Zero-Valent Iron Nanoparticles Remediate Nickel-Contaminated Aqueous Solutions and Biosolids-Amended Agricultural Soil
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sampling and Preparation of Soil, Biosolids, and nZVI
2.2. Incubation Experiment
2.3. Nickel Sorption and Removal Efficiency
2.4. Adsorption Isotherm Models
2.5. Nickel Sorption Kinetics
2.6. Kinetics Modeling
2.7. Nickel Fractions in Biosolid-Amended Soil
2.8. Nickel Release
2.9. Packed-Column Experiment
3. Results and Discussion
3.1. Characterization of nZVI Particles
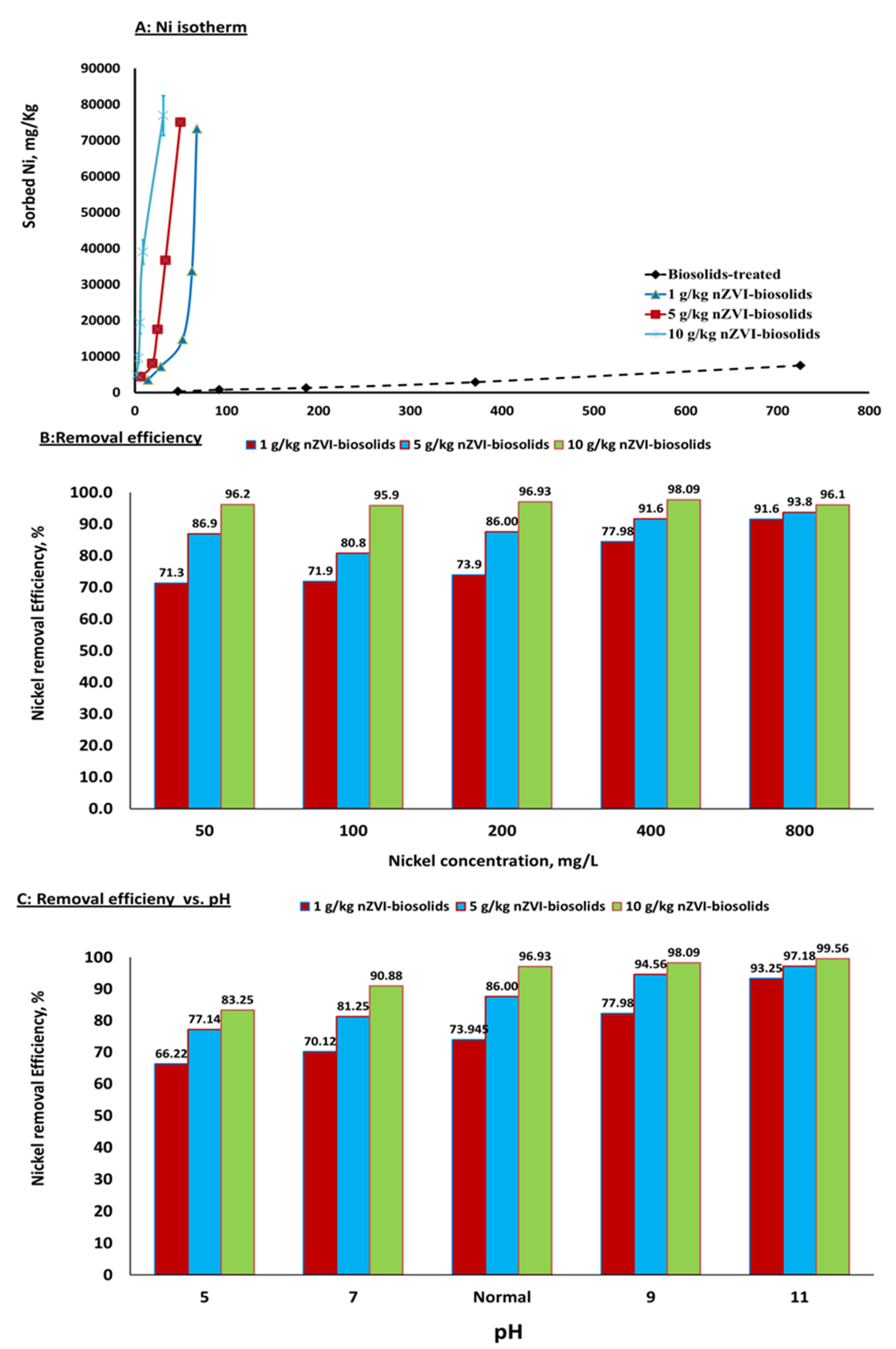
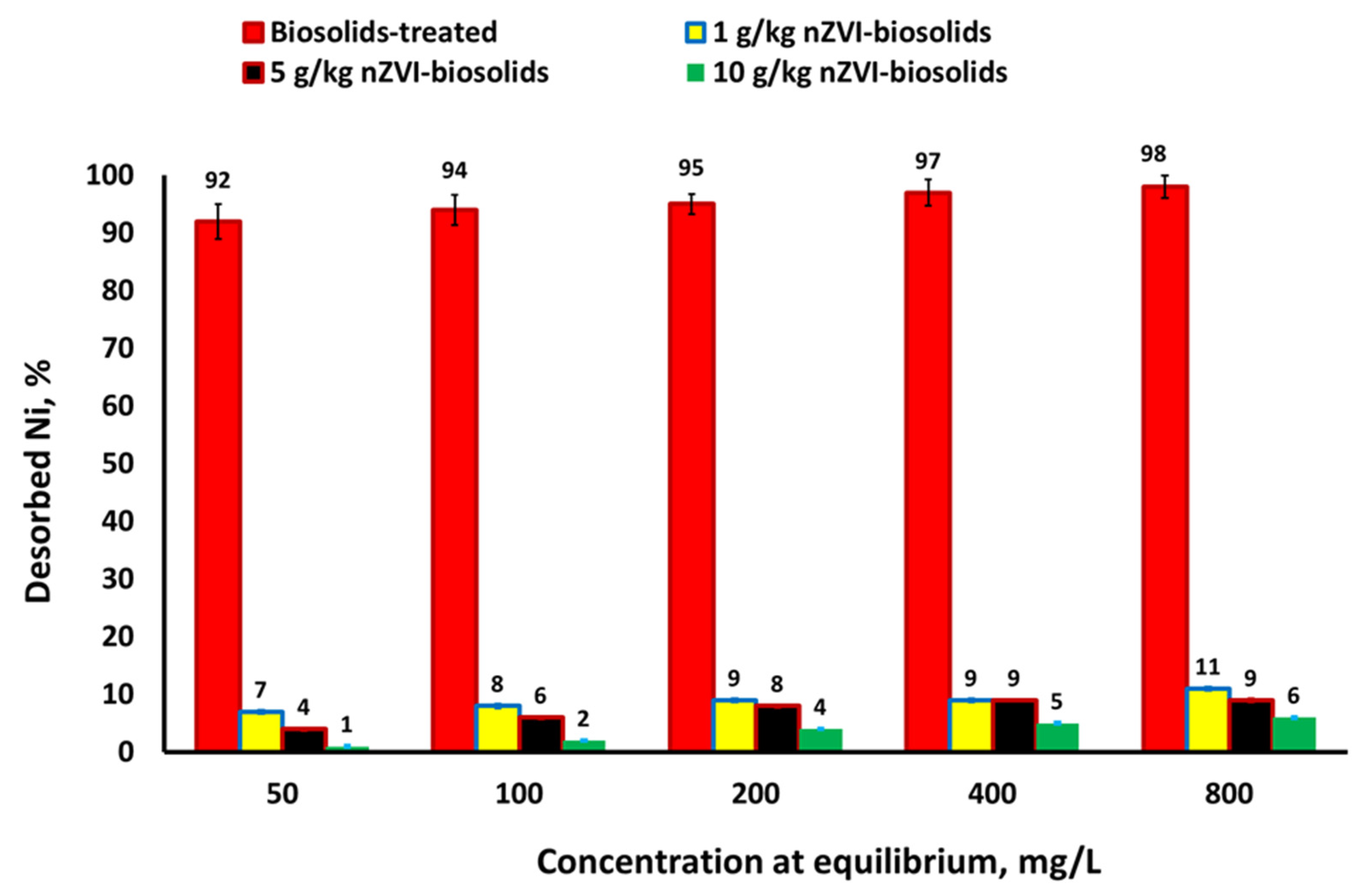
3.2. Sorption Isotherms, Removal Efficiency from Water, Removal Mechanism, and Release of Ni+2
3.3. Iron Nanoparticles as a Reductant and Sorbent
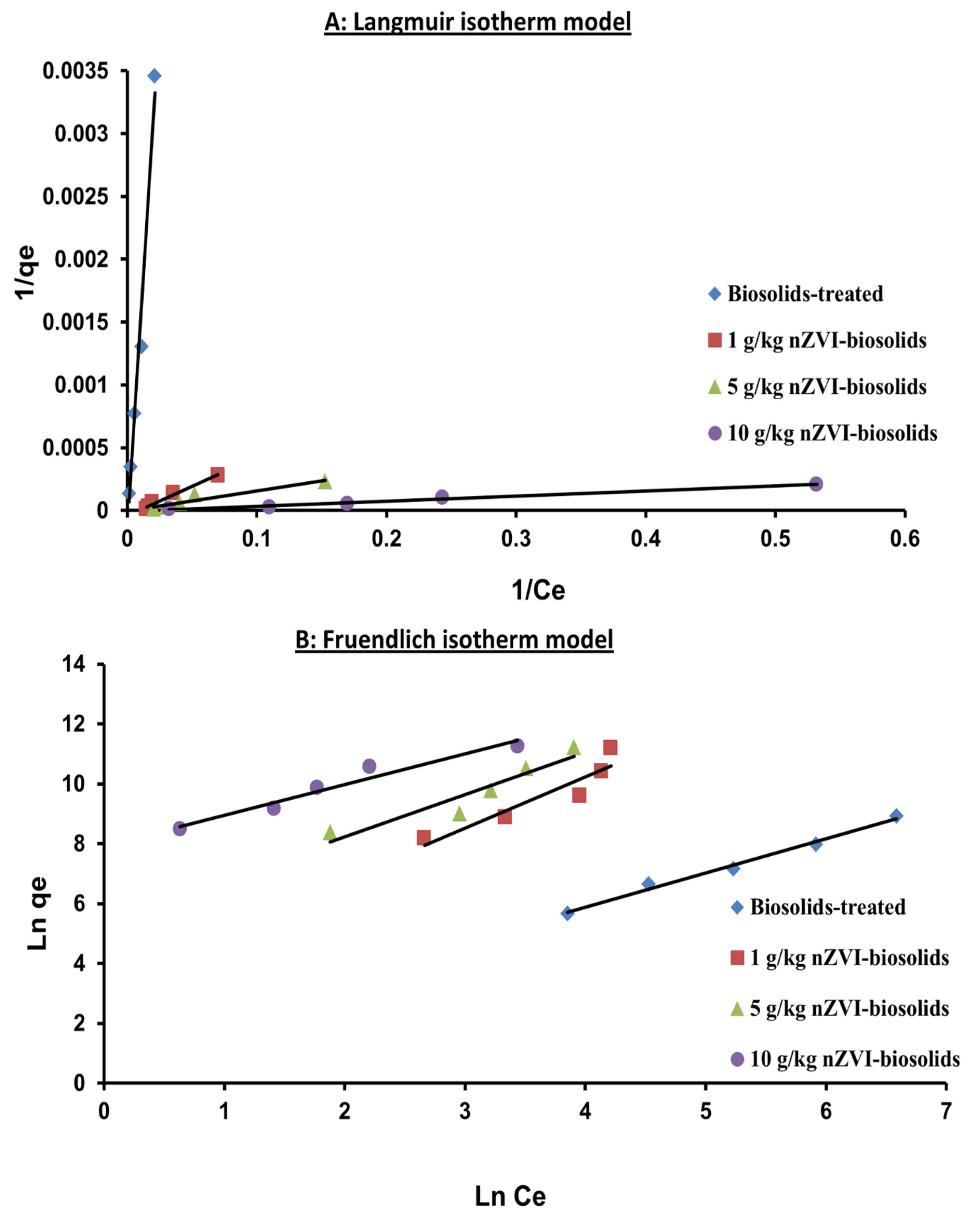
3.4. Sorption Isotherm Modeling
3.5. Effect of Reaction Time and Kinetic Models Fitting
| Models | Parameter | Biosolids-Treated Soil | nZVI Added to Biosolids-Treated Soil, g/kg | ||
|---|---|---|---|---|---|
| 1 | 5 | 10 | |||
| First-order Ln (qo − q) = a − ka t [42] | Ka µg g −1 min−1 | 0.971 | 1.253 | 1.023 | 0.832 |
| a µg g −1 | 10.05 | 10.17 | 9.54 | 7.43 | |
| R2 | 0.92 | 0.98 | 0.97 | 0.96 | |
| SE | 0.665 | 1.490 | 1.095 | 1.203 | |
| Power Function q = kaCo t1/m [43] | Ka min−1 | 2439.49 | 68,092.61 | 70,778.28 | 75,840.29 |
| 1/m | 0.176 | 0.012 | 0.009 | 0.002 | |
| R2 | 0.85 | 0.80 | 0.79 | 0.72 | |
| SE | 0.065 | 0.005 | 0.004 | 0.001 | |
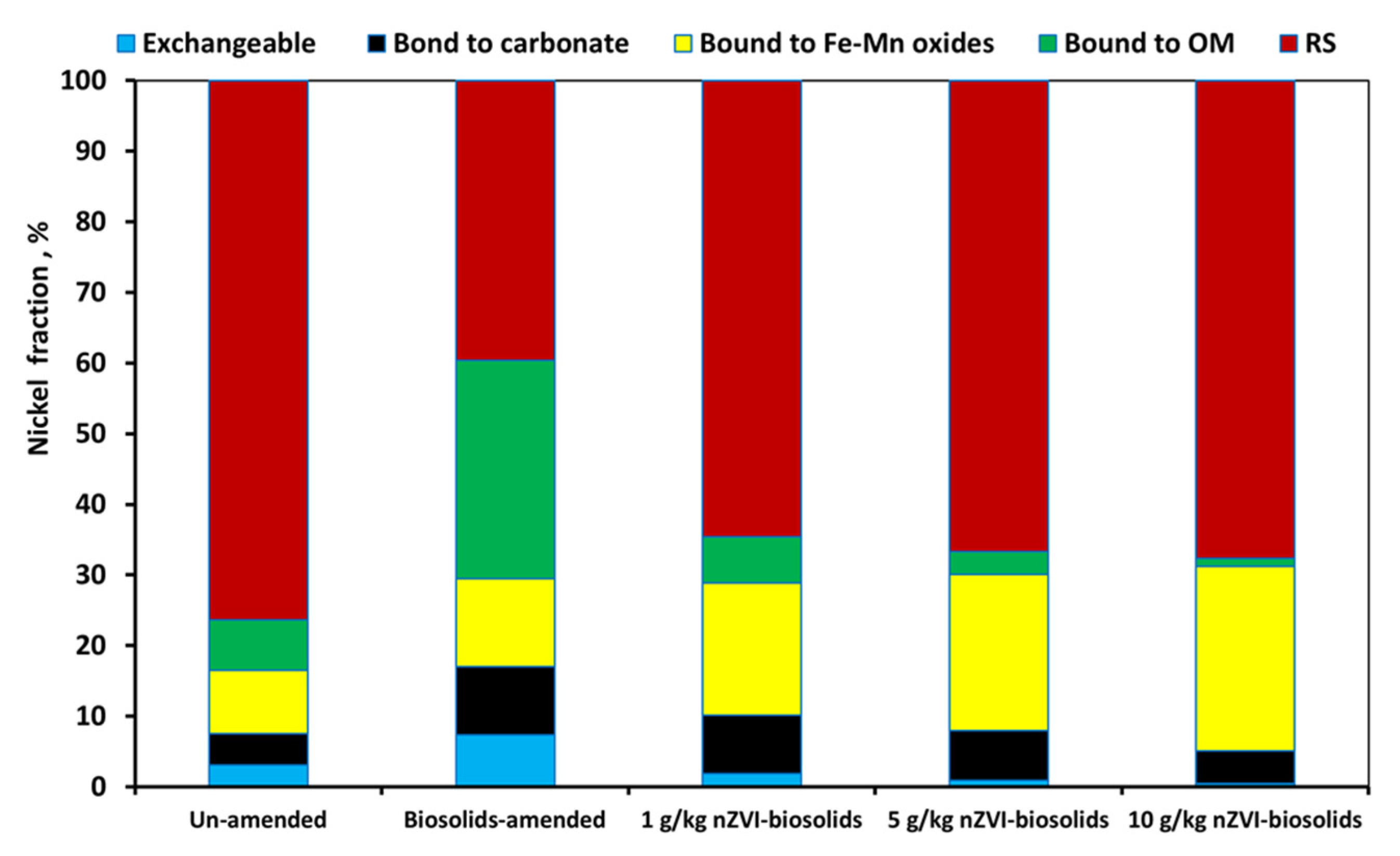
3.6. Effect of nZVI on Nickel Fractions in Biosolid-Amended Soil
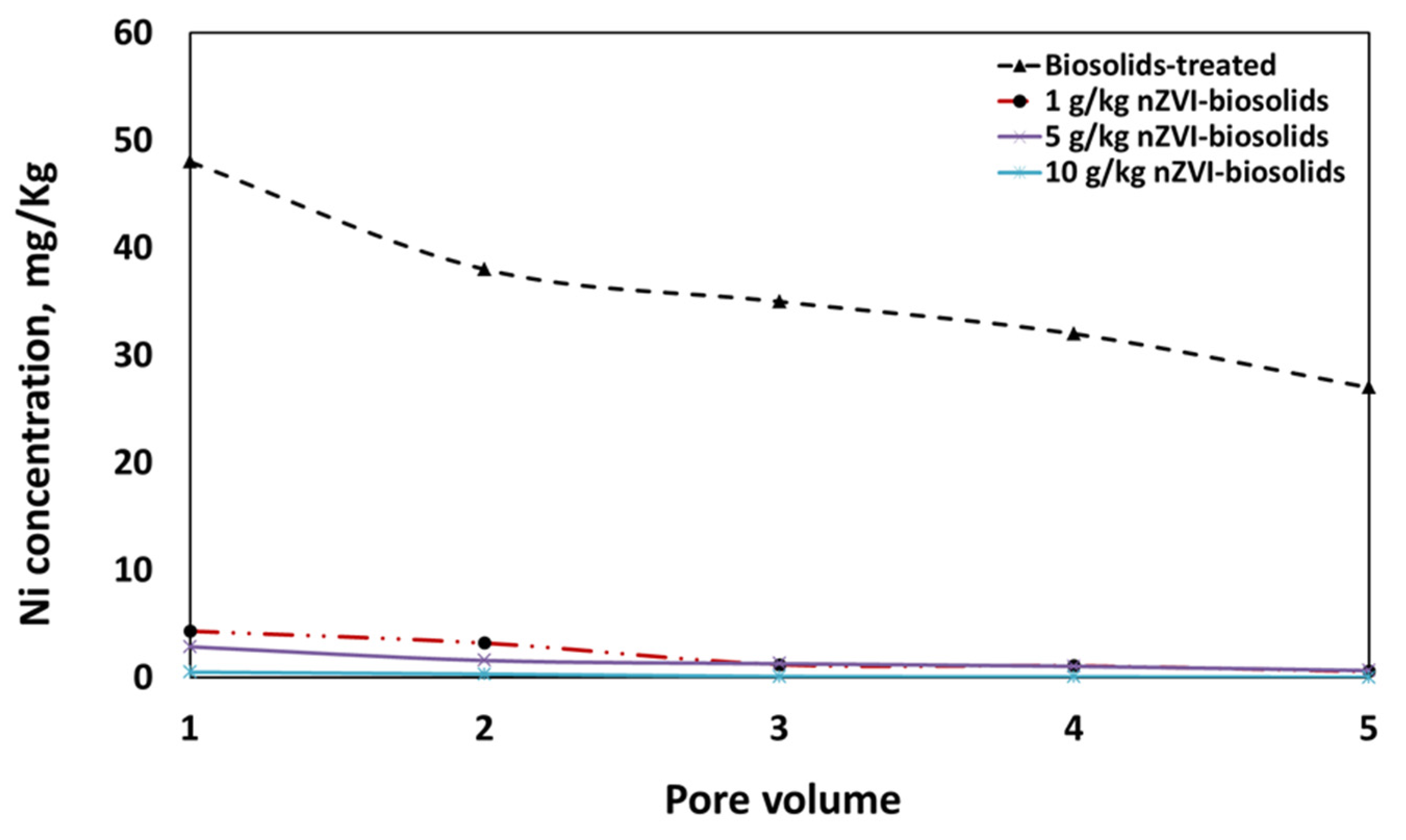
3.7. Effect of nZVI on the Mobility of Nickel through Packed-Column
