Deterioration of Portland Cement Pervious Concrete in Sponge Cities Subjected to Acid Rain
Abstract
1. Introduction
2. Research Significance
3. Experiment Detail
3.1. Materials
3.2. Specimens Preparation
3.3. Test Methods
3.3.1. Strength
3.3.2. Abrasion Resistance
3.3.3. Water Permeability Coefficient
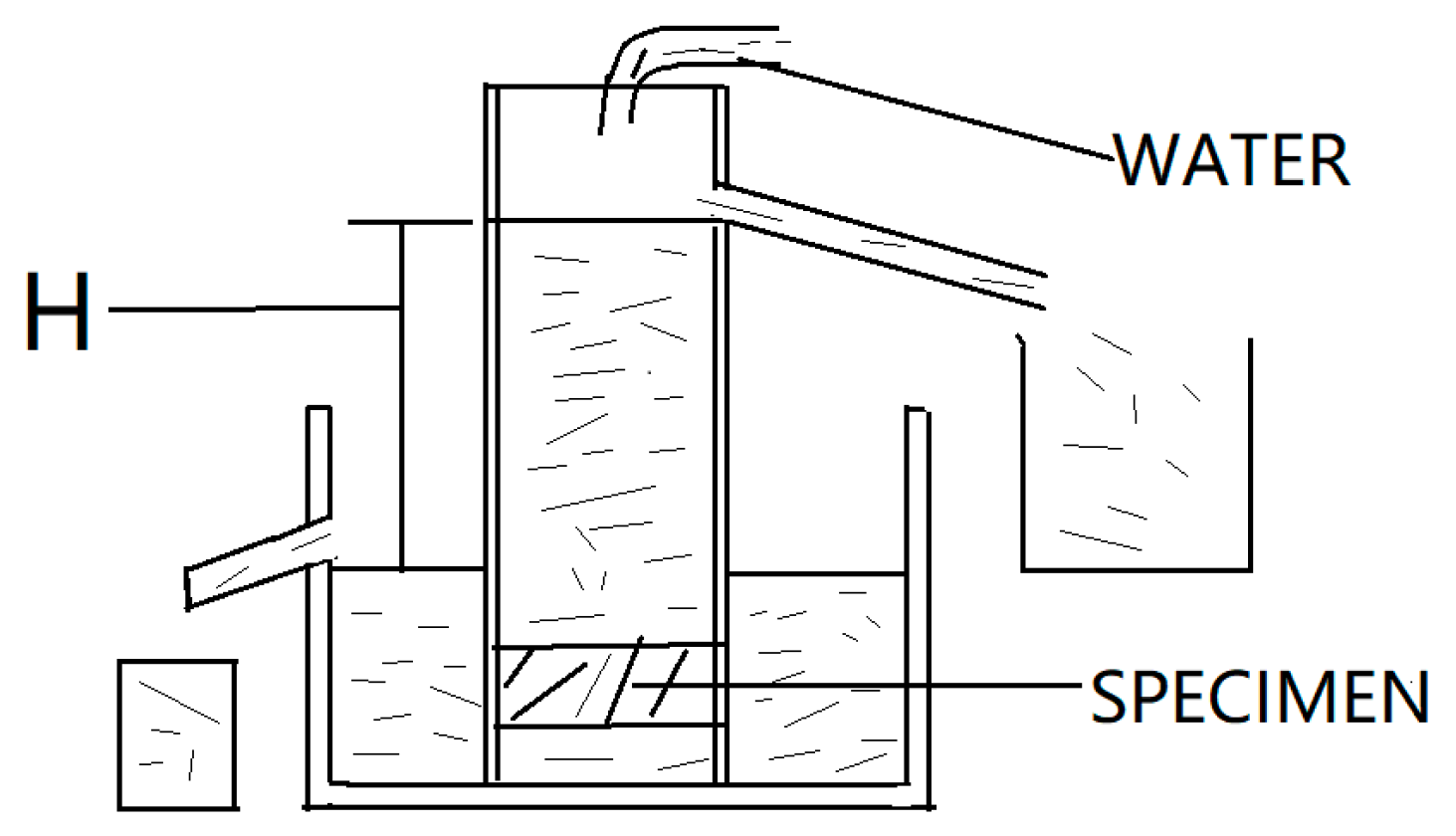
