.65 ± 0.31 | 2.09 ± 0.76 | 6.39 ± 0.72 |
| aRP (L/mg) | 1.02 ± 0.17 | 1.33 ± 0.44 | 1.59 ± 0.51 | 1.08 ± 0.12 | 1.31 ± 0.27 | 1.53 ± 0.23 | |
| bRP | 0.22 ± 0.03 | 0.16 ± 0.01 | 0.08 ± 0.01 | 0.20 ± 0.01 | 0.12 ± 0.01 | 0.09 ± 0.03 | |
| R2 | 0.992 | 0.998 | 0.991 | 0.990 | 0.991 | 0.991 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szewczuk-Karpisz, K.; Tomczyk, A.; Celińska, M.; Sokołowska, Z.; Kuśmierz, M. Carboxin and Diuron Adsorption Mechanism on Sunflower Husks Biochar and Goethite in the Single/Mixed Pesticide Solutions. Materials 2021, 14, 2584. https://doi.org/10.3390/ma14102584
Szewczuk-Karpisz K, Tomczyk A, Celińska M, Sokołowska Z, Kuśmierz M. Carboxin and Diuron Adsorption Mechanism on Sunflower Husks Biochar and Goethite in the Single/Mixed Pesticide Solutions. Materials. 2021; 14(10):2584. https://doi.org/10.3390/ma14102584
Chicago/Turabian StyleSzewczuk-Karpisz, Katarzyna, Agnieszka Tomczyk, Magdalena Celińska, Zofia Sokołowska, and Marcin Kuśmierz. 2021. "Carboxin and Diuron Adsorption Mechanism on Sunflower Husks Biochar and Goethite in the Single/Mixed Pesticide Solutions" Materials 14, no. 10: 2584. https://doi.org/10.3390/ma14102584
APA StyleSzewczuk-Karpisz, K., Tomczyk, A., Celińska, M., Sokołowska, Z., & Kuśmierz, M. (2021). Carboxin and Diuron Adsorption Mechanism on Sunflower Husks Biochar and Goethite in the Single/Mixed Pesticide Solutions. Materials, 14(10), 2584. https://doi.org/10.3390/ma14102584