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhai, X.; Li, Z.; Huang, B.; Luo, N.; Huang, M.; Zhang, Q.; Zeng, G. Remediation of multiple heavy metal contaminated soil through the combination of soil washing and in situ immobilization. Sci. Total Environ. 2018, 635, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Vilardi, G.; Rodriguez-Rodriguez, J.; Ochando-Pulido, J.M.; Di Palma, L.; Verdone, N. Fixed-bed reactor scale-up and modelling for Cr (VI) removal using nano iron-based coated biomass as packing material. Chem. Eng. J. 2019, 361, 990–998. [Google Scholar] [CrossRef]
- Fu, F.; Wang, Q. Removal of heavy metal ions from wastewaters: A review. J. Environ. Manag. 2011, 92, 407–418. [Google Scholar] [CrossRef]
- Jabeen, H.; Kemp, K.C.; Chandra, V. Synthesis of nano zerovalent iron nanoparticles–graphene composite for the treatment of lead contaminated water. J. Environ. Manag. 2013, 130, 429–435. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; He, M.C. Theoretical study of heavy metal Cd, Cu, Hg, and Ni (II) adsorption on the kaolinite (0 0 1) surface. Appl. Surf. Sci. 2014, 317, 718–723. [Google Scholar] [CrossRef]
- Vilardi, G.; Di Palma, L.; Verdone, N. Competitive reaction modelling in aqueous systems: The case of contemporary reduction of dichromates and nitrates by nZVI. Chem. Eng. Trans. 2017, 60, 175–180. [Google Scholar]
- Gueye, M.T.; Di Palma, L.; Allahverdiyeva, G.; Bavasso, I.; Petrucci, E.; Stoller, M.; Vilardi, G. The influence of heavy metals and organic matter on hexavalent chromium reduction by nano zero valent iron in soil. Chem. Eng. Trans. 2016, 47, 289–294. [Google Scholar]