3.3.4. Wet-Dry Cycles
4. Results and Discussion
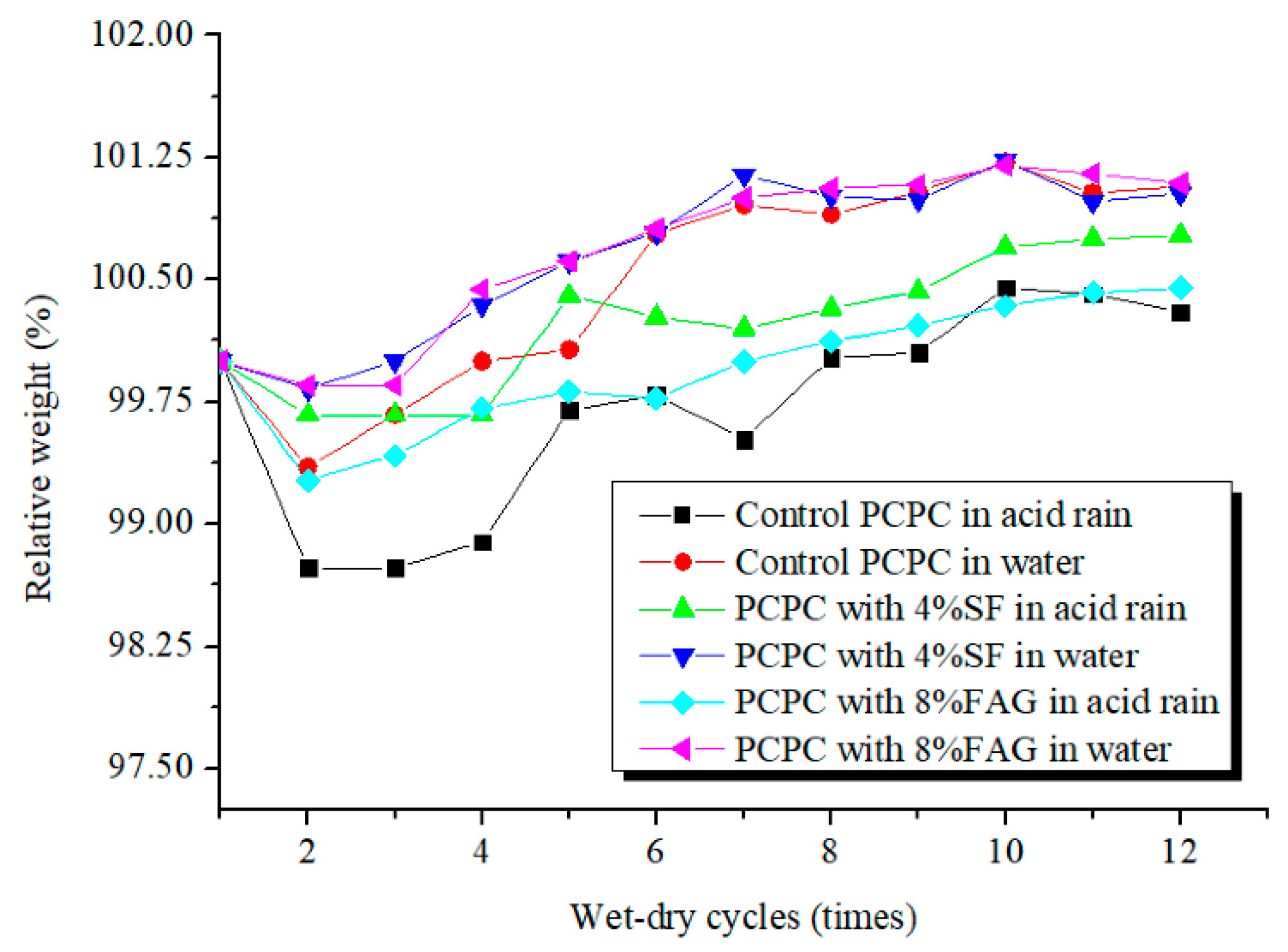
4.1. Weight Change
