One-Dimensional (1D) Nanostructured Materials for Energy Applications
Abstract
:1. Introduction
2. Synthesis
3. Applications
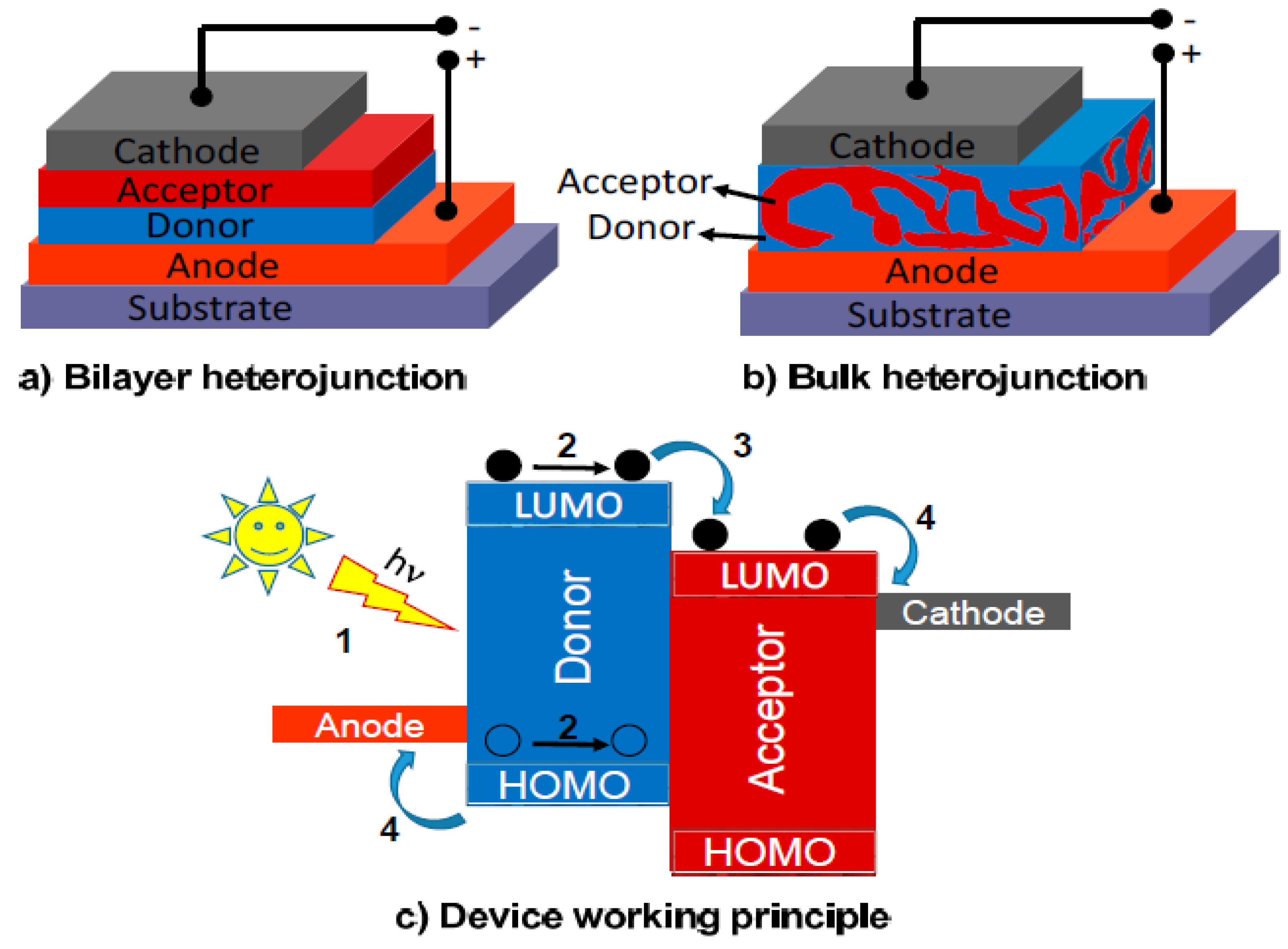
3.1. Photochemical Applications
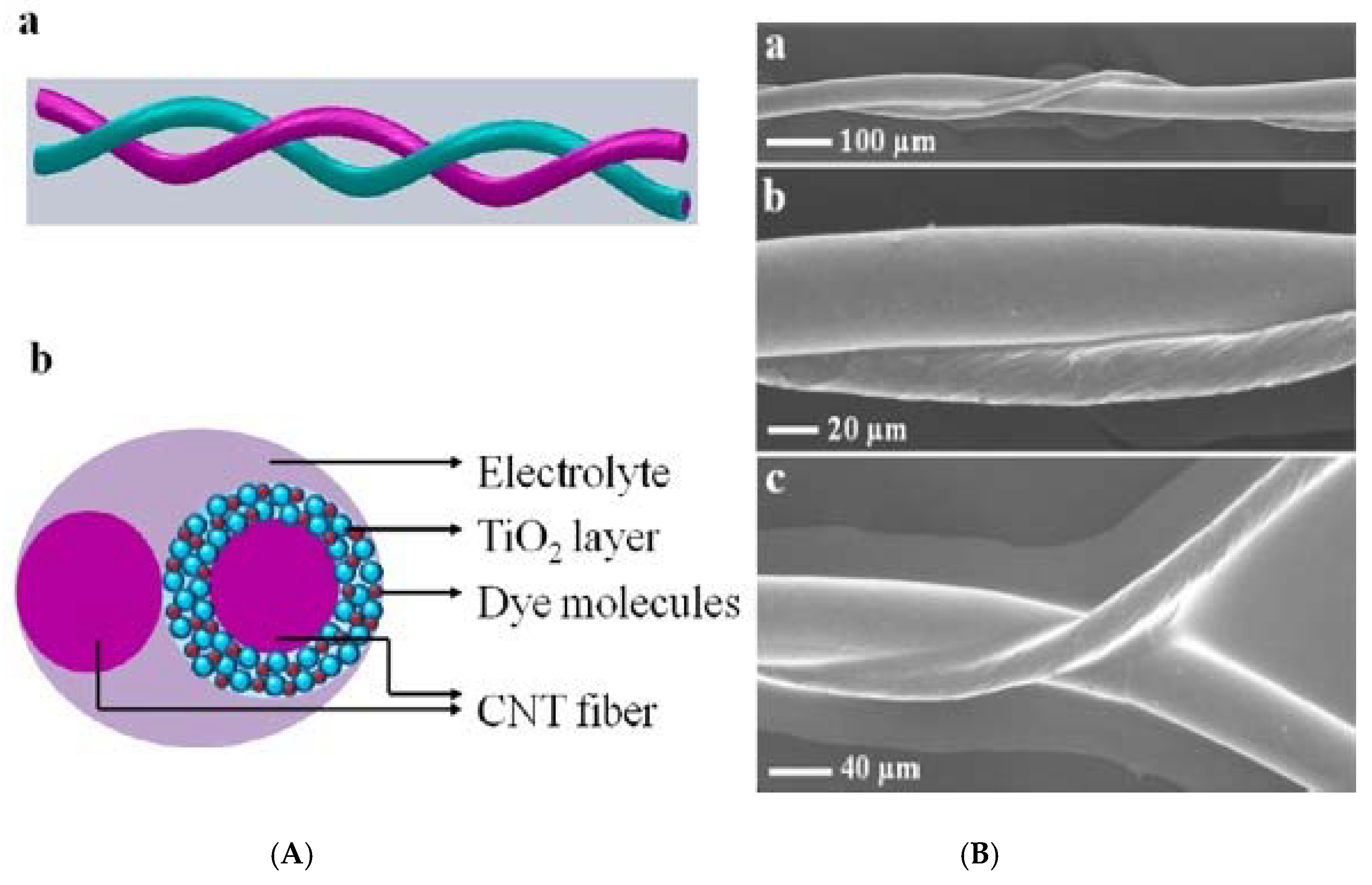
3.1.1. Photovoltaic Cells
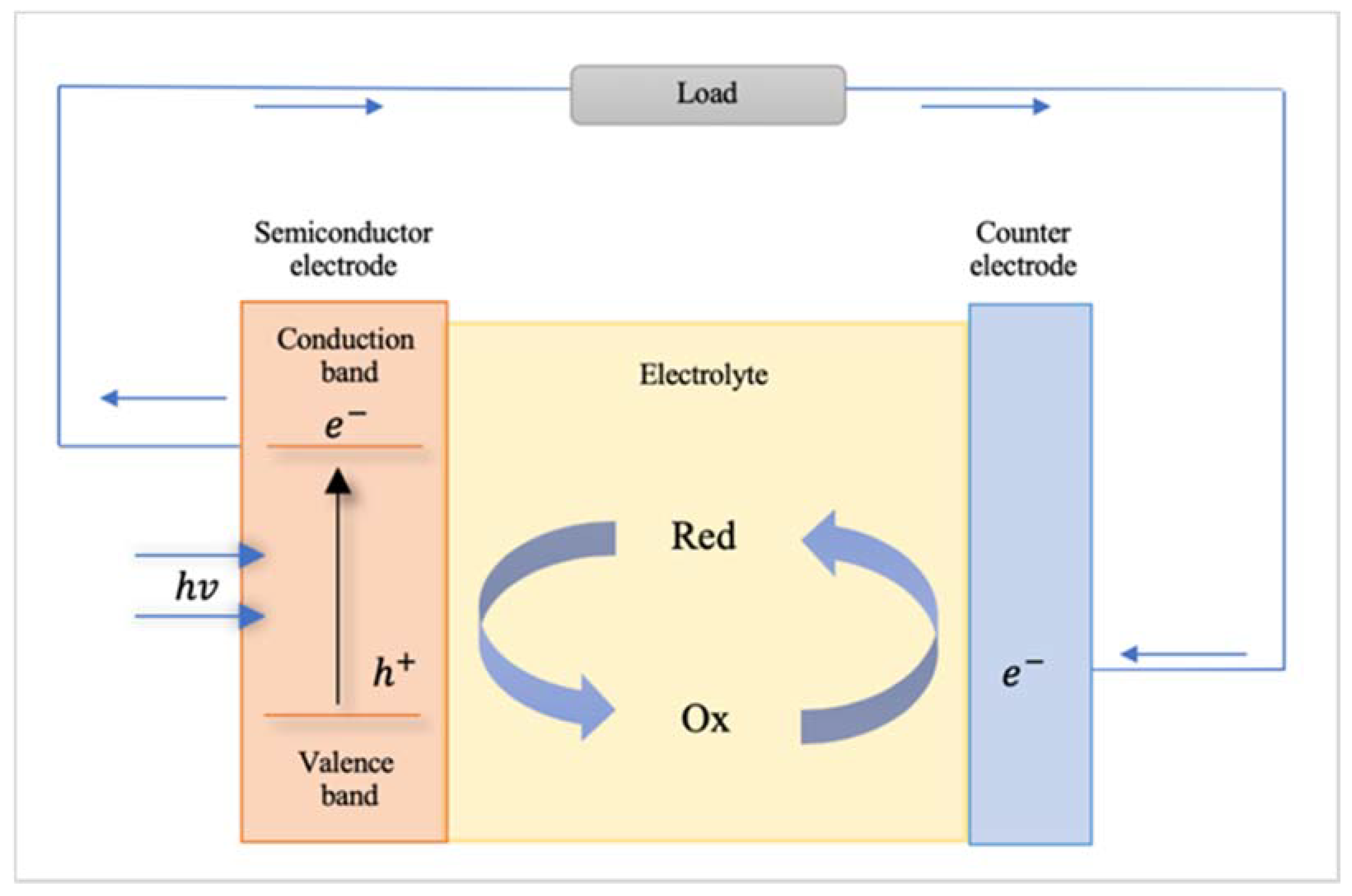
3.1.2. Photochemical Cells
3.1.3. Hydrogen Production
3.2. Piezoelectric and Thermoelectric Materials
3.3. Electrochemical Energy Storage
3.3.1. Batteries
One Dimensional Active Material
- Intercalation
- Alloying
- Conversion
One Dimensional Conductive Agent
3.3.2. Supercapacitors
EDLCs Materials
Pseudocapacitors
- Doped Carbonaceous Materials
- Conductive Polymers
- TMOs
4. Summary and Outlook
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vajtai, R. Springer Handbook of Nanomaterials; Springer-Verlag: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Piner, R.D.; Zhu, J.; Xu, F.; Hong, S.; Mirkin, C.A. “Dip-Pen” Nanolithography. Science 1999, 283, 661–663. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.J.; Lambers, E.S.; Pearton, S.J.; Ostling, M.C.; Zetterling, M.; Grow, J.M.; Ren, F.; Shul, R.J. Inductively coupled plasma etching of bulk 6H-SiC and thin-film SiCN in NF3 chemistries. J. Vac. Sci. Technol. A 1998, 16, 2204–2209. [Google Scholar] [CrossRef]
- Naureen, S.; Sanatinia, R.; Shahid, N.; Anand, S. High optical quality InP-based nanopillars fabricated by a top-down approach. Nano Lett. 2011, 11, 4805–4811. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Liang, X.; Wang, Y.; Wang, B.; Zhang, T.; Yi, F. Preparation of CdS nanorods on silicon nanopillars surface by hydrothermal method. Mater. Res. Bull. 2019, 120, 110591. [Google Scholar] [CrossRef]
- Priolo, F.; Gregorkiewicz, T.; Galli, M.; Krauss, T.F. Silicon nanostructures for photonics and photovoltaics. Nat. Nanotechnol. 2014, 9, 19–32. [Google Scholar] [CrossRef] [PubMed]
- Pinilla, S.; Barrio, R.; González, N.; Pérez, R.; Márquez, F.; Sanz, J.M.; Morant, C. Role of hydrogen in the preparation of amorphous silicon nanowires by metal-assisted chemical etching. J. Phys. Chem. C 2018, 122, 22667–22674. [Google Scholar] [CrossRef]
- Schierning, G. Silicon nanostructures for thermoelectric devices: A review of the current state of the art. Phys. Status Solidi A 2014, 211, 1235–1249. [Google Scholar] [CrossRef]
- Kara, S.A.; Keffous, A.; Giovannozzi, A.M.; Rossi, A.M.; Cara, E.; D’Ortenzi, L.; Sparnacci, K.; Boarino, L.; Gabouzeb, N.; Soukanea, S. Fabrication of flexible silicon nanowires by self-assembled metal assisted chemical etching for surface enhanced Raman spectroscopy. RSC Adv. 2016, 6, 93649–93659. [Google Scholar] [CrossRef]
- Chen, C.-Y.; Wong, C.-P. Morphological transition of Si surfaces from solid nanowires to porous nanobelts at room temperature. Chem. Commun. 2013, 49, 7295–7297. [Google Scholar] [CrossRef]
- Kim, Y.; Tsao, A.; Lee, D.H.; Maboudian, R. Solvent-induced formation of unidirectionally curved and tilted Si nanowires during metal-assisted chemical etching. J. Mater. Chem. C 2013, 1, 220–224. [Google Scholar] [CrossRef]
- Hochbaum, A.I.; Gargas, D.; Hwang, Y.J.; Yang, P. Single Crystalline Mesoporous Silicon Nanowires. Nano Lett. 2009, 9, 3550–3554. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kikuchi, T.; Kawashima, J.; Natsui, S.; Suzuki, R.O. Fabrication of porous tungsten oxide via anodizing in an ammonium nitrate/ethylene glycol/water mixture for visible light-driven photocatalyst. Appl. Surf. Sci. 2017, 422, 130–137. [Google Scholar] [CrossRef]
- Zaraska, L.; Gawlak, K.; Gurgul, M.; Chlebda, D.K.; Socha, R.P.; Sulka, G.D. Controlled synthesis of nanoporous tin oxide layers with various pore diameters and their photoelectrochemical properties. Electrochim. Acta 2017, 254, 238–245. [Google Scholar] [CrossRef]
- Zaraska, L.; Mika, K.; Syrek, K.; Sulka, G.D. Formation of ZnO nanowires during anodic oxidation of zinc in bicarbonate electrolytes. J. Electroanal. Chem. 2017, 801, 511–520. [Google Scholar] [CrossRef]
- Pisarek, M.; Krajczewski, J.; Wierzbicka, E.; Hołdyński, M.; Sulka, G.D.; Nowakowski, R.; Kudelski, A.; Janik-Czachor, M. Influence of the silver deposition method on the activity of platforms for chemometric surface-enhanced Raman scattering measurements: Silver films on ZrO2 nanopore arrays. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2017, 182, 124–129. [Google Scholar] [CrossRef] [PubMed]
- Stojadinović, S.; Tadić, N.; Radić, N.; Stefanov, P.; Grbić, B.; Vasilić, R. Anodic luminescence, structural, photoluminescent, and photocatalytic properties of anodic oxide films grown on niobium in phosphoric acid. Appl. Surf. Sci. 2015, 355, 912–920. [Google Scholar] [CrossRef]
- Ohta, T.; Masegi, H.; Noda, K. Photocatalytic decomposition of gaseous methanol over anodized iron oxide nanotube arrays in high vacuum. Mater. Res. Bull. 2018, 99, 367–376. [Google Scholar] [CrossRef]
- Wierzbicka, E.; Sulka, G.D. Fabrication of highly ordered nanoporous thin Au films and their application for electrochemical determination of epinephrine. Sens. Actuators B Chem. 2016, 222, 270–279. [Google Scholar] [CrossRef]
- Kumeria, T.; Rahman, M.M.; Santos, A.; Ferré-Borrull, J.; Marsal, L.F.; Losic, D. Nanoporous Anodic Alumina Rugate Filters for Sensing of Ionic Mercury: Toward Environmental Point-of-Analysis Systems. ACS Appl. Mater. Interfaces 2014, 6, 12971–12978. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.; Yoo, J.H.; Rohatgi, C.V.; Kumeria, T.; Wang, Y.; Losic, D. Realisation and advanced engineering of true optical rugate filters based on nanoporous anodic alumina by sinusoidal pulse anodisation. Nanoscale 2016, 8, 1360–1373. [Google Scholar] [CrossRef]
- Attaluri, A.C.; Huang, Z.; Belwalkar, A.; Van Geertruyden, W.; Gao, D.; Misiolek, W. Evaluation of Nano-Porous Alumina Membranes for Hemodialysis Application. ASAIO J. 2009, 55, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Law, C.S.; Santos, A.; Kumeria, T.; Losic, D. Engineered Therapeutic-Releasing Nanoporous Anodic Alumina-Aluminum Wires with Extended Release of Therapeutics. ACS Appl. Mater. Interfaces 2015, 7, 3846–3853. [Google Scholar] [CrossRef]
- Feng, X.; Shankar, K.; Paulose, M.; Grimes, C.A. Tantalum-Doped Titanium Dioxide Nanowire Arrays for Dye-Sensitized Solar Cells with High Open-Circuit Voltage. Angew. Chem. Int. Ed. 2009, 121, 8239–8242. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, L.; Wang, H.; Shen, H.; Cheng, Q.; Yan, C.; Park, S. Electrodeposition of Rhodium Nanowires Arrays and Their Morphology-Dependent Hydrogen Evolution Activity. Nanomaterials 2017, 7, 103. [Google Scholar] [CrossRef] [PubMed]
- Palmero, E.M.; Bran, C.; Del Real, R.P.; Magen, C.; Vazquez, M. Magnetic behavior of NiCu nanowire arrays: Compositional, geometry and temperature dependence. J. Appl. Phys. 2014, 116, 033908. [Google Scholar] [CrossRef] [Green Version]
- Masuda, H.; Fukuda, K. Ordered Metal Nanohole Arrays Made by a Two-Step Replication of Honeycomb Structures of Anodic Alumina. Science 1995, 268, 1466–1468. [Google Scholar] [CrossRef]
- Wu, M.T.; Leu, I.C.; Hon, M.H. Effect of polishing pretreatment on the fabrication of ordered nanopore arrays on aluminum foils by anodization. J. Vac. Sci. Technol. B Microelectron. Nanometer Struct. 2002, 20, 776. [Google Scholar] [CrossRef]
- Chu, S.; Wada, K.; Inoue, S.; Isogai, M.; Yasumori, A. Fabrication of ideally ordered nanoporous alumina films and integrated alumina nanotubule arrays by high-field anodization. Adv. Mater. 2005, 17, 2115–2119. [Google Scholar] [CrossRef]
- Poinern, G.E.J.; Ali, N.; Fawcett, D. Progress in Nano-Engineered Anodic Aluminum Oxide Membrane Development. Materials 2011, 4, 487–526. [Google Scholar] [CrossRef] [Green Version]
- Masuda, H.; Yada, K.; Osaka, A. Self-Ordering of Cell Configuration of Anodic Porous Alumina with Large-Size Pores in Phosphoric Acid Solution. Jpn. J. Appl. Phys. 1998, 37, L1340–L1342. [Google Scholar] [CrossRef]