- Garg, A.; Visht, S.; Sharma, P.K.; Kumar, N. Formulation, characterization and application on nanoparticle: A review. Der Pharmacia Sin. 2011, 2, 17–26. [Google Scholar]
- Guo, D.; Xie, G.; Luo, J. Mechanical properties of NPs: Basics and applications. J. Phys. D Appl. Phys. 2014, 47, 13001–13026. [Google Scholar] [CrossRef] [Green Version]
- Prabhakar, R.; Samadder, S.R. Low cost and easy synthesis of aluminium oxide nanoparticles for arsenite removal from groundwater: A complete batch study. J. Mol. Liq. 2018, 250, 192–201. [Google Scholar] [CrossRef]
- Jiang, D.; Zeng, G.; Huang, D.; Chen, M.; Zhang, C.; Huang, C.; Wan, J. Remediation of contaminated soils by enhanced nanoscale zero valent iron. Environ. Res. 2018, 163, 217–227. [Google Scholar] [CrossRef] [PubMed]
- Bardos, P.; Bone, B.; Daly, P.; Elliott, D.; Jones, S.; Lowry, G.; Merly, C. A Risk/Benefit Appraisal for the Application of Nano-Scale Zero Valent Iron (nZVI) for the Remediation of Contaminated Sites. Wp9 Nanorem Project. 2014. Available online: https://www.researchgate.net/publication/264788812_A_RiskBenefit_Appraisal_for_the_Application_of_Nano-Scale_Zero_Valent_Iron_nZVI_for_the_Remediation_of_Contaminated_Sites (accessed on 14 May 2021).
- EPA. Federal Remediation Technologies Roundtable Meeting, Arlington, VA, USA, 9 June 2004. Available online: http://www.frtr.gov/pdf/meetings/frtr1298.pdf (accessed on 1 May 2021).
- Karn, B.; Kuiken, T.; Otto, M. Nanotechnology and in situ remediation: A review of the benefits and potential risks. Environ. Health Perspect. 2009, 117, 1813–1831. [Google Scholar] [CrossRef] [Green Version]