4.2. Mechanical Properties of PCPC
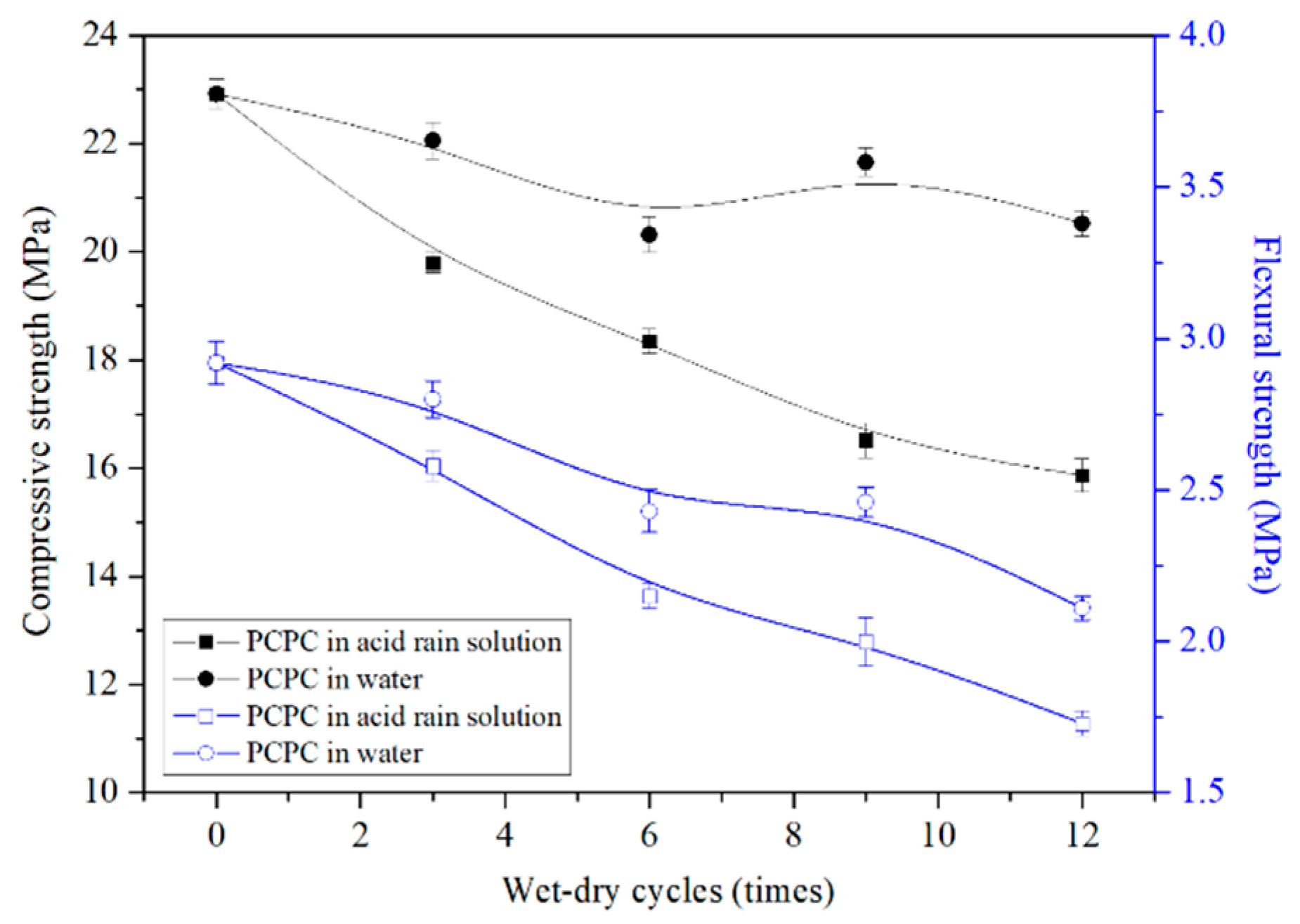
4.2.1. Strength of Control PCPC