- Parkhutik, V.P.; Shershulsky, V.I. Theoretical modelling of porous oxide growth on aluminium. J. Phys. D Appl. Phys. 1992, 25, 1258–1263. [Google Scholar] [CrossRef]
- Zhao, X.; Seo, S.-K.; Lee, U.-J.; Lee, K.-H. Controlled Electrochemical Dissolution of Anodic Aluminum Oxide for Preparation of Open-Through Pore Structures. J. Electrochem. Soc. 2007, 154, C553–C557. [Google Scholar] [CrossRef] [Green Version]
- Wagner, R.S.; Ellis, W.C. Vapor-liquid-solid mechanism of single crystal growth. Appl. Phys. Lett. 1964, 4, 89–90. [Google Scholar] [CrossRef]
- Ozin, G.A.; Arsenault, A.C. Nanochemistry: A Chemical Approach to Nanomaterials; Royal Society of Chemistry: Cambridge, UK, 2009. [Google Scholar]
- Maruyama, S.; Kojima, R.; Miyauchi, Y.; Chiashi, S.; Kohno, M. Low-temperature synthesis of high-purity single-walled carbon nanotubes from alcohol. Chem. Phys. Lett. 2002, 360, 229–234. [Google Scholar] [CrossRef]
- Maruyama, S.; Einarsson, E.; Murakami, Y.; Edamura, T. Growth process of vertically aligned single-walled carbon nanotubes. Chem. Phys. Lett. 2005, 403, 320–323. [Google Scholar] [CrossRef] [Green Version]
- Hata, K.; Futaba, D.N.; Mizuno, K.; Namai, T.; Yumura, M.; Iijima, S. Water-assisted highly efficient synthesis of impurity-free single walled carbon nanotubes. Science 2004, 306, 1362–1364. [Google Scholar] [CrossRef] [Green Version]
- Glaspell, G.; Abdelsayed, V.; Saoud, K.M.; El-Shall, M.S. Vapor-phase synthesis of metallic and intermetallic nanoparticles and nanowires: Magnetic and catalytic properties. Pure Appl. Chem. 2006, 78, 1667–1689. [Google Scholar] [CrossRef]
- Liz-Marzán, L.M.; Kamat, P.V. Nanoscale Materials; Kluwer Academic: Dordrecht, The Netherlands, 2003. [Google Scholar]
- Güniat, L.; Caroff, P.; Morral, A.F.I. Vapor Phase Growth of Semiconductor Nanowires: Key Developments and Open Questions. Chem. Rev. 2019, 119, 8958–8971. [Google Scholar] [CrossRef] [Green Version]
- Guo, Y.N.; Zou, J.; Paladugu, M.C.; Wang, H.; Gao, Q.; Tan, H.H.; Jagadish, C. Structural characteristics of GaSb∕GaAs nanowire heterostructures grown by metal-organic chemical vapor deposition. Appl. Phys. Lett. 2006, 89, 231917. [Google Scholar] [CrossRef] [Green Version]
- Larsson, M.W.; Wagner, J.B.; Wallin, M.; Håkansson, P.; Fröberg, L.E.; Samuelson, L.; Wallenberg, L.R. Strain mapping in free-standing heterostructured wurtzite InAs/InP nanowires. Nanotechnology 2006, 18, 015504. [Google Scholar] [CrossRef]
- Caroff, P.; Messing, M.E.; Borg, B.M.; Dick, K.A.; Deppert, K.; Wernersson, L.-E. InSb heterostructure nanowires: MOVPE growth under extreme lattice mismatch. Nanotechnology 2009, 20, 495606. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, B.W.; Ayres, V.M.; Petkov, M.P.; Halpern, J.B.; He, M.; Baczewski, A.D.; McElroy, K.; Crimp, M.A.; Zhang, J.; Shaw, H.C. Electronic and Structural Characteristics of Zinc-Blende Wurtzite Biphasic Homostructure GaN Nanowires. Nano Lett. 2007, 7, 1435–1438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joyce, H.J.; Wong-Leung, J.; Gao, Q.; Tan, H.H.; Jagadish, C. Phase Perfection in Zinc Blende and Wurtzite III−V Nanowires Using Basic Growth Parameters. Nano Lett. 2010, 10, 908–915. [Google Scholar] [CrossRef] [PubMed]
- Sukrittanon, S.; Dobrovolsky, A.; Kang, W.-M.; Jang, J.-S.; Kim, B.-J.; Chen, W.; Buyanova, I.A.; Tu, C.W. Growth and characterization of dilute nitride GaNxP1−x nanowires and GaNxP1−x/GaNyP1−y core/shell nanowires on Si (111) by gas source molecular beam epitaxy. Appl. Phys. Lett. 2014, 105, 072107. [Google Scholar] [CrossRef] [Green Version]
- Namazi, L.; Ghalamestani, S.G.; Lehmann, S.; Zamani, R.R.; Dick, K.A. Direct nucleation, morphology and compositional tuning of InAs1−xSbx nanowires on InAs (111) B substrates. Nanotechnology 2017, 28, 165601. [Google Scholar] [CrossRef]
- Maliakkal, C.B.; Jacobsson, D.; Tornberg, M.; Persson, A.R.; Johansson, J.; Wallenberg, R.; Dick, K.A. In situ analysis of catalyst composition during gold catalyzed GaAs nanowire growth. Nat. Commun. 2019, 10, 4577. [Google Scholar] [CrossRef] [Green Version]
- Goktas, N.I.; Wilson, P.; Ghukasyan, A.; Wagner, D.; McNamee, S.; Lapierre, R.R. Nanowires for energy: A review. Appl. Phys. Rev. 2018, 5, 041305. [Google Scholar] [CrossRef]
- Soto-Vázquez, L.; Rolón-Delgado, F.; Rivera, K.; Cotto, M.C.; Ducongé, J.; Morant, C.; Pinilla, S.; Márquez-Linares, F.M. Catalytic use of TiO2 nanowires in the photodegradation of Benzophenone-4 as an active ingredient in sunscreens. J. Environ. Manag. 2019, 247, 822–828. [Google Scholar] [CrossRef]
- Pinilla, S.; Machín, A.; Park, S.H.; Arango, J.C.; Nicolosi, V.; Márquez-Linares, F.; Morant, C. TiO2-Based nanomaterials for the production of hydrogen and the development of lithium-ion batteries. J. Phys. Chem. B 2018, 122, 972–983. [Google Scholar] [CrossRef]
- Lee, W.; Park, S.-J. Porous Anodic Aluminum Oxide: Anodization and Templated Synthesis of Functional Nanostructures. Chem. Rev. 2014, 114, 7487–7556. [Google Scholar] [CrossRef]
- Marquez, F.; Morant, C.; Campo, T.; Sanz, J.M.; Elizalde, E. Ordered metal nanotube arrays fabricated by PVD. J. Nanosci. Nanotechnol. 2010, 10, 1115–1119. [Google Scholar] [CrossRef] [PubMed]
- Masuda, H.; Yamada, H.; Satoh, M.; Asoh, H.; Nakao, M.; Tamamura, T. Highly ordered nanochannel-array architecture in anodic alumina. Appl. Phys. Lett. 1997, 71, 2770–2772. [Google Scholar] [CrossRef]
- Márquez, F.; Morant, C.; Pirota, K.; Elizalde, E.; Borrás, A.; Sanz, J.M. Fabrication of Ordered Crystalline Zirconium Nanoporous Membranes by an One-Step Procedure. Nano Today 2009, 4, 21–26. [Google Scholar] [CrossRef]
- Ganapathi, A.; Swaminathan, P.; Neelakantan, L. Anodic Aluminum Oxide Template Assisted Synthesis of Copper Nanowires using a Galvanic Displacement Process for Electrochemical Denitrification. ACS Appl. Nano Mater. 2019, 2, 5981–5988. [Google Scholar] [CrossRef]
- Sun, B.; Long, Y.; Zhang, H.; Li, M.; Duvail, J.; Jiang, X.; Yin, H. Advances in three-dimensional nanofibrous macrostructures via electrospinning. Prog. Polym. Sci. 2014, 39, 862–890. [Google Scholar] [CrossRef]
- Reneker, D.H.; Fong, H. Polymeric Nanofibers: Introduction; Reneker, D.H., Fong, H., Eds.; American Chemical Society: Washington, DC, USA, 2006. [Google Scholar]
- Bhardwaj, N.; Kundu, S.C. Electrospinning: A fascinating fiber fabrication technique. Biotechnol. Adv. 2010, 28, 325–347. [Google Scholar] [CrossRef]
- Xue, J.; Wu, T.; Dai, Y.; Xia, Y. Electrospinning and Electrospun Nanofibers: Methods, Materials, and Applications. Chem. Rev. 2019, 119, 5298–5415. [Google Scholar] [CrossRef] [PubMed]
- Dou, Y.; Zhang, W.; Kaiser, A. Electrospinning of Metal–Organic Frameworks for Energy and Environmental Applications. Adv. Sci. 2019, 7, 1902590. [Google Scholar] [CrossRef] [Green Version]
- Husain, A.A.; Hasan, W.Z.W.; Shafie, S.; Hamidon, M.N.; Pandey, S.S. A review of transparent solar photovoltaic technologies. Renew. Sustain. Energy Rev. 2018, 94, 779–791. [Google Scholar] [CrossRef]
- Kumaresan, P.; Vegiraju, S.; Ezhumalai, Y.; Yau, S.L.; Kim, C.; Lee, W.-H.; Chen, M.-C. Fused-Thiophene Based Materials for Organic Photovoltaics and Dye-Sensitized Solar Cells. Polymers 2014, 6, 2645–2669. [Google Scholar] [CrossRef]
- Bhowmik, S.; Ali, O.A. Highly Efficient Ultra-thin Film CIGS Solar Cell with SnS BSF Layer. IOSR J. Electr. Electron. Eng. 2019, 14, 49–54. [Google Scholar]
- Kobitski, A.Y.; Zhuravlev, K.S.; Wagner, H.P.; Zahn, D.R.T. Self-trapped exciton recombination in silicon nanocrystals. Phys. Rev. B Condens. Matter Mater. Phys. 2001, 63, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Robel, I.; Bunker, B.A.; Kamat, P.V.; Kuno, M. Exciton Recombination Dynamics in CdSe Nanowires: Bimolecular to Three-Carrier Auger Kinetics. Nano Lett. 2006, 6, 1344–1349. [Google Scholar] [CrossRef]