- Piquepaille, R. Cleaning the Environment with Iron Nanoparticles. Roland Piquepaille’s Technology Trends. 2003. Available online: http://radio.weblogs.com/0105910/2003/09/05.html#a577 (accessed on 1 May 2021).
- Makris, K.C.; Harris, W.G. Time dependency and irreversibility of water desorption by drinking-water treatment residuals: Implications for sorption mechanisms. J. Colloid Interface Sci. 2006, 294, 151–154. [Google Scholar] [CrossRef]
- Tan, K.H. Soil Sampling, Preparation, and Analysis; CRC Press: Boca Raton, FL, USA, 2005. [Google Scholar]
- Mahdy, A.M.; Salem, Z.M.; Ali, A.M.; Ali, H.M. Optimum Operating Conditions for the Removal of Phosphate from Water Using of Wood-Branch Nanoparticles from Eucalyptus Camaldulensis. Materials 2020, 13, 1851. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Langmuir, I. The constitution and fundamental properties of solids and liquids. Part I. Solids. J. Am. Chem. Soc. 1916, 38, 2221–2295. [Google Scholar] [CrossRef] [Green Version]
- Freundlich, H.M.F. Over the adsorption in solution. J. Phys. Chem. 1906, 57, 1100–1107. [Google Scholar]
- Amacher, M.C.; Selim, H.M.; Iskandar, I.K. Kinetics of chromium (VI) and cadmium retention in soils; a nonlinear multireaction model. Soil Sci. Soc. Am. J. 1988, 52, 398–408. [Google Scholar] [CrossRef]
- Laidler, K.J.; Polanyi, J.C. Theories of the kinetics of bimolecular reactions. Prog. React. Kinet. 1965, 3, 1–61. [Google Scholar]
- Elkhatib, E.A.; Elshebiny, G.M.; Balba, A.M. Kinetics of lead sorption in calcareous soils. Arid Land Res. Manag. 1992, 6, 297–310. [Google Scholar] [CrossRef]