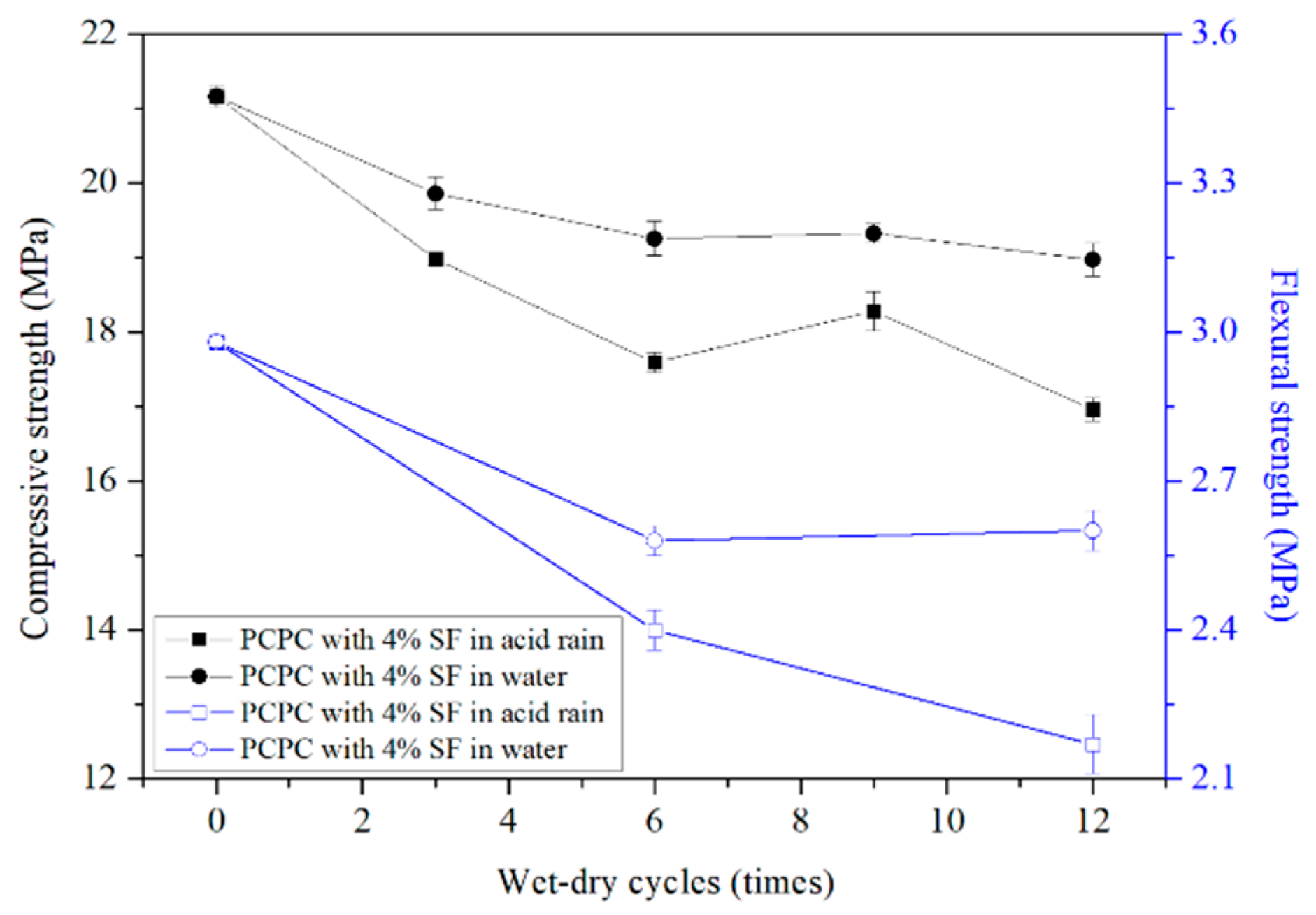
4.2.2. Strength of 4% SF PCPC
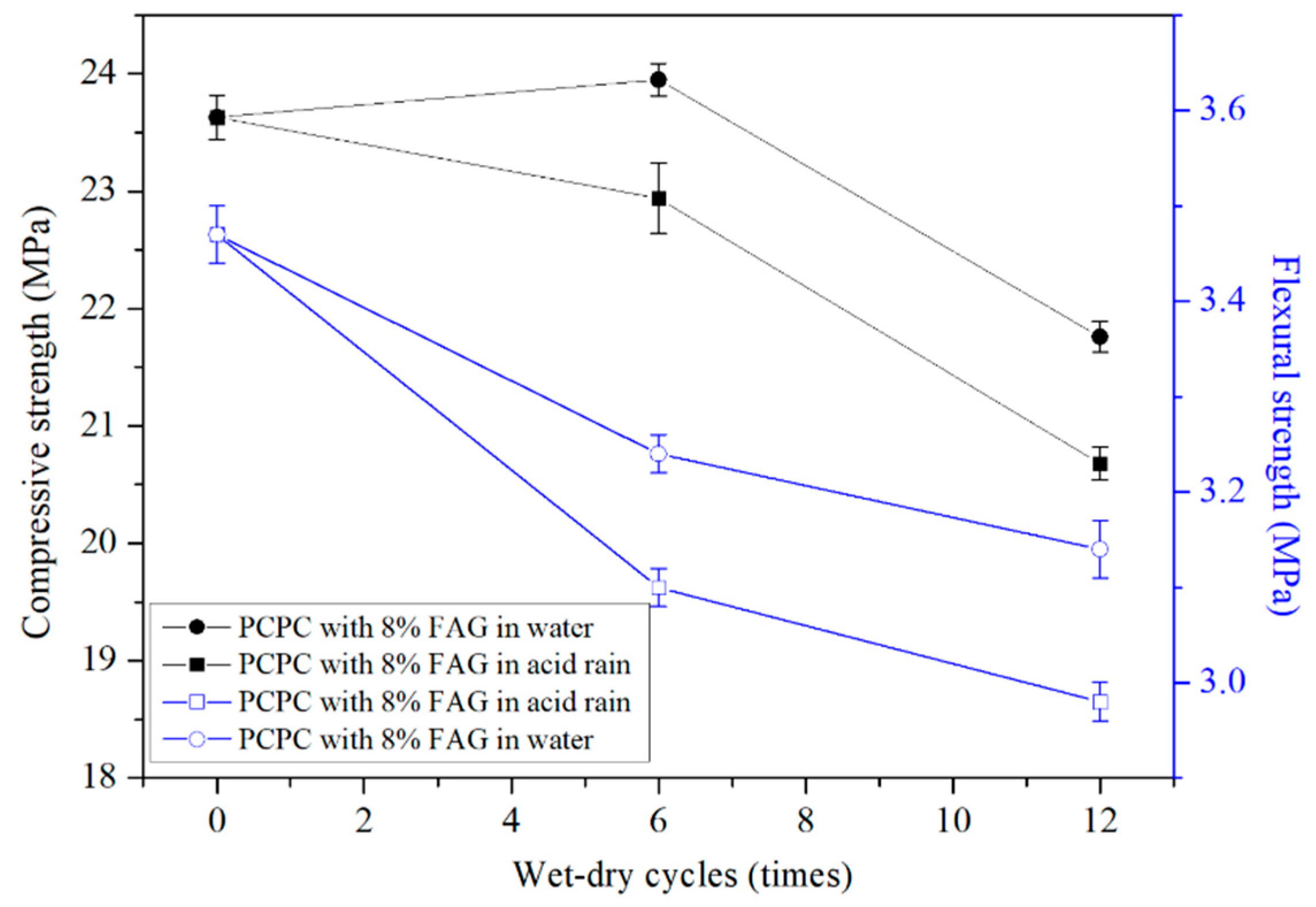