- Zhuang, H.L.; Hennig, R.G. Stability and magnetism of strongly correlated single-layer VS2. Phys. Rev. B 2016, 93, 054429. [Google Scholar] [CrossRef] [Green Version]
- Razykov, T.; Ferekides, C.; Morel, D.; Stefanakos, E.; Ullal, H.; Upadhyaya, H. Solar photovoltaic electricity: Current status and future prospects. Sol. Energy 2011, 85, 1580–1608. [Google Scholar] [CrossRef]
- Bedeloglu, A.; Demir, A.; Bozkurt, Y.; Sariciftci, N.S. A Photovoltaic Fiber Design for Smart Textiles. Text. Res. J. 2009, 80, 1065–1074. [Google Scholar] [CrossRef]
- Liu, J.; Namboothiry, M.; Carroll, D.L. Optical geometries for fiber-based organic photovoltaics. Appl. Phys. Lett. 2007, 90, 133515. [Google Scholar] [CrossRef]
- Fan, X.; Chu, Z.Z.; Wang, F.Z.; Zhang, C.; Chen, L.; Tang, Y.W.; Zou, D.C. Wire-Shaped Flexible Dye-sensitized Solar Cells. Adv. Mater. 2008, 20, 592–595. [Google Scholar] [CrossRef]
- Kempa, T.J.; Cahoon, J.F.; Kim, S.-K.; Day, R.W.; Bell, D.C.; Park, H.-G.; Lieber, C.M. Coaxial multishell nanowires with high-quality electronic interfaces and tunable optical cavities for ultrathin photovoltaics. Proc. Natl. Acad. Sci. USA 2012, 109, 1407–1412. [Google Scholar] [CrossRef] [Green Version]
- Sun, H.; Li, H.; You, X.; Yang, Z.; Deng, J.; Qiu, L.; Peng, H. Quasi-solid-state, coaxial, fiber-shaped dye-sensitized solar cells. J. Mater. Chem. A 2013, 2, 345–349. [Google Scholar] [CrossRef]
- Fu, Y.; Lv, Z.; Hou, S.; Wu, H.; Wang, D.; Zhang, C.; Chu, Z.; Cai, X.; Fan, X.; Wang, Z.L.; et al. Conjunction of fiber solar cells with groovy micro-reflectors as highly efficient energy harvesters. Energy Environ. Sci. 2011, 4, 3379–3383. [Google Scholar] [CrossRef]
- Hou, S.; Cai, X.; Fu, Y.; Lv, Z.; Wang, D.; Wu, H.; Zhang, C.; Chu, Z.; Zou, D. Transparent conductive oxide-less, flexible, and highly efficient dye-sensitized solar cells with commercialized carbon fiber as the counter electrode. J. Mater. Chem. 2011, 21, 13776–13779. [Google Scholar] [CrossRef]
- Huang, S.; Guo, X.; Huang, X.; Zhang, Q.; Sun, H.; Li, D.; Luo, Y.; Meng, Q. Highly efficient fibrous dye-sensitized solar cells based on TiO2 nanotube arrays. Nanotechnology 2011, 22, 315402. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z. High performance fiber-shaped solar cells. Pure Appl. Chem. 2016, 88, 113–117. [Google Scholar] [CrossRef]
- Qu, L.; Dai, L.; Stone, M.; Xia, Z.; Wang, Z.L. Carbon Nanotube Arrays with Strong Shear Binding-On and Easy Normal Lifting-Off. Science 2008, 322, 238–242. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Shi, E.; Ji, C.; Li, Z.; Li, P.; Shang, Y.; Li, Y.; Wei, J.; Wang, K.; Zhu, H.; et al. Fiber and fabric solar cells by directly weaving carbon nanotube yarns with CdSe nanowire-based electrodes. Nanoscale 2012, 4, 4954. [Google Scholar] [CrossRef]
- Zhang, L.; Shi, E.; Li, Z.; Li, P.; Jia, Y.; Ji, C.; Wei, J.; Wang, K.; Zhu, H.; Wu, D.; et al. Wire-supported CdSe nanowire array photoelectrochemical solar cells. Phys. Chem. Chem. Phys. 2012, 14, 3583–3588. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Qiu, L.; Cai, Z.; Gong, F.; Yang, Z.; Wang, Z.; Peng, H. Intertwined Aligned Carbon Nanotube Fiber Based Dye-Sensitized Solar Cells. Nano Lett. 2012, 12, 2568–2572. [Google Scholar] [CrossRef]
- Chen, H.; Zhu, L.; Liu, H.; Li, W. Growth of ZnO nanowires on fibers for one-dimensional flexible quantum dot-sensitized solar cells. Nanotechnology 2012, 23, 075402. [Google Scholar] [CrossRef] [PubMed]
- Pan, S.; Yang, Z.; Li, H.; Qiu, L.; Sun, H.; Peng, H. Efficient Dye-Sensitized Photovoltaic Wires Based on an Organic Redox Electrolyte. J. Am. Chem. Soc. 2013, 135, 10622–10625. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Yang, Z.; Chen, X.; Qiu, L.; You, X.; Chen, P.; Peng, H. Photovoltaic Wire with High Efficiency Attached onto and Detached from a Substrate Using a Magnetic Field. Angew. Chem. 2013, 125, 8434–8438. [Google Scholar] [CrossRef]
- Zhang, S.; Ji, C.; Bian, Z.; Yu, P.; Zhang, L.; Liu, D.; Shi, E.; Shang, Y.; Peng, H.; Cheng, Q.; et al. Porous, Platinum Nanoparticle-Adsorbed Carbon Nanotube Yarns for Efficient Fiber Solar Cells. ACS Nano 2012, 6, 7191–7198. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Ding, Y.; Niu, J.; Xia, Z.; Roy, A.K.; Chen, H.; Qu, J.; Wang, Z.L.; Dai, L. Rationally designed graphene-nanotube 3D architectures with a seamless nodal junction for efficient energy conversion and storage. Sci. Adv. 2015, 1, e1400198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- López-Covarrubias, J.G.; Soto-Muñoz, L.; Iglesias, A.L.; Villarreal-Gómez, L.J. Electrospun Nanofibers Applied to Dye Solar Sensitive Cells: A Review. Materials 2019, 12, 3190. [Google Scholar] [CrossRef] [Green Version]
- Joly, D.; Jung, J.-W.; Kim, I.-D.; Demadrille, R. Electrospun materials for solar energy conversion: Innovations and trends. J. Mater. Chem. C 2016, 4, 10173–10197. [Google Scholar] [CrossRef]
- Xia, Y.; Yang, P.; Sun, Y.; Wu, Y.; Mayers, B.; Gates, B.; Yin, Y.; Kim, F.; Yan, H. One-Dimensional Nanostructures: Synthesis, Characterization, and Applications. Adv. Mater. 2003, 15, 353–389. [Google Scholar] [CrossRef]
- Fu, Y.; Zhu, H.; Chen, J.; Hautzinger, M.P.; Zhu, X.-Y.; Jin, S. Metal halide perovskite nanostructures for optoelectronic applications and the study of physical properties. Nat. Rev. Mater. 2019, 4, 169–188. [Google Scholar] [CrossRef]
- Moore, D.T.; Sai, H.; Tan, K.W.; Smilgies, D.-M.; Zhang, W.; Snaith, H.J.; Wiesner, U.; Estroff, L.A. Crystallization Kinetics of Organic–Inorganic Trihalide Perovskites and the Role of the Lead Anion in Crystal Growth. J. Am. Chem. Soc. 2015, 137, 2350–2358. [Google Scholar] [CrossRef]
- Zhu, H.; Fu, Y.; Meng, F.; Wu, X.; Gong, Z.; Ding, Q.; Gustafsson, M.V.; Trinh, M.T.; Jin, S.; Zhu, X.-Y. Lead halide perovskite nanowire lasers with low lasing thresholds and high quality factors. Nat. Mater. 2015, 14, 636–642. [Google Scholar] [CrossRef]
- Ha, S.T.; Liu, X.; Zhang, Q.; Giovanni, D.; Sum, T.C.; Xiong, Q. Synthesis of Organic-Inorganic Lead Halide Perovskite Nanoplatelets: Towards High-Performance Perovskite Solar Cells and Optoelectronic Devices. Adv. Opt. Mater. 2014, 2, 838–844. [Google Scholar] [CrossRef]
- Chen, J.; Fu, Y.; Samad, L.; Dang, L.; Zhao, Y.; Shen, S.; Guo, L.; Jin, S. Vapor-Phase Epitaxial Growth of Aligned Nanowire Networks of Cesium Lead Halide Perovskites (CsPbX3, X = Cl, Br, I). Nano Lett. 2017, 17, 460–466. [Google Scholar] [CrossRef]
- Schmidt, L.C.; Pertegás, A.; González-Carrero, S.; Malinkiewicz, O.; Agouram, S.; Mínguez-Espallargas, G.; Bolink, H.J.; Galian, R.E.; Pérez-Prieto, J. Nontemplate synthesis of CH3NH3PbBr3 perovskite nanoparticles. J. Am. Chem. Soc. 2014, 136, 850–853. [Google Scholar] [CrossRef] [PubMed]
- Ashley, M.J.; O’Brien, M.N.; Hedderick, K.R.; Mason, J.A.; Ross, M.B.; Mirkin, C.A. Templated Synthesis of Uniform Perovskite Nanowire Arrays. J. Am. Chem. Soc. 2016, 138, 10096–10099. [Google Scholar] [CrossRef] [PubMed]
- Horváth, E.; Spina, M.; Szekrényes, Z.; Kamarás, K.; Gaal, R.; Gachet, D.; Forró, L. Nanowires of Methylammonium Lead Iodide (CH3NH3PbI3) prepared by low temperature solution-mediated crystallization. Nano Lett. 2014, 14, 6761–6766. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, S.; Kershaw, S.V.; Rogach, A.L. 25th Anniversary Article: Ion Exchange in Colloidal Nanocrystals. Adv. Mater. 2013, 25, 6923–6944. [Google Scholar] [CrossRef] [PubMed]
- Pan, D.; Fu, Y.; Chen, J.; Czech, K.J.; Wright, J.C.; Jin, S. Visualization and Studies of Ion-Diffusion Kinetics in Cesium Lead Bromide Perovskite Nanowires. Nano Lett. 2018, 18, 1807–1813. [Google Scholar] [CrossRef] [PubMed]