- Tessier, A.; Campbell, P.G.; Bisson, M. Sequential extraction procedure for the speciation of particulate trace metals. Anal. Chem. 1979, 51, 844–851. [Google Scholar] [CrossRef]
- Liu, H.; Bruton, T.A.; Doyle, F.M.; Sedlak, D.L. In situ chemical oxidation of contaminated groundwater by persulfate: Decomposition by Fe (III)-and Mn (IV)-containing oxides and aquifer materials. Environ. Sci. Technol. 2014, 48, 10330–10336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dongsheng, Z.; Wenqiang, G.; Guozhang, C.; Shuai, L.; Weizhou, J.; Youzhi, L. Removal of heavy metal lead (II) using nanoscale zero-valent iron with different preservation methods. Adv. Powder Technol. 2019, 30, 581–589. [Google Scholar] [CrossRef]
- Li, X.Q.; Zhang, W.X. Iron nanoparticles: The core–shell structure and unique properties for Ni (II) sequestration. Langmuir 2006, 22, 4638–4642. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wang, W.; Liang, F.; Zhang, W.X. Heavy metal removal using nanoscale zero-valent iron (nZVI): Theory and application. J. Hazard. Mater. 2017, 322, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.; Lu, T.; Peng, L.; Shao, J.; Zeng, Q.; Gu, J.D. Synthesis of nanoscale zero-valent iron immobilized in alginate microcapsules for removal of Pb (II) from aqueous solution. J. Mater. Chem. A 2014, 2, 15463–15472. [Google Scholar] [CrossRef]
- Ali, A.M.; Mahdy, A.M.; Salem, M.Z. Characterization of phosphate biosorption on nanoparticles of different woody-sawdust used for controlling P runoff and a supplemental P-fertilizer. Desalination Water Treat. 2021, 209, 71–81. [Google Scholar] [CrossRef]