4.2.3. Strength of PCPC with 8% FAG
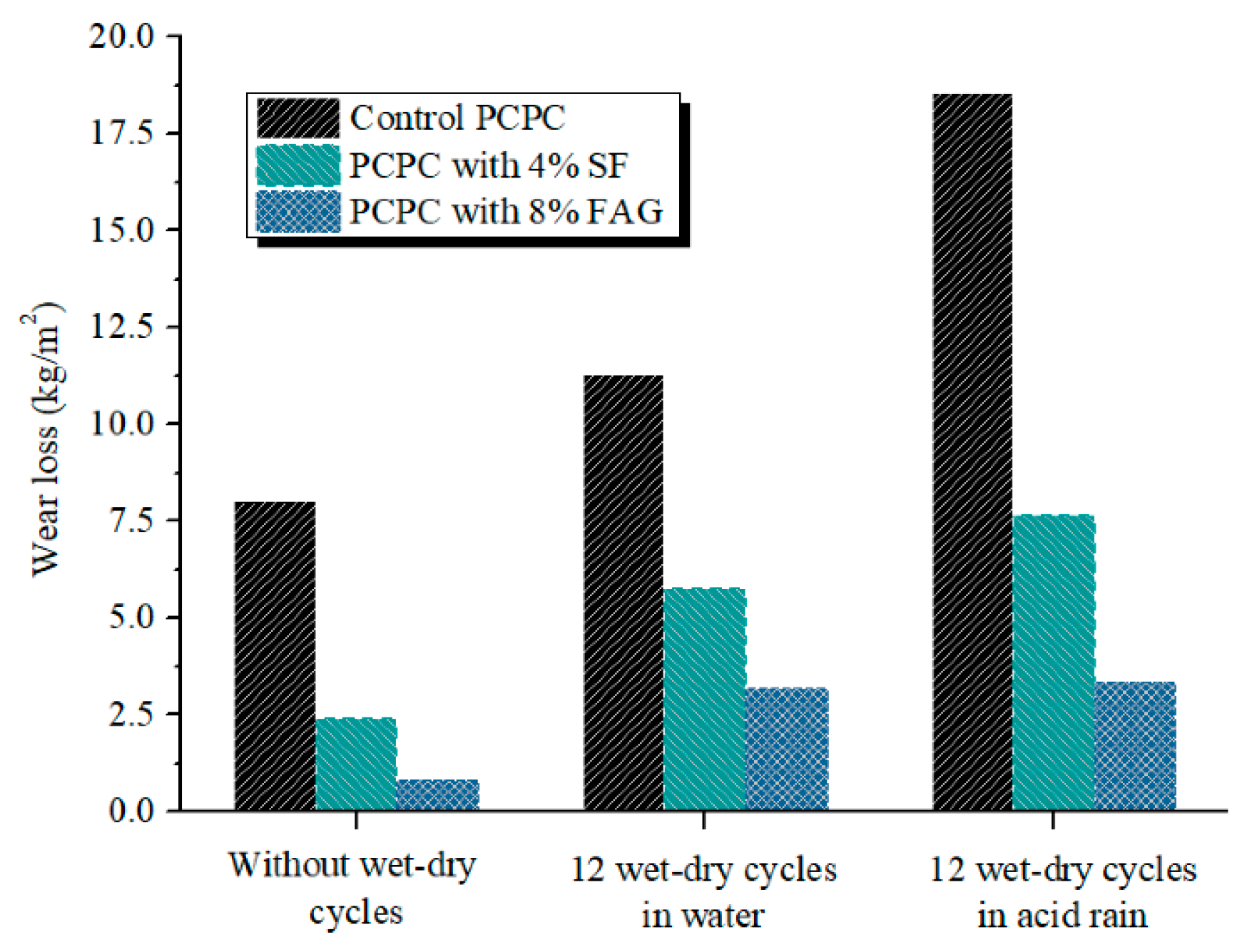
4.3. Abrasion Resistance