- Grätzel, M. Photoelectrochemical cells. Nat. Cell Biol. 2001, 414, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Salomão, P.E.; Gomes, D.S.; Ferreira, E.J.; Moura, F.; Nascimento, L.L.; Patrocínio, A.O.; Pereira, M.C. Photoelectrochemical hydrogen production from water splitting using heterostructured nanowire arrays of Bi2O3/BiAl oxides as a photocathode. Sol. Energy Mater. Sol. Cells 2019, 194, 276–284. [Google Scholar] [CrossRef]
- Li, J.; Jin, X.; Li, R.; Zhao, Y.; Wang, X.; Liu, X.; Jiao, H. Copper oxide nanowires for efficient photoelectrochemical water splitting. Appl. Catal. B Environ. 2019, 240, 1–8. [Google Scholar] [CrossRef]
- Feng, W.; Lin, L.; Li, H.; Chi, B.; Pu, J.; Li, J. Hydrogenated TiO2/ZnO heterojunction nanorod arrays with enhanced performance for photoelectrochemical water splitting. Int. J. Hydrogen Energy 2017, 42, 3938–3946. [Google Scholar] [CrossRef]
- Wei, R.-B.; Kuang, P.-Y.; Cheng, H.; Chen, Y.-B.; Long, J.-Y.; Zhang, M.-Y.; Liu, Z.-Q. Plasmon-Enhanced Photoelectrochemical Water Splitting on Gold Nanoparticle Decorated ZnO/CdS Nanotube Arrays. ACS Sustain. Chem. Eng. 2017, 5, 4249–4257. [Google Scholar] [CrossRef]
- Wang, L.; Lu, W.; Zhu, W.; Wu, H.; Wang, F.; Xu, X. A photoelectrochemical sensor for highly sensitive detection of glucose based on Au–NiO1–x hybrid nanowires. Sens. Actuators B Chem. 2019, 304, 1–8. [Google Scholar] [CrossRef]
- Ongaro, M.; Signoretto, M.; Trevisan, V.; Stortini, A.M.; Ugo, P. Arrays of TiO2 Nanowires as Photoelectrochemical Sensors for Hydrazine Detection. Chemosensors 2015, 3, 146–156. [Google Scholar] [CrossRef] [Green Version]
- Zu, M.; Zheng, M.; Zhang, S.; Xing, C.; Zhou, M.; Liu, H.; Zhou, X.; Zhang, S. Designing robust anatase-branch@hydrogenated-rutile-nanorod TiO2 as accurate and sensitive photoelectrochemical sensors. Sens. Actuators B Chem. 2020, 321, 128504. [Google Scholar] [CrossRef]
- Han, F.; Song, Z.; Nawaz, M.H.; Dai, M.; Han, D.; Han, L.; Fan, Y.; Xu, J.; Han, D.; Niu, L. MoS2/ZnO Heterostructures Based Label-free, Visible-Light-Excited Photoelectro-chemical Sensor for Sensitive and Selective Determination of Synthetic Antioxidant Propyl Gallate. Anal. Chem. 2019, 91, 10657–10662. [Google Scholar] [CrossRef] [PubMed]
- Han, H.-X.; Shi, C.; Yuan, L.; Sheng, G.-P. Enhancement of methyl orange degradation and power generation in a photoelectrocatalytic microbial fuel cell. Appl. Energy 2017, 204, 382–389. [Google Scholar] [CrossRef]
- Zhou, Z.; Wu, Z.; Xu, Q.; Zhao, G. A solar-charged photoelectrochemical wastewater fuel cell for efficient and sustainable hydrogen production. J. Mater. Chem. A Mater. 2017, 5, 25450–25459. [Google Scholar] [CrossRef]
- Wu, J.; Han, X.; Li, D.; Logan, B.E.; Liu, J.; Zhang, Z.; Feng, Y. Efficient CO2 conversion to formic acid in a novel microbial photoelectrochemical cell using a visible-light responsive Co3O4 nanorod-arrayed photocathode. Appl. Catal. B Environ. 2020, 276, 119102. [Google Scholar] [CrossRef]
- Wang, D.; Li, Y.; Puma, G.L.; Wang, C.; Wang, P.; Zhang, W.; Wang, Q. Dye-sensitized photoelectrochemical cell on plasmonic Ag/AgCl @ chiral TiO2 nanofibers for treatment of urban wastewater effluents, with simultaneous production of hydrogen and electricity. Appl. Catal. B Environ. 2015, 168–169, 25–32. [Google Scholar] [CrossRef] [Green Version]
- Sherif, S.; Barbir, F.; Veziroglu, T. Towards a Hydrogen Economy. Electr. J. 2005, 18, 62–76. [Google Scholar] [CrossRef]
- Schley, N.D.; Blakemore, J.D.; Subbaiyan, N.K.; Incarvito, C.D.; D’Souza, F.; Crabtree, R.H.; Brudvig, G.W. Distinguishing Homogeneous from Heterogeneous Catalysis in Electrode-Driven Water Oxidation with Molecular Iridium Complexes. J. Am. Chem. Soc. 2011, 133, 10473–10481. [Google Scholar] [CrossRef]
- Gross, E.; Krier, J.M.; Heinke, L.; Somorjai, G.A. Building Bridges in Catalysis Science. Monodispersed Metallic Nanoparticles for Homogeneous Catalysis and Atomic Scale Characterization of Catalysts under Reaction Conditions. Top. Catal. 2012, 55, 13–23. [Google Scholar] [CrossRef]
- Adleman, J.R.; Boyd, D.A.; Goodwin, D.G.; Psaltis, D. Heterogenous Catalysis Mediated by Plasmon Heating. Nano Lett. 2009, 9, 4417–4423. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.J.; Lee, G.-J. Advanced Nanomaterials for Water Splitting and Hydrogen Generation. In Nanomaterials for Green Energy; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Banerjee, S.; Dionysiou, D.D.; Pillai, S.C. Self-cleaning applications of TiO2 by photo-induced hydrophilicity and photocatalysis. Appl. Catal. B Environ. 2015, 176–177, 396–428. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.-F.; Wang, A.; Qiao, B.; Li, J.; Liu, J.; Zhang, T. Single-Atom Catalysts: A New Frontier in Heterogeneous Catalysis. Acc. Chem. Res. 2013, 46, 1740–1748. [Google Scholar] [CrossRef] [PubMed]
- Pelaez, M.; Nolan, N.T.; Pillai, S.C.; Seery, M.K.; Falaras, P.; Kontos, A.G.; Dunlop, P.S.M.; Hamilton, J.W.J.; Byrne, J.A.; O’Shea, K.E.; et al. A review on the visible light active titanium dioxide photocatalysts for environmental applications. Appl. Catal. B Environ. 2012, 125, 1–52. [Google Scholar] [CrossRef] [Green Version]
- Schneider, J.; Matsuoka, M.; Takeuchi, M.; Zhang, J.; Horiuchi, Y.; Anpo, M.; Bahnemann, D.W. Understanding TiO2 Photocatalysis: Mechanisms and Materials. Chem. Rev. 2014, 114, 9919–9986. [Google Scholar] [CrossRef]
- Spasiano, D.; Marotta, R.; Malato, S.; Fernández, P.; Di-Somma, I. Solar photocatalysis: Materials, reactors, some comercial, and pre-industrialized applications. A comprehensive approach. Appl. Catal. B Environ. 2015, 170, 90–123. [Google Scholar] [CrossRef]
- Ahmad, H.; Kamarudin, S.; Minggu, L.; Kassim, M. Hydrogen from photo-catalytic water splitting process: A review. Renew. Sustain. Energy Rev. 2015, 43, 599–610. [Google Scholar] [CrossRef]
- Leung, D.Y.C.; Fu, X.; Wang, C.; Ni, M.; Leung, M.K.H.; Wang, X.; Fu, X. Hydrogen Production over Titania-Based Photocatalysts. ChemSusChem 2010, 3, 681–694. [Google Scholar] [CrossRef] [PubMed]
- Nanowires Defined What Nanowires Are, What They Are Made from and How They Are Used. AZoNano. 2006. Available online: https://www.azonano.com/article.aspx?ArticleID=1735 (accessed on 20 January 2021).
- Li, W.; Xie, S.; Li, M.; Ouyang, X.; Cui, G.; Lu, X.; Tong, Y. CdS/CeOx heterostructured nanowires for photocatalytic hydrogen production. J. Mater. Chem. A 2013, 1, 4190–4193. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, Y.; Jin, J.; Zhang, J.; Lin, Z.; Huang, F.; Yu, J. Efficient Visible-Light Photocatalytic Hydrogen Evolution and Enhanced Photostability of Core/Shell CdS/g-C3N4 Nanowires. ACS Appl. Mater. Interfaces 2013, 5, 10317–10324. [Google Scholar] [CrossRef]
- Zhou, X.; Jin, J.; Zhu, X.; Huang, J.; Yu, J.; Wong, W.-Y. New Co(OH)2/CdS nanowires for efficient visible light photocatalytic hydrogen production. J. Mater. Chem. A 2016, 4, 5282–5287. [Google Scholar] [CrossRef]
- Rao, B.G.; Mukherjee, D.; Reddy, B.M. Novel approaches for preparation of nanoparticles. In Nanostructures for Novel Therapy. Synthesis, Characterization and Applications; Elsevier: Amsterdam, The Netherlands, 2017; pp. 1–36. [Google Scholar]
- Machín, A.; Cotto, M.C.; Duconge, J. Synthesis and Characterization of Au@TiO2 NWs and their Catalytic Activity by Water Splitting: A Comparative Study with Degussa P25. Am. J. Eng. Appl. Sci. 2017, 10, 298–311. [Google Scholar] [CrossRef] [Green Version]
- Machín, A.; Cotto, M.C.; Duconge, J. Hydrogen production via water splitting using different Au@ZnO catalysts under UV–vis irradiation. J. Photochem. Photobiol. A 2018, 353, 385–394. [Google Scholar] [CrossRef]
- Ghassan, A.A.; Mijan, N.A.; Taufiq-Yap, Y.H. Nanomaterials: An Overview of Nanorods Synthesis and Optimization. In Nanorods—An Overview from Synthesis to Emerging Device Applications; IntechOpen: London, UK, 2019. [Google Scholar]
- Liu, Z.; Bai, H.; Xu, S. Hierarchical CuO/ZnO “corn-like” architecture for photocatalytic hydrogen generation. Int. J. Hydrogen Energy 2011, 36, 13473–13480. [Google Scholar] [CrossRef]