- Binupriya, A.R.; Sathishkumar, M.; Jung, S.H.; Song, S.H.; Yun, S.I. A novel method in utilization of bokbunja seed wastes from wineries in liquid-phase sequestration of reactive blue 4. Int. J. Environ. Res. 2009, 3, 1–12. [Google Scholar]
- Saravanane, R.; Sundararajan, T.; Reddy, S.S. Efficiency of chemically modified low cost adsorbents for the removal of heavy metals from waste water: A comparative study. Indian J. Environ. Health 2002, 44, 78–87. [Google Scholar]
- Kim, H.N.; Ren, W.X.; Kim, J.S.; Yoon, J. Fluorescent and colorimetric sensors for detection of lead, cadmium, and mercury ions. Chem. Soc. Rev. 2012, 41, 3210–3244. [Google Scholar] [CrossRef] [PubMed]
- Arancibia-Miranda, N.; Baltazar, S.E.; García, A.; Romero, A.H.; Rubio, M.A.; Altbir, D. Lead removal by nano-scale zero valent iron: Surface analysis and pH effect. Mater. Res. Bull. 2014, 59, 341–348. [Google Scholar] [CrossRef]
- Zhang, W.X.; Wang, C.B.; Lien, H.L. Treatment of chlorinated organic contaminants with nanoscale bimetallic particles. Catal. Today 1998, 40, 387–395. [Google Scholar] [CrossRef]
- Zhang, W.X. Nanoscale iron particles for environmental remediation: An overview. J. Nanoparticle Res. 2003, 5, 323–332. [Google Scholar] [CrossRef]
- Dzombak, D.A.; Morel, F.M. Surface Complexation Modeling: Hydrous Ferric Oxide; John Wiley & Sons: Hoboken, NJ, USA, 1990. [Google Scholar]
- Stumm, W. Chemistry of the Solid-Water Interface: Processes at the Mineral-Water and Particle-Water Interface in Natural Systems; John Wiley & Son Inc.: Hoboken, NJ, USA, 1992. [Google Scholar]
- Stumm, W.; Morgan, J.J. Aquatic Chemistry; John Wiley & Sons. Inc.: New York, NY, USA, 1996. [Google Scholar]