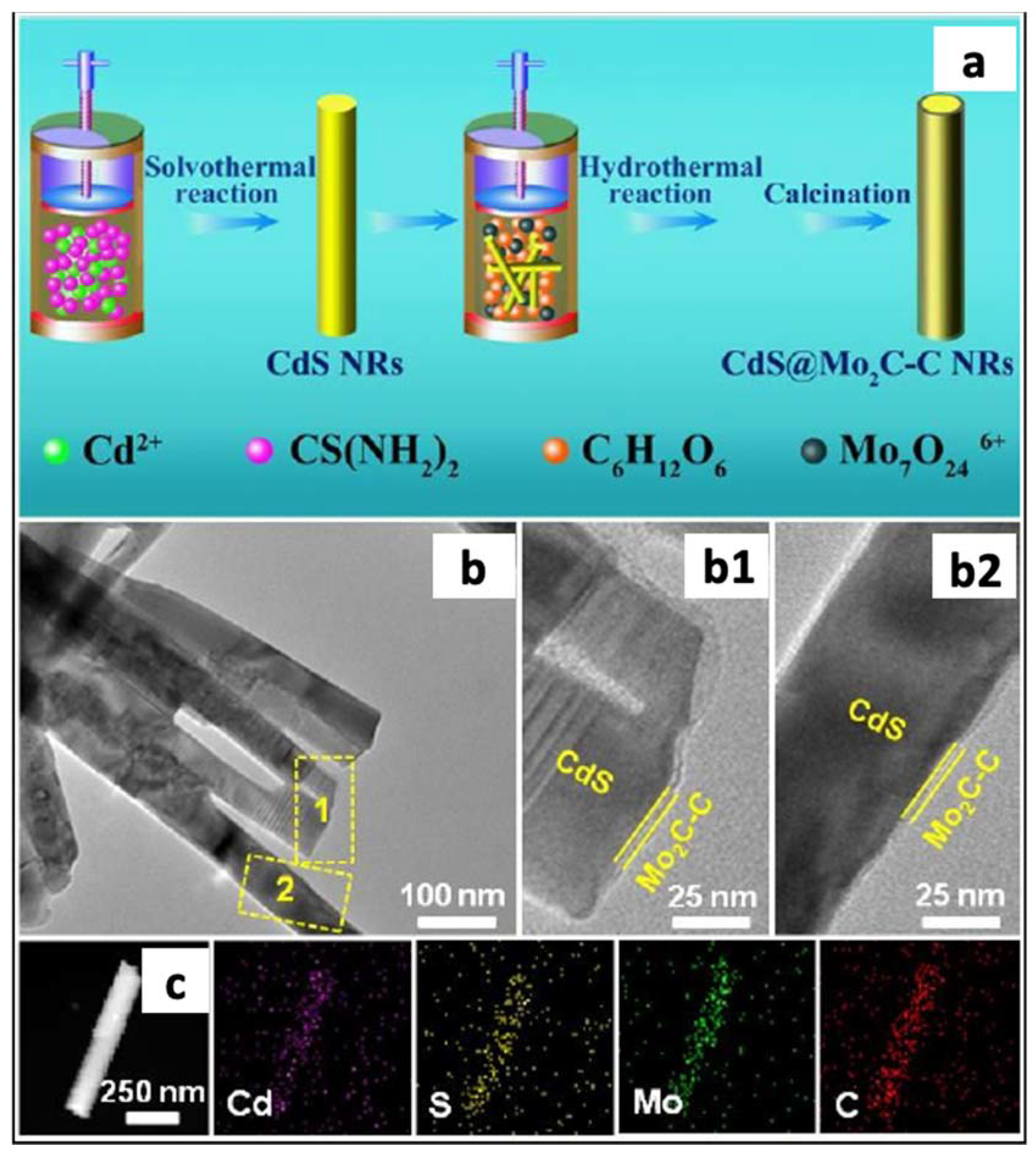
- Yi, S.S.; Yan, J.M.; Wulanb, B.R. Efficient Visible-Light-Driven Hydrogen Generation from Water Splitting Catalyzed by Highly Stable CdS@Mo2C-C Core-Shell Nanorods. J. Mater. Chem. A 2017, 5, 15862–15868. [Google Scholar] [CrossRef]
- Chen, W.; Li, X.; Wang, F.; Javaid, S.; Pang, Y.; Chen, J.; Yin, Z.; Wang, S.; Li, Y.; Jia, G. Nonepitaxial Gold-Tipped ZnSe Hybrid Nanorods for Efficient Photocatalytic Hydrogen Production. Small 2020, 16, e1902231. [Google Scholar] [CrossRef] [PubMed]
- García-Mendoza, C.; Oros-Ruiz, S.; Hernández-Gordillo, A. Suitable preparation of Bi2S3 nanorod—TiO2 heterojunction semiconductors with improved photocatalytic hydrogen production from water/methanol decomposition. J. Chem. Technol. Biotechnol. 2016, 91, 2198–2204. [Google Scholar] [CrossRef]
- Govindaraju, N.; Singh, R. Synthesis and Properties of Boron Nitride Nanotubes. In Nanotube Superfiber Materials—Changing Engineering Design; Elsevier: Amsterdam, The Netherlands, 2014; pp. 243–265. [Google Scholar]
- Xu, S.; Du, A.J.; Liu, J.; Ng, J.; Sun, D.D. Highly efficient CuO incorporated TiO2 nanotube photocatalyst for hydrogen production from water. Int. J. Hydrogen Energy 2011, 36, 6560–6568. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, L.; Lu, G.M.; Yao, X.; Guo, L. Nanoparticles enwrapped with nanotubes: A unique architecture of CdS/titanate nanotubes for efficient photocatalytic hydrogen production from water. J. Mater. Chem. 2011, 21, 5134–5141. [Google Scholar] [CrossRef]
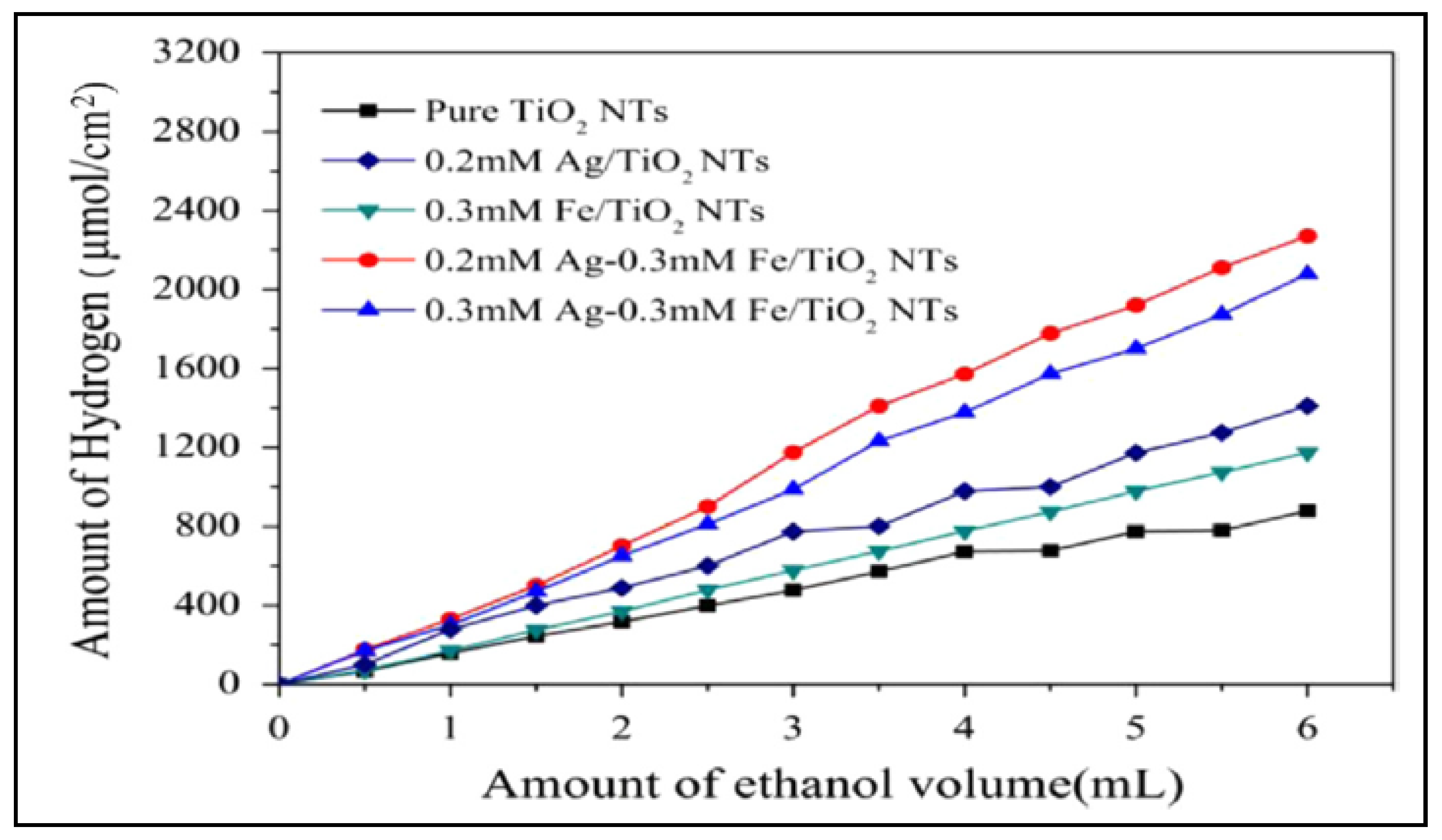
- Fan, X.; Fan, J.; Hu, X.; Liu, E.; Kang, L.; Tang, C.; Ma, Y.; Wu, H.; Li, Y. Preparation and characterization of Ag deposited and Fe doped TiO2 nanotube arrays for photocatalytic hydrogen production by water splitting. Ceram. Int. 2014, 40, 15907–15917. [Google Scholar] [CrossRef]
- Muneshwar, T.; Miao, M.; Borujeny, E.R. Atomic Layer Deposition: Fundamentals, Practice, and Challenges. In Handbook of Thin Film Deposition; Elsevier: Amsterdam, The Netherlands, 2018; pp. 359–377. [Google Scholar]
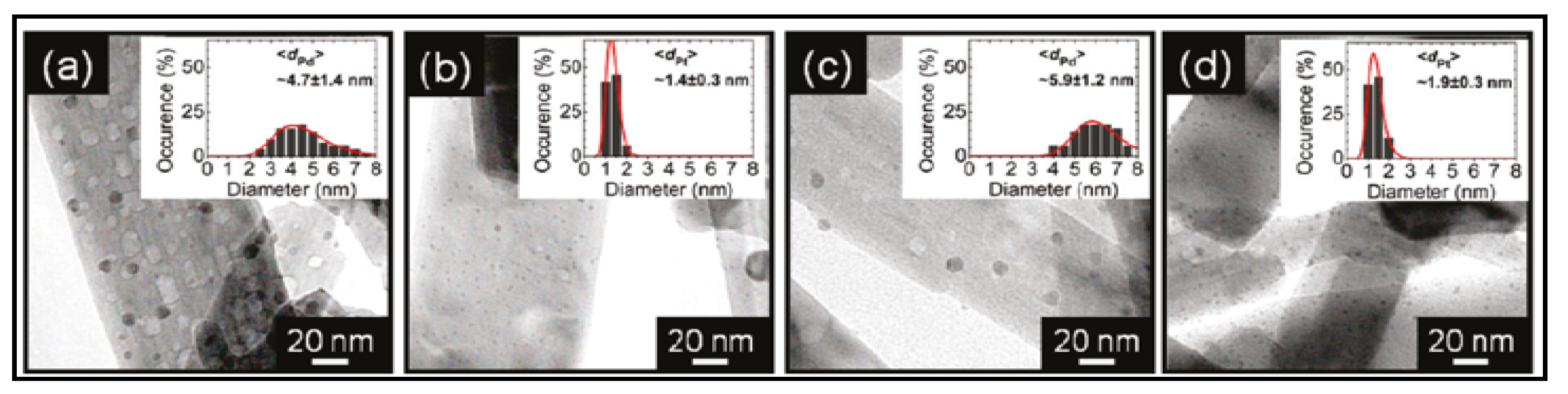
- Zhang, J.; Yu, Z.; Gao, Z. Porous TiO2 Nanotubes with Spatially Separated Platinum and CoOx Cocatalysts Produced by Atomic Layer Deposition for Photocatalytic Hydrogen Production. Angew. Chem. 2016, 128, 1–6. [Google Scholar]
- Kenry; Lim, C.T. Nanofiber technology: Current status and emerging developments. Prog. Polym. Sci. 2017, 70, 1–17. [Google Scholar] [CrossRef]
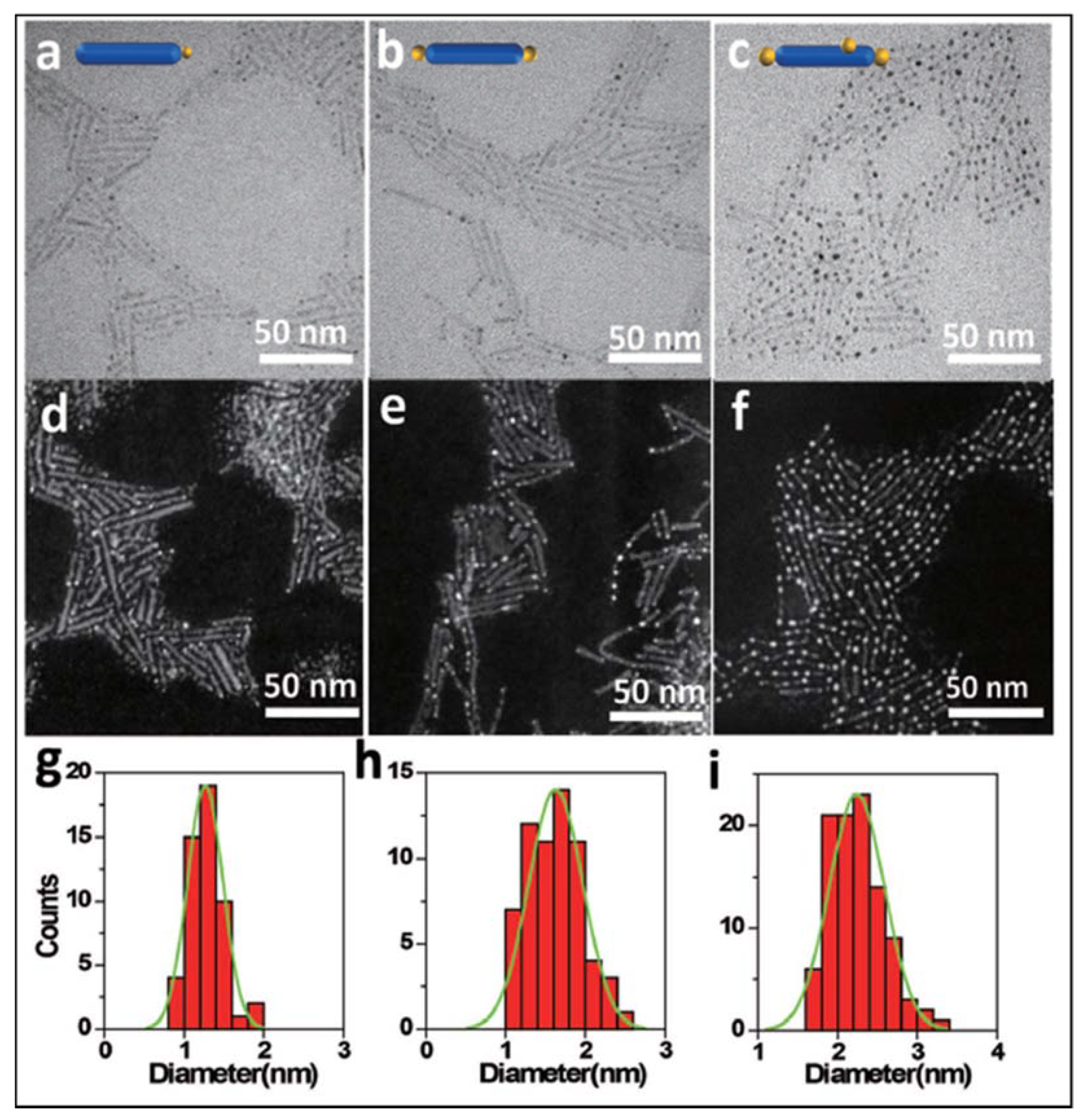
- Wu, M.-C.; Hiltunen, J.; Sápi, A.; Avila, A.; Larsson, W.; Liao, H.-C.; Huuhtanen, M.; Tóth, G.; Shchukarev, A.; Laufer, N.; et al. Nitrogen-Doped Anatase Nanofibers Decorated with Noble Metal Nanoparticles for Photocatalytic Production of Hydrogen. ACS Nano 2011, 5, 5025–5030. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, A.; Cao, S.-W.; Bosman, M.; Li, S.; Xue, C. Direct evidence of plasmon enhancement on photocatalytic hydrogen generation over Au/Pt-decorated TiO2 nanofibers. Nanoscale 2014, 6, 5217–5222. [Google Scholar] [CrossRef]
- Zhang, L.; Zhao, H.; Xu, S.; Liu, Q.; Li, T.; Luo, Y.; Gao, S.; Shi, X.; Asiri, A.M.; Sun, X. Recent Advances in 1D Electrospun Nanocatalysts for Electrochemical Water Splitting. Small Struct. 2021, 2, 2000048. [Google Scholar] [CrossRef]
- Hu, J.; Wang, L.; Zhang, P.; Liang, C.; Shao, G. Construction of solid-state Z-scheme carbon-modified TiO2/WO3 nanofibers with enhanced photocatalytic hydrogen production. J. Power Sources 2016, 328, 28–36. [Google Scholar] [CrossRef]
- Wang, Z.L.; Song, J.H. Piezoelectric nanogenerators based on zinc oxide nanowire arrays. Science 2006, 312, 242–246. [Google Scholar] [CrossRef]
- Wang, Z.L. Progress in Piezotronics and Piezo-Phototronics. Adv. Mater. 2012, 24, 4632–4646. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Qin, Y.; Dai, L.; Wang, Z.L. Power generation with laterally packaged piezoelectric fine wires. Nat. Nanotechnol. 2009, 4, 34–39. [Google Scholar] [CrossRef]
- Huang, C.-T.; Song, J.; Lee, W.-F.; Ding, Y.; Gao, Z.; Hao, Y.; Chen, L.-J.; Wang, Z.L. GaN Nanowire Arrays for High-Output Nanogenerators. J. Am. Chem. Soc. 2010, 132, 4766–4771. [Google Scholar] [CrossRef]
- Hou, T.-C.; Yang, Y.; Lin, Z.-H.; Ding, Y.; Park, C.; Pradel, K.C.; Chen, L.-J.; Wang, Z.L. Nanogenerator based on zinc blende CdTe micro/nanowires. Nano Energy 2013, 2, 387–393. [Google Scholar] [CrossRef]
- Ku, N.-J.; Wang, C.-H.; Huang, J.-H.; Fang, H.-C.; Huang, P.-C.; Liu, C.-P. Energy Harvesting from the Obliquely Aligned InN Nanowire Array with a Surface Electron-Accumulation Layer. Adv. Mater. 2012, 25, 861–866. [Google Scholar] [CrossRef] [PubMed]
- Tjong, S.C. Nanocrystalline Materials: Their Synthesis-Structure-Property Relationships and Applications; Elsevier: London, UK, 2014. [Google Scholar]
- Chen, X.; Xu, S.; Yao, N.; Shi, Y. 1.6 V Nanogenerator for Mechanical Energy Harvesting Using PZT Nanofibers. Nano Lett. 2010, 10, 2133–2137. [Google Scholar] [CrossRef]
- Acosta, M.; Novak, N.; Rojas, V.; Patel, S.; Vaish, R.; Koruza, J.; Jrossetti, G.A.R.; Rödel, J. BaTiO3-based piezoelectrics: Fundamentals, current status, and perspectives. Appl. Phys. Rev. 2017, 4, 041305. [Google Scholar] [CrossRef] [Green Version]
- Xu, S.; Hansen, B.J.; Wang, Z.L. Piezoelectric-nanowire-enabled power source for driving wireless microelectronics. Nat. Commun. 2010, 1, 93. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.-H.; Yang, Y.; Wu, J.M.; Liu, Y.; Zhang, F.; Wang, Z.L. BaTiO3 Nanotubes-Based Flexible and Transparent Nanogenerators. J. Phys. Chem. Lett. 2012, 3, 3599–3604. [Google Scholar] [CrossRef] [Green Version]
- Lin, Y.M.; Rabin, O.; Cronin, S.B.; Ying, J.Y.; Dresselhaus, M.S. Semimetal–semiconductor transition in Bi1−xSbx alloy nanowires and their thermoelectric properties. Appl. Phys. Lett. 2002, 81, 2403–2405. [Google Scholar] [CrossRef]
- Goldsmid, H.J.; Balise, P.L. Applications of Thermoelectricity (Methuen, London, 1969). Phys. Today 1961, 14, 72. [Google Scholar] [CrossRef]
- Lin, Y.-M.; Dresselhaus, M.S. Thermoelectric properties of superlattice nanowires. Phys. Rev. B 2003, 68, 075304. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Tang, G.; Pan, J.; Wang, H. Synthesis and Thermoelectric Property of 1D Flexible PEDOT: P-TSA/Glass Fiber. J. Miner. Mater. Charact. Eng. 2018, 6, 448–463. [Google Scholar] [CrossRef] [Green Version]
- Bounioux, C.; Díaz-Chao, P.; Campoy-Quiles, M.; Martín-González, M.S.; Goñi, A.R.; Yerushalmi-Rozen, R.; Müller, C. Thermoelectric Composites of Poly(3-hexylthiophene) and Carbon Nanotubes with a Large Power Factor. Energy Environ. Sci. 2013, 6, 918–925. [Google Scholar] [CrossRef]
- Wang, Y.; Cai, K.; Yao, X. Facile Fabrication and Thermoelectric Properties of PbTe-Modified Poly(3,4-ethylenedioxythiophene) Nanotubes. ACS Appl. Mater. Interfaces 2011, 3, 1163–1166. [Google Scholar] [CrossRef] [PubMed]
- Wei, Q.; Xiong, F.; Tan, S.; Huang, L.; Lan, E.H.; Dunn, B.; Mai, L. Energy Storage: Porous One-Dimensional Nanomaterials: Design, Fabrication and Applications in Electrochemical Energy Storage. Adv. Mater. 2017, 29, 1602300. [Google Scholar] [CrossRef]
- Mai, L.; Tian, X.; Xu, X.; Chang, L.; Xu, L. Nanowire Electrodes for Electrochemical Energy Storage Devices. Chem. Rev. 2014, 114, 11828–11862. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Gan, L.; Guo, K.; Ke, L.; Wei, Y.; Shen, G.; Zhai, T. Self-supported Zn3P2 Nanowires Arrays Grafted on Carbon Fabrics as an Advanced Integrated Anode for Flexible Lithium Ion Battery. Nanoscale 2016, 8, 8666–8672. [Google Scholar] [CrossRef]
- Wang, L.; Gong, H.; Wang, C.; Wang, D.; Tang, K.; Qian, Y. Facile synthesis of novel tunable highly porous CuO nanorods for high rate lithium battery anodes with realized long cycle life and high reversible capacity. Nanoscale 2012, 4, 6850–6855. [Google Scholar] [CrossRef]
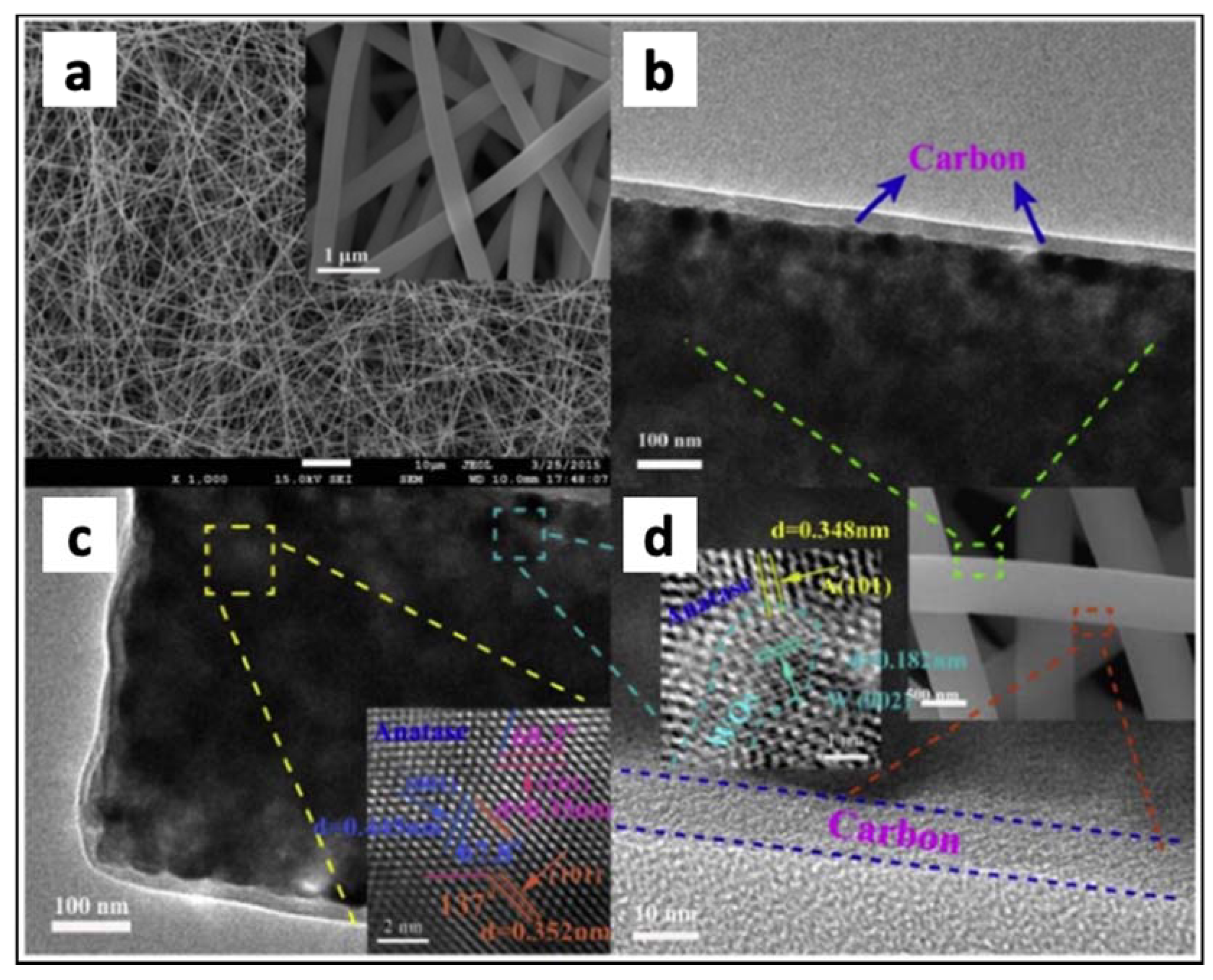
- Pinilla, S.; Park, S.H.; Fontánez, K.; Márquez, F.; Nicolosi, V.; Morant, C. 0D-1D Hybrid Silicon Nanocomposite as Lithium-Ion Batteries Anodes. Nanomaterials 2020, 10, 515. [Google Scholar] [CrossRef] [Green Version]
- Lukatskaya, M.R.; Dunn, B.; Gogotsi, M.R.L.Y. Multidimensional materials and device architectures for future hybrid energy storage. Nat. Commun. 2016, 7, 12647. [Google Scholar] [CrossRef]
- Wu, J.; Gao, X.; Yu, H.; Ding, T.; Yan, Y.; Yao, B.; Yao, X.; Chen, D.; Liu, M.; Huang, L. A Scalable Free-Standing V2O5/CNT Film Electrode for Supercapacitors with a Wide Operation Voltage (1.6 V) in an Aqueous Electrolyte. Adv. Funct. Mater. 2016, 26, 6114–6120. [Google Scholar] [CrossRef]
- Li, D.; Lv, C.; Liu, L.; Chunxiao, L.; She, X.; Guo, S.; Yang, D. Egg-Box Structure in Cobalt Alginate: A New Approach to Multifunctional Hierarchical Mesoporous N-Doped Carbon Nanofibers for Efficient Catalysis and Energy Storage. ACS Cent. Sci. 2015, 1, 261–269. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.; Augustyn, V.; Jia, X.; Xiao, Q.; Dunn, B.; Lu, Y. High-Performance Sodium-Ion Pseudocapacitors Based on Hierarchically Porous Nanowire Composites. ACS Nano 2012, 6, 4319–4327. [Google Scholar] [CrossRef]
- Wei, Q.; Fu, Y.; Zhang, G.; Yang, D.; Meng, G.; Sun, S. Rational design of novel nanostructured arrays based on porous AAO templates for electrochemical energy storage and conversion. Nano Energy 2019, 55, 234–259. [Google Scholar] [CrossRef]
- Li, Y.; Tan, B.; Wu, Y. Mesoporous Co3O4 Nanowire Arrays for Lithium Ion Batteries with High Capacity and Rate Capability. Nano Lett. 2008, 8, 265–270. [Google Scholar] [CrossRef]
- Nan, D.; Huang, Z.-H.; Lv, R.; Yang, L.; Wang, J.-G.; Shen, W.; Lin, Y.; Yu, X.; Ye, L.; Sun, H.; et al. Nitrogen-enriched electrospun porous carbon nanofiber networks as high-performance free-standing electrode materials. J. Mater. Chem. A 2014, 2, 19678–19684. [Google Scholar] [CrossRef]
- Cabana, J.; Monconduit, L.; Larcher, D.; Palacín, M.R. Beyond Intercalation-Based Li-Ion Batteries: The State of the Art and Challenges of Electrode Materials Reacting Through Conversion Reactions (Adv. Mater. 35/2010). Adv. Mater. 2010, 22, E170–E192. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Wu, H.; Zou, M.; Shi, X.; Yuan, Y.; Bai, W.; Cao, A. Short-range ordered graphitized-carbon nanotubes with large cavity as high-performance lithium-ion battery anodes. Carbon 2020, 158, 642–650. [Google Scholar] [CrossRef]
- Cha, G.; Mohajernia, S.; Nguyen, N.T.; Mazare, A.; Denisov, N.; Hwang, I.; Schmuki, P. Li + Pre-Insertion Leads to Formation of Solid Electrolyte Interface on TiO2 Nanotubes That Enables High-Performance Anodes for Sodium Ion Batteries. Adv. Energy Mater. 2019, 10, 1903448. [Google Scholar] [CrossRef] [Green Version]
- Armstrong, G.; Armstrong, A.R.; Bruce, P.G.; Reale, P.; Scrosati, B. TiO2(B) Nanowires as an Improved Anode Material for Lithium-Ion Batteries Containing LiFePO4 or LiNi0.5Mn1.5O4 Cathodes and a Polymer Electrolyte. Adv. Mater. 2006, 18, 2597–2600. [Google Scholar] [CrossRef]
- Yuan, T.; Zhao, B.; Cai, R.; Zhou, Y.; Shao, Z. Electrospinning based fabrication and performance improvement of film electrodes for lithium-ion batteries composed of TiO2 hollow fibers. J. Mater. Chem. 2011, 21, 15041–15048. [Google Scholar] [CrossRef]
- Shi, F.; Chen, C.; Xu, Z.-L. Recent Advances on Electrospun Nanofiber Materials for Post-lithium Ion Batteries. Adv. Fiber Mater. 2021, 3, 1–27. [Google Scholar] [CrossRef]
- Jung, J.-W.; Lee, C.-L.; Yu, S.; Kim, I.-D. Electrospun nanofibers as a platform for advanced secondary batteries: A comprehensive review. J. Mater. Chem. A 2016, 4, 703–750. [Google Scholar] [CrossRef]
- Luo, S.; Zhang, P.; Yuan, T.; Ruan, J.; Peng, C.; Pang, Y.; Sun, H.; Yang, J.; Zheng, S. Molecular self-assembly of a nanorod N-Li4Ti5O12/TiO2/C anode for superior lithium ion storage. J. Mater. Chem. A 2018, 6, 15755–15761. [Google Scholar] [CrossRef]
- Zhang, W.-J. A review of the electrochemical performance of alloy anodes for lithium-ion batteries. J. Power Source 2011, 196, 13–24. [Google Scholar] [CrossRef]
- Park, C.-M.; Kim, J.-H.; Kim, H.; Sohn, H.-J. Li-alloy based anode materials for Li secondary batteries. Chem. Soc. Rev. 2010, 39, 3115–3141. [Google Scholar] [CrossRef]
- Ge, M.; Rong, J.; Fang, X.; Zhou, C. Porous Doped Silicon Nanowires for Lithium Ion Battery Anode with Long Cycle Life. Nano Lett. 2012, 12, 2318–2323. [Google Scholar] [CrossRef] [PubMed]
- Hwang, I.-S.; Kim, J.-C.; Seo, S.-D.; Lee, S.; Lee, J.-H.; Kim, D.-W. A binder-free Ge-nanoparticle anode assembled on multiwalled carbon nanotube networks for Li-ion batteries. Chem. Commun. 2012, 48, 7061. [Google Scholar] [CrossRef] [PubMed]
- Feng, K.; Li, M.; Liu, W.; Kashkooli, A.G.; Xiao, X.; Cai, M.; Chen, Z. Silicon-Based Anodes for Lithium-Ion Batteries: From Fundamentals to Practical Applications. Small 2018, 14, 1702737. [Google Scholar] [CrossRef]
- Tian, H.; Xin, F.; Wang, X.; He, W.; Han, W. High capacity group-IV elements (Si, Ge, Sn) based anodes for lithium-ion batteries. J. Mater. 2015, 1, 153–169. [Google Scholar] [CrossRef] [Green Version]
- Armand, M.; Tarascon, J.M. Building better batteries. Nature 2008, 451, 652–657. [Google Scholar] [CrossRef]
- Aricò, A.S.; Bruce, P.; Scrosati, B.; Tarascon, J.-M.; Van Schalkwijk, W. Nanostructured materials for advanced energy conversion and storage devices. Nat. Mater. 2005, 4, 366–377. [Google Scholar] [CrossRef] [PubMed]
- Roy, P.; Srivastava, S.K. Nanostructured anode materials for lithium ion batteries. J. Mater. Chem. A 2015, 3, 2454–2484. [Google Scholar] [CrossRef]
- Landi, B.J.; Ganter, M.J.; Cress, C.D.; Dileo, R.A.; Raffaelle, R.P. Carbon nanotubes for lithium ion batteries. Energy Environ. Sci. 2009, 2, 638–654. [Google Scholar] [CrossRef]
- Zhang, B.; Kang, F.; Tarascon, J.-M.; Kim, J.-K. Recent advances in electrospun carbon nanofibers and their application in electrochemical energy storage. Prog. Mater. Sci. 2016, 76, 319–380. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, Y.; Xu, S.; Zhang, C.; Hou, L.; Yuan, C. Scalable Synthesis of One-Dimensional Mesoporous ZnMnO3 Nanorods with Ultra-Stable and High Rate Capability for Efficient Lithium Storage. Chem. A Eur. J. 2019, 25, 16683–16691. [Google Scholar] [CrossRef] [PubMed]
- Cao, F.-F.; Deng, J.-W.; Xin, S.; Ji, H.-X.; Schmidt, O.G.; Wan, L.-J.; Guo, Y.-G. Cu-Si Nanocable Arrays as High-Rate Anode Materials for Lithium-Ion Batteries. Adv. Mater. 2011, 23, 4415–4420. [Google Scholar] [CrossRef] [PubMed]
- Carbone, M. Zn defective ZnCo2O4 nanorods as high capacity anode for lithium ion batteries. J. Electroanal. Chem. 2018, 815, 151–157. [Google Scholar] [CrossRef]
- Cui, L.F.; Ruffo, R.; Chan, C.K.; Peng, H.; Cui, Y. Crystalline-Amorphous Core−Shell Silicon Nanowires for High Capacity and High Current Battery Electrodes. Nano Lett. 2009, 9, 491–495. [Google Scholar] [CrossRef]
- Cui, Z.; Wang, S.; Zhang, Y.; Cao, M. High-performance lithium storage of Co3O4 achieved by constructing porous nanotube structure. Electrochim. Acta 2015, 182, 507–515. [Google Scholar] [CrossRef]
- Kim, M.; Lee, J.; Lee, S.; Seo, S.; Bae, C.; Shin, H. Nanotubular Heterostructure of Tin Dioxide/Titanium Dioxide as a Binder-Free Anode in Lithium-Ion Batteries. ChemSusChem 2015, 8, 2363–2371. [Google Scholar] [CrossRef] [PubMed]
- de Juan, L.M.Z.; Maggay, I.V.B.; Nguyen, M.T.; Liu, W.R.; Yonezawa, T. β-Sn Nanorods with Active (001) Tip Induced LiF-Rich SEI Layer for Stable Anode Material in Lithium Ion Battery. ACS Appl. Nano Mater. 2018, 1, 3509–3519. [Google Scholar] [CrossRef]