- Kurniawan, T.A.; Lo, W.H. Removal of refractory compounds from stabilized landfill leachate using an integrated H2O2 oxidation and granular activated carbon (GAC) adsorption treatment. Water Res. 2009, 43, 4079–4091. [Google Scholar] [CrossRef] [PubMed]
- Christensen, T.H. Cadmium soil sorption at low concentrations: VIII. Correlation with soil parameters. Water Air Soil Pollut. 1989, 44, 71–82. [Google Scholar] [CrossRef]
- Elkhatib, E.A.; Hern, J.L. Kinetics of potassium desorption from Appalachian soils. Soil Sci. 1988, 145, 11–19. [Google Scholar] [CrossRef]
- Kuo, S.; Lotse, E.G. Kinetics of phosphate adsorption and desorption by hematite and gibbsite1. Soil Sci. 1973, 116, 400–406. [Google Scholar] [CrossRef]
- Wang, W.; Hua, Y.; Li, S.; Yan, W.; Zhang, W.X. Removal of Pb (II) and Zn (II) using lime and nanoscale zero-valent iron (nZVI): A comparative study. Chem. Eng. J. 2016, 304, 79–88. [Google Scholar] [CrossRef]
- Saha, P.; Chowdhury, S.; Gupta, S.; Kumar, I. Insight into adsorption equilibrium, kinetics and thermodynamics of Malachite Green onto clayey soil of Indian origin. Chem. Eng. J. 2010, 165, 874–882. [Google Scholar] [CrossRef]
- Kocur, C.M.; O’Carroll, D.M.; Sleep, B.E. Impact of nZVI stability on mobility in porous media. J. Contam. Hydrol. 2013, 145, 17–25. [Google Scholar] [CrossRef] [PubMed]

| Characteristics | Soil | Biosolids |
|---|---|---|
| pH * | 7.75 ± 0.06 | 6.38 ± 0.05 |
| Electrical Conductivity (EC) (dS/m) | 2.13 ± 0.04 | 8.33 ± 0.17 |
| Organic Matter (g/kg) | 3.80 ± 0.18 | 410.00 ± 3.77 |
| Cation Exchange Capacity (CEC) (cmol (+)/kg) | 12.00 ± 1.07 | 77.77 ± 2.58 |
| Sand (g/kg) | 738.00 ± 3.70 | N/A† |
| Silt (g/kg) | 106.40 ± 1.90 | N/A |
| Clay (g/kg) | 155.60 ± 3.20 | N/A |