- Zhang, L.; Zhu, S.; Cao, H.; Hou, L.; Yuan, C. Hierarchical Porous ZnMn2O4 Hollow Nanotubes with Enhanced Lithium Storage toward Lithium-Ion Batteries. Chem. A Eur. J. 2015, 21, 10771–10777. [Google Scholar] [CrossRef]
- Cheng, J.; Wang, B.; Park, C.-M.; Wu, Y.; Huang, H.; Nie, F. CNT@Fe3O4@C Coaxial Nanocables: One-Pot, Additive-Free Synthesis and Remarkable Lithium Storage Behavior. Chem. A Eur. J. 2013, 19, 9866–9874. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-M.; Abel, P.R.; Heller, A.; Mullins, C.B. α-Fe2O3 Nanorods as Anode Material for Lithium Ion Batteries. J. Phys. Chem. Lett. 2011, 2, 2885–2891. [Google Scholar] [CrossRef]
- Kennedy, T.; Mullane, E.; Geaney, H.; Osiak, M.; O’Dwyer, C.; Ryan, K.M. High-Performance Germanium Nanowire-Based Lithium-Ion Battery Anodes Extending over 1000 Cycles Through in Situ Formation of a Continuous Porous Network. Nano Lett. 2014, 14, 716–723. [Google Scholar] [CrossRef] [Green Version]
- Cheong, J.Y.; Kim, C.; Jung, J.-W.; Yoon, K.R.; Kim, I.-D. Porous SnO2-CuO nanotubes for highly reversible lithium storage. J. Power Source 2018, 373, 11–19. [Google Scholar] [CrossRef]
- Shen, L.; Yuan, C.; Luo, H.; Zhang, X.; Xu, K.; Zhang, F. In situ growth of Li4Ti5O12 on multi-walled carbon nanotubes: Novel coaxial nanocables for high rate lithium ion batteries. J. Mater. Chem. 2010, 21, 761–767. [Google Scholar] [CrossRef]
- Yin, D.; Huang, G.; Na, Z.; Wang, X.; Li, Q.; Wang, L. CuO Nanorod Arrays Formed Directly on Cu Foil from MOFs as Superior Binder-Free Anode Material for Lithium-Ion Batteries. ACS Energy Lett. 2017, 2, 1564–1570. [Google Scholar] [CrossRef]
- Lim, Y.R.; Cha, E.H.; Jung, C.S.; Im, H.S.; Park, K.; Cho, W.I. Zn2GeO4 and Zn2SnO4 nanowires for high-capacity lithium- and sodium-ion batteries. J. Mater. Chem. A 2016, 4, 10691–10699. [Google Scholar] [CrossRef]
- Wu, H.; Chan, G.; Choi, J.W.; Ryu, I.; Yao, Y.; McDowell, M.T.; Lee, S.W.; Jackson, A.; Yang, Y.; Hu, L.; et al. Stable cycling of double-walled silicon nanotube battery anodes through solid–electrolyte interphase control. Nat. Nanotechnol. 2012, 7, 310–315. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Hu, L.; Meng, Y.S.; Li, Q. Electrodeposited three-dimensional Ni–Si nanocable arrays as high performance anodes for lithium ion batteries. Nanoscale 2013, 5, 10376–10383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Fu, J.; Pan, Z.; Su, J.; Xu, J.; Gao, B.; Peng, X.; Wang, L.; Zhang, X.; Chu, P.K. Peapod-like V2O3 nanorods encapsulated into carbon as binder-free and flexible electrodes in lithium-ion batteries. J. Power Source 2016, 331, 58–66. [Google Scholar] [CrossRef]
- Gao, L.; Wang, X.; Xie, Z.; Song, W.; Wang, L.; Wu, X.; Qu, F.; Chen, D.; Shen, G. High-performance energy-storage devices based on WO3 nanowire arrays/carbon cloth integrated electrodes. J. Mater. Chem. A 2013, 1, 7167–7173. [Google Scholar] [CrossRef]
- Xu, J.; Wu, H.; Wang, F.; Xia, Y.; Zheng, G. Zn4Sb3 Nanotubes as Lithium Ion Battery Anodes with High Capacity and Cycling Stability. Adv. Energy Mater. 2012, 3, 286–289. [Google Scholar] [CrossRef]
- Geng, H.; Ge, D.; Lu, S.; Wang, J.; Ye, Z.; Yang, Y.; Zheng, J.; Gu, H. Preparation of a γ-Fe2O3/Ag Nanowire Coaxial Nanocable for High-Performance Lithium-Ion Batteries. Chem. A Eur. J. 2015, 21, 11129–11133. [Google Scholar] [CrossRef] [PubMed]
- Niu, H.; Zhou, H.; Lin, T. Electrospun carbon nanofiber as electrode materials for supercapacitor applications. In Electrospun Polymers and Composites: Ultrafine Materials, High Performance Fibers and Wearables. A Volume in Series in Composites Science and Engineering; Woodhead Publishing: Shaston, UK, 2020. [Google Scholar]
- Liang, J.; Zhao, H.; Yue, L.; Fan, G.; Li, T.S.; Lu, S.; Chen, G.; Gao, S.; Asiri, A.M.; Sun, X. Recent advances in electrospun nanofibers for supercapacitors. J. Mater. Chem. A 2020, 8, 16747–16789. [Google Scholar] [CrossRef]
- Xu, B.; Wu, F.; Su, Y.; Cao, G.; Chen, S.; Zhou, Z.; Yang, Y. Competitive effect of KOH activation on the electrochemical performances of carbon nanotubes for EDLC: Balance between porosity and conductivity. Electrochim. Acta 2008, 53, 7730–7735. [Google Scholar] [CrossRef]
- Kim, B.; Chung, H.; Kim, W. High-performance supercapacitors based on vertically aligned carbon nanotubes and nonaqueous electrolytes. Nanotechnology 2012, 23, 155401. [Google Scholar] [CrossRef]
- Qiu, Y.; Li, G.; Hou, Y.; Pan, Z.; Li, H.; Li, W.; Liu, M.; Ye, F.; Yang, X.; Zhang, Y. Vertically Aligned Carbon Nanotubes on Carbon Nanofibers: A Hierarchical Three-Dimensional Carbon Nanostructure for High-Energy Flexible Supercapacitors. Chem. Mater. 2015, 27, 1194–1200. [Google Scholar] [CrossRef]
- Arcila-Velez, M.R.; Zhu, J.; Childress, A.; Karakaya, M.; Podila, R.; Rao, A.M.; Roberts, M.E. Roll-to-roll synthesis of vertically aligned carbon nanotube electrodes for electrical double layer capacitors. Nano Energy 2014, 8, 9–16. [Google Scholar] [CrossRef]
- Brousse, T.; Bélanger, D.; Long, J.W. To Be or Not To Be Pseudocapacitive? J. Electrochem. Soc. 2015, 162, A5185–A5189. [Google Scholar] [CrossRef] [Green Version]
- Mathis, T.S.; Kurra, N.; Wang, X.; Pinto, D.; Simon, P.; Gogotsi, Y. Energy Storage Data Reporting in Perspective—Guidelines for Interpreting the Performance of Electrochemical Energy Storage Systems. Adv. Energy Mater. 2019, 9, 1902007. [Google Scholar] [CrossRef]
- Wang, Y.; Song, Y.; Xia, Y. Electrochemical capacitors: Mechanism, materials, systems, characterization and applications. Chem. Soc. Rev. 2016, 45, 5925–5950. [Google Scholar] [CrossRef] [PubMed]
- Okubo, M.; Hosono, E.; Kim, J.; Enomoto, M.; Kojima, N.; Kudo, T.; Zhou, A.H.; Honma, I. Nanosize Effect on High-Rate Li-Ion Intercalation in LiCoO2 Electrode. J. Am. Chem. Soc. 2007, 129, 7444–7452. [Google Scholar] [CrossRef]
- Simon, P.; Gogotsi, Y.; Dunn, B. Where Do Batteries End and Supercapacitors Begin? Science 2014, 343, 1210–1211. [Google Scholar] [CrossRef] [Green Version]
- Tie, D.; Huang, S.; Wang, J.; Ma, J.; Zhang, J.; Zhao, Y. Hybrid energy storage devices: Advanced electrode materials and matching principles. Energy Storage Mater. 2019, 21, 22–40. [Google Scholar] [CrossRef]
- Zuo, W.; Li, R.; Zhou, C.; Li, Y.; Xia, J.; Liu, J. Battery-Supercapacitor Hybrid Devices: Recent Progress and Future Prospects. Adv. Sci. 2017, 4, 1600539. [Google Scholar] [CrossRef]
- Yang, Z.; Ren, J.; Zhang, Z.; Chen, X.; Guan, G.; Qiu, L.; Zhang, Y.; Peng, H. Recent Advancement of Nanostructured Carbon for Energy Applications. Chem. Rev. 2015, 115, 5159–5223. [Google Scholar] [CrossRef]
- Deng, Y.; Xie, Y.; Zou, K.; Ji, X. Review on recent advances in nitrogen-doped carbons: Preparations and applications in supercapacitors. J. Mater. Chem. A 2016, 4, 1144–1173. [Google Scholar] [CrossRef]
- Wang, K.; Wu, H.; Meng, Y.; Wei, Z. Conducting Polymer Nanowire Arrays for High Performance Supercapacitors. Small 2014, 10, 14–31. [Google Scholar] [CrossRef]
- Simon, P.; Gogotsi, Y. Materials for electrochemical capacitors. Nat. Mater. 2008, 7, 845–854. [Google Scholar] [CrossRef] [Green Version]
- Zhou, G.; Xu, L.; Hu, G.; Mai, L.; Cui, Y. Nanowires for Electrochemical Energy Storage. Chem. Rev. 2019, 119, 11042–11109. [Google Scholar] [CrossRef]
- Huang, J.; Wang, K.; Wei, Z. Conducting polymer nanowire arrays with enhanced electrochemical performance. J. Mater. Chem. 2010, 20, 1117–1121. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, L.; Zhang, J. A review of electrode materials for electrochemical supercapacitors. Chem. Soc. Rev. 2012, 41, 797–828. [Google Scholar] [CrossRef] [Green Version]
- Yu, Z.; Tetard, L.; Zhai, L.; Thomas, J. Supercapacitor electrode materials: Nanostructures from 0 to 3 dimensions. Energy Environ. Sci. 2015, 8, 702–730. [Google Scholar] [CrossRef] [Green Version]
- Conway, B.E. Proceedings of the 34th International Power Sources Symposium, Cherry Hill, NJ, USA, 25–26 June 1990; IEEE: Piscataway, NJ, USA, 1990; pp. 319–327.
- Yuan, C.; Hou, L.; Li, D.; Yang, L.; Li, J. Enhanced Supercapacitance of Hydrous Ruthenium Oxide/Mesocarbon Microbeads Composites toward Electrochemical Capacitors. Int. J. Electrochem. 2012, 2012, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Frackowiak, E.; Metenier, K.; Bertagna, V.; Beguin, F. Supercapacitor electrodes from multiwalled carbon nanotubes. Appl. Phys. Lett. 2000, 77, 2421–2423. [Google Scholar] [CrossRef]
- An, K.H.; Kim, W.S.; Park, Y.S.; Choi, Y.C.; Lee, S.M.; Bae, D.C.C.D.J.; Lim, S.C.; Lee, Y.H. Supercapacitors Using Single-Walled Carbon Nanotube Electrodes. Adv. Mater. 2001, 13, 497–500. [Google Scholar] [CrossRef]
- Le, V.T.; Kim, H.; Ghosh, A.; Kim, J.; Chang, J.; Vu, Q.A.; Pham, D.T.; Lee, J.-H.; Kim, S.-W.; Lee, Y.H. Coaxial Fiber Supercapacitor Using All-Carbon Material Electrodes. ACS Nano 2013, 7, 5940–5947. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.; Chung, H.; Kim, W. Supergrowth of Aligned Carbon Nanotubes Directly on Carbon Papers and Their Properties as Supercapacitors. J. Phys. Chem. C 2010, 114, 15223–15227. [Google Scholar] [CrossRef]
- Iglesias, D.; Senokos, E.; Alemán, B.; Cabana, L.; Navío, C.; Marcilla, R.; Prato, M.; Vilatela, J.J.; Marchesan, S. Gas-Phase Functionalization of Macroscopic Carbon Nanotube Fiber Assemblies: Reaction Control, Electrochemical Properties, and Use for Flexible Supercapacitors. ACS Appl. Mater. Interfaces 2018, 10, 5760–5770. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Qu, L.; Henry, K.; Dai, L. High performance electrochemical capacitors from aligned carbon nanotube electrodes and ionic liquid electrolytes. J. Power Source 2009, 189, 1270–1277. [Google Scholar] [CrossRef]
- Chen, Q.-L.; Xue, K.-H.; Shen, W.; Tao, F.-F.; Yin, S.-Y.; Xu, W. Fabrication and electrochemical properties of carbon nanotube array electrode for supercapacitors. Electrochim. Acta 2004, 49, 4157–4161. [Google Scholar] [CrossRef]
- Cheng, Y.; Huang, L.; Xiao, X.; Yao, B.; Yuan, L.; Li, T.; Hu, Z.; Wang, B.; Wan, J.; Zhou, J. Flexible and cross-linked N-doped carbon nanofiber network for high performance freestanding supercapacitor electrode. Nano Energy 2015, 15, 66–74. [Google Scholar] [CrossRef]
- Dubal, D.P.; Chodankar, N.R.; Caban-Huertas, Z.; Wolfart, F.; Vidotti, M.; Holze, R.; Lokhande, C.D.; Gomez-Romero, P. Synthetic approach from polypyrrole nanotubes to nitrogen doped pyrolyzed carbon nanotubes for asymmetric supercapacitors. J. Power Source 2016, 308, 158–165. [Google Scholar] [CrossRef]
- Hu, C.-C.; Chang, K.-H.; Lin, A.M.-C.; Wu, Y.-T. Design and Tailoring of the Nanotubular Arrayed Architecture of Hydrous RuO2 for Next Generation Supercapacitors. Nano Lett. 2006, 6, 2690–2695. [Google Scholar] [CrossRef]
- Xia, H.; Feng, J.; Wang, H.; Lai, M.O.; Lu, L. MnO2 nanotube and nanowire arrays by electrochemical deposition for supercapacitors. J. Power Source 2010, 195, 4410–4413. [Google Scholar] [CrossRef]
- Jiang, H.; Zhao, T.; Ma, J.; Yan, C.; Li, C. Ultrafine manganese dioxide nanowire network for high-performance supercapacitors. Chem. Commun. 2011, 47, 1264–1266. [Google Scholar] [CrossRef]
- Qu, Q.; Zhu, Y.; Gao, X.; Wu, Y. Core-Shell Structure of Polypyrrole Grown on V2O5 Nanoribbon as High Performance Anode Material for Supercapacitors. Adv. Energy Mater. 2012, 2, 950–955. [Google Scholar] [CrossRef]
- Zhou, K.; He, Y.; Xu, Q.; Zhang, Q.; Zhou, A.; Lu, Z.; Yang, L.-K.; Jiang, Y.; Ge, D.; Liu, X.Y.; et al. A Hydrogel of Ultrathin Pure Polyaniline Nanofibers: Oxidant-Templating Preparation and Supercapacitor Application. ACS Nano 2018, 12, 5888–5894. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Zhang, L.; Hu, N.; Yang, Z.; Su, Y.; Xu, S.; Li, M.; Yao, L.; Hong, M.; Zhang, Y. Rational design of sandwiched polyaniline nanotube/layered graphene/polyaniline nanotube papers for high-volumetric supercapacitors. Chem. Eng. J. 2017, 309, 89–97. [Google Scholar] [CrossRef]
- Yu, P.; Zhao, X.; Li, Y.; Zhang, Q. Controllable growth of polyaniline nanowire arrays on hierarchical macro/mesoporous graphene foams for high-performance flexible supercapacitors. Appl. Surf. Sci. 2017, 393, 37–45. [Google Scholar] [CrossRef]




















| Nanorods | Nanowires | Nanotubes | Nanocables |
|---|---|---|---|
| 3 ZnMnO3 [197] | 2 Si [170] | 1 g-CNTs [179] | 2 Cu-Si [198] |
| 950 mAh/g (0.5 A/g) 500 cycles | 1200 mAh/g (2 A/g) 500 cycles | 200 mAh/g (0.5 A/g) 400 cycles | 1500 mAh/g (1.4 A/g) 100 cycles |
| 3 ZnCo2O4 [199] | 2 Si [200] | 3 Co3O4 [201] | 1,2 SnO2-TiO2 [202] |
| 1050 mAh/g (0.4 A/g) 200 cycles | 900 mAh/g (0.2 C) 100 cycles | 1800 mAh/g (0.3 A/g) 100 cycles | 300 mAh/g (0.1 C) 50 cycles |
| 2 β-Sn [203] | 1 TiO2 [52] | 3 ZnMn2O4 [204] | 3 CNT@Fe3O4@C [205] |
| 600 mAh/g (0.2 C) 100 cycles | 350 mAh/g (0.02 A/g) 35 cycles | 670 mAh/g (0.2 A/g) 280 cycles | 700 mAh/g (2 A/g) 200 cycles |
| 3 α-Fe2O3 [206] | 2 Ge [207] | 2,3 SnO2-CuO [208] | 1 MWNT@LTO [209] |
| 970 mAh/g (0.5 C) 100 cycles | 900 mAh/g (0.5 C) 1100 cycles | 600 mAh/g (0.5 A/g) 100 cycles | 130 mAh/g (10 C) 100 cycles |
| 3 CuO [210] | 2,3 Zn2GeO4 [211] | 2 Si [212] | 2 Ni-Si [213] |
| 670 mAh/g (0.1 A/g) 150 cycles | 1200 mAh/g (0.1 C) 100 cycles | 600 mAh/g (12 C) 6000 cycles | 1100 mAh/g (0.5 C) 100 cycles |
| 3 V2O3 [214] | 3 WO3 [215] | 3 Zn4Sb3 [216] | 2,3 Ag@γ-Fe2O3 [217] |
| 200 mAh/g (0.1 C) 125 cycles | 660 mAh/g (0.28 C) 140 cycles | 450 mAh/g (0.1 A/g) 100 cycles | 890 mAh/g (0.1 C) 60 cycles |
| Storage Mechanism | Active Material | Electrode Composition | Capacitance (F/g) | P–E * | Ref. |
|---|---|---|---|---|---|
| EDLCs | Carbon | MWCNTs/CB/PVDF (85/5/10) | 135 F/g (1 mV/s) | - | [241] |
| EDLCs | Carbon | Single-wall CNTs | 150 F/g | 20 k W/Kg 6.5 Wh/kg | [242] |
| EDLCs | Carbon | Carbon nanofibers + CNTs | 130 F/g (5 mV/s) | [243] | |
| EDLCs | Carbon | Vertically Aligned CNTs + CNFs | 180 F/g (150 A/g) | 40 kW/Kg 20 Wh/Kg | [244] |
| PS | Functionalized Carbon | Oxygen functionalized CNT fibres | 46 F/g (50mV/s) | 20 kW/Kg 1.29 Wh/Kg | [245] |
| PS | Functionalized Carbon | Vertically aligned, Oxygen functionalized CNTs | 440 F/g | 100 kW/Kg 100 Wh/Kg | [246] |
| PS | Functionalized Carbon | Template based, vertically aligned CNTs | 365 F/g (2 A/g) | - | [247] |
| PS | Functionalized Carbon | N–doped CNF network | 175 F/g (50 A/g) | 1200 W/Kg 5.9 Wh/Kg | [248] |
| PS | Functionalized Carbon | N–doped CNTs | 228 F/g (1 mA/cm2) | 7.75 kW/Kg 29 Wh/Kg | [249] |
| PS | TMO–RuO2 | Hydrous RuO2 nanotubular array | ≈1000 F/g (100 mV/s) | 4320 kW/Kg 7.5 Wh/Kg | [250] |
| PS | TMO–MnO2 | MnO2 nanotube array | 325 F/g (2 A/g) | - | [251] |
| PS | TMO–MnO2 | MnO2 NW (80%)/CB (15%)/PTFE (5%) | 279 F/g (1 A/g) | - | [252] |
| PS | TMO–V2O5 | V2O5 nanowires/CNTs | 216 F/g (5 mV/s)–460 F/cm2 | 6.5 kW/L 29 Wh/L | [173] |
| PS | TMO–V2O5 | V2O5 + PPy | 308 F/g (0.1 A/g) | 2.5 KW/Kg 24 Wh/kg | [253] |
| PS | Conductive polymer | PPy nanowires arrays | 250 F/g (2.75 A/g) | 10 kW/Kg 50 Wh/Kg | [236] |
| PS | Conductive polymer | PPy nanowire network | 332 F/g (1 mA/cm2) | 7.75 kW/Kg 29 Wh/Kg | [249] |
| PS | Conductive polymer | PANI nanowire hydrogel | 636 F/g (2 A/g) | - | [254] |
| PS | Conductive polymer | RGO–PANI nanowires paper PANI nw/RGO/PANI nw sandwich | 956 F/g (1 A/g)–172 F/cm3 363 F/g (1 A/g)–722 F/cm3 | [255] | |
| PS | Conductive polymer | PANI arrays/graphene foams | 936 F/g (1 A/g) | 103 kW/Kg 21 Wh/Kg | [256] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Machín, A.; Fontánez, K.; Arango, J.C.; Ortiz, D.; De León, J.; Pinilla, S.; Nicolosi, V.; Petrescu, F.I.; Morant, C.; Márquez, F. One-Dimensional (1D) Nanostructured Materials for Energy Applications. Materials 2021, 14, 2609. https://doi.org/10.3390/ma14102609
Machín A, Fontánez K, Arango JC, Ortiz D, De León J, Pinilla S, Nicolosi V, Petrescu FI, Morant C, Márquez F. One-Dimensional (1D) Nanostructured Materials for Energy Applications. Materials. 2021; 14(10):2609. https://doi.org/10.3390/ma14102609
Chicago/Turabian StyleMachín, Abniel, Kenneth Fontánez, Juan C. Arango, Dayna Ortiz, Jimmy De León, Sergio Pinilla, Valeria Nicolosi, Florian I. Petrescu, Carmen Morant, and Francisco Márquez. 2021. "One-Dimensional (1D) Nanostructured Materials for Energy Applications" Materials 14, no. 10: 2609. https://doi.org/10.3390/ma14102609