| Soil Texture | Sandy loam | N/A |
| CaCO3 (g/kg) | 33.20 ± 3.69 | N/A |
| Total Ni (mg/kg) | 13.50 ± 0.11 | 6.23 ± 0.58 |
| Exchangeable Ni (mg/kg) | 6.07 ± 0.08 | 0.16 ± 0.06 |
| Soluble Ni (mg/kg) | 0.24 ± 0.01 | 0.09 ± 0.02 |
| Models | Parameter | Biosolids-Treated Soil | nZVI Added to Biosolids-Treated Soil, g/kg | ||
|---|---|---|---|---|---|
| 1 | 5 | 10 | |||
| Freundlich qe = KFCe1/n | KF (mL g−1) | 3.69 | 30.32 | 228.69 | 2757.28 |
| 1/n | 1.14 | 1.71 | 1.4 | 1.03 | |
| R2 | 0.99 | 0.87 | 0.89 | 0.94 | |
| SE | 0.14 | 0.51 | 0.44 | 0.29 | |
| Langmuir qe = qmax(KL Ce/1 + KLCe) | qmax (μgg1) | 5000 | 25,000 | 33,333.30 | 125,000 |
| KL (L mg−1) | 3.2 × 10−5 | 1.88 × 10−7 | 4.80 × 10−9 | 3.2 × 10−9 | |
| R2 | 0.98 | 0.98 | 0.92 | 0.98 | |
| SE | 0.0002 | 1.62 × 10−5 | 2.94 × 10−5 | 1.23 × 10−5 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahdy, A.M.; Zhang, T.; Lin, Z.-Q.; Fathi, N.O.; Badr Eldin, R.M. Zero-Valent Iron Nanoparticles Remediate Nickel-Contaminated Aqueous Solutions and Biosolids-Amended Agricultural Soil. Materials 2021, 14, 2655. https://doi.org/10.3390/ma14102655
Mahdy AM, Zhang T, Lin Z-Q, Fathi NO, Badr Eldin RM. Zero-Valent Iron Nanoparticles Remediate Nickel-Contaminated Aqueous Solutions and Biosolids-Amended Agricultural Soil. Materials. 2021; 14(10):2655. https://doi.org/10.3390/ma14102655
Chicago/Turabian StyleMahdy, Ahmed M., Tiequan Zhang, Zhi-Qing Lin, Nieven O. Fathi, and Rasha M. Badr Eldin. 2021. "Zero-Valent Iron Nanoparticles Remediate Nickel-Contaminated Aqueous Solutions and Biosolids-Amended Agricultural Soil" Materials 14, no. 10: 2655. https://doi.org/10.3390/ma14102655