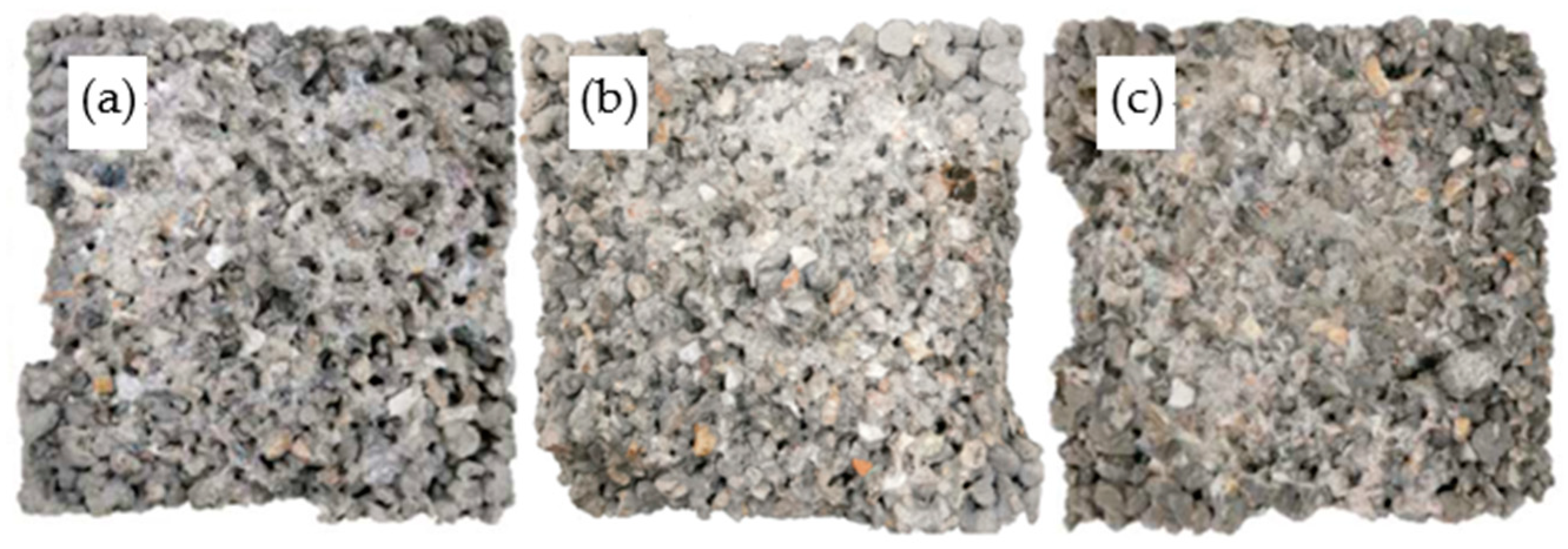
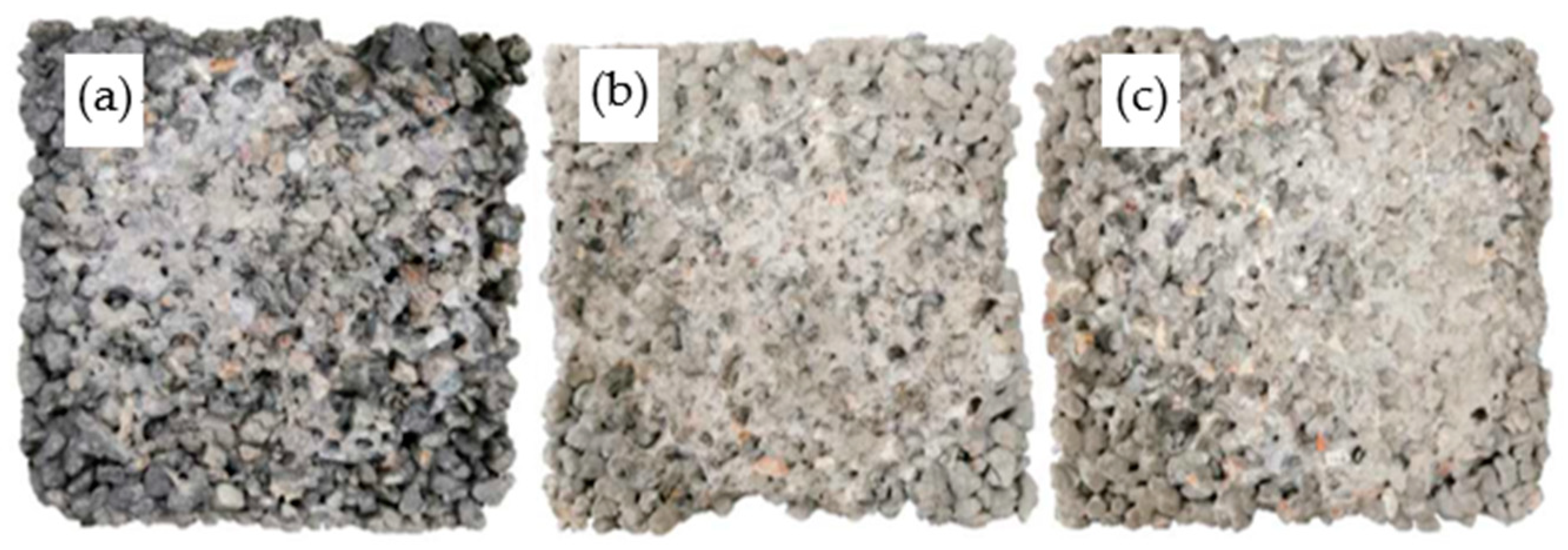
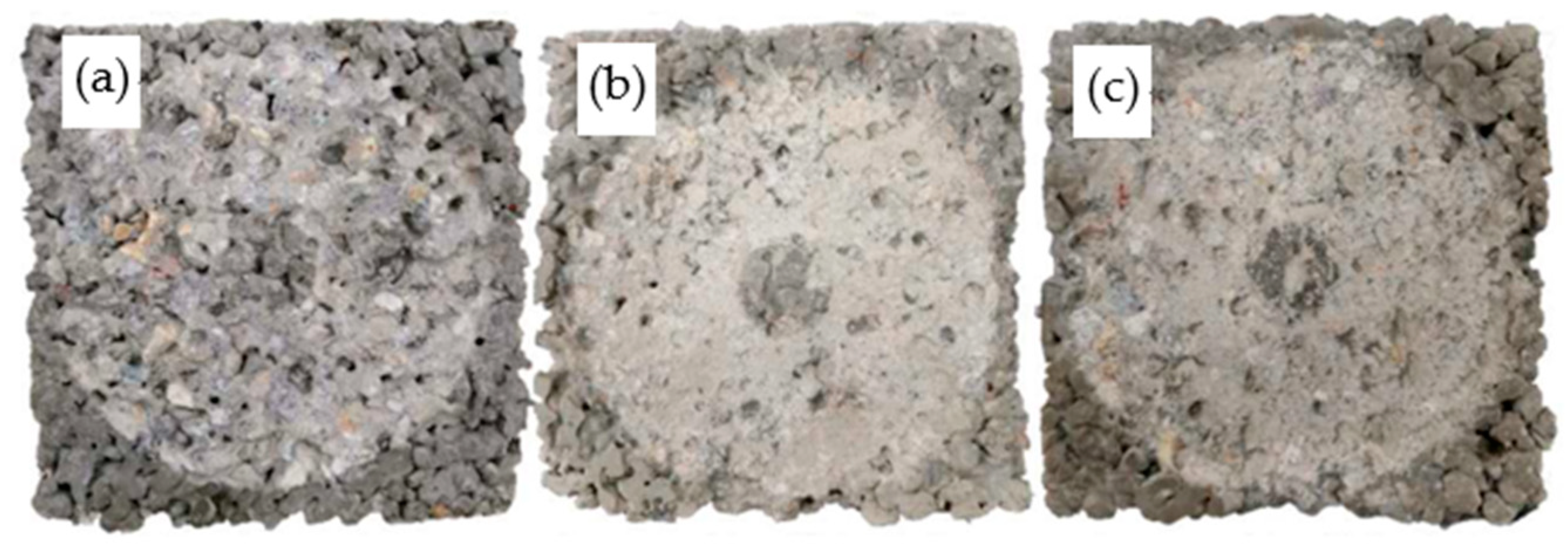
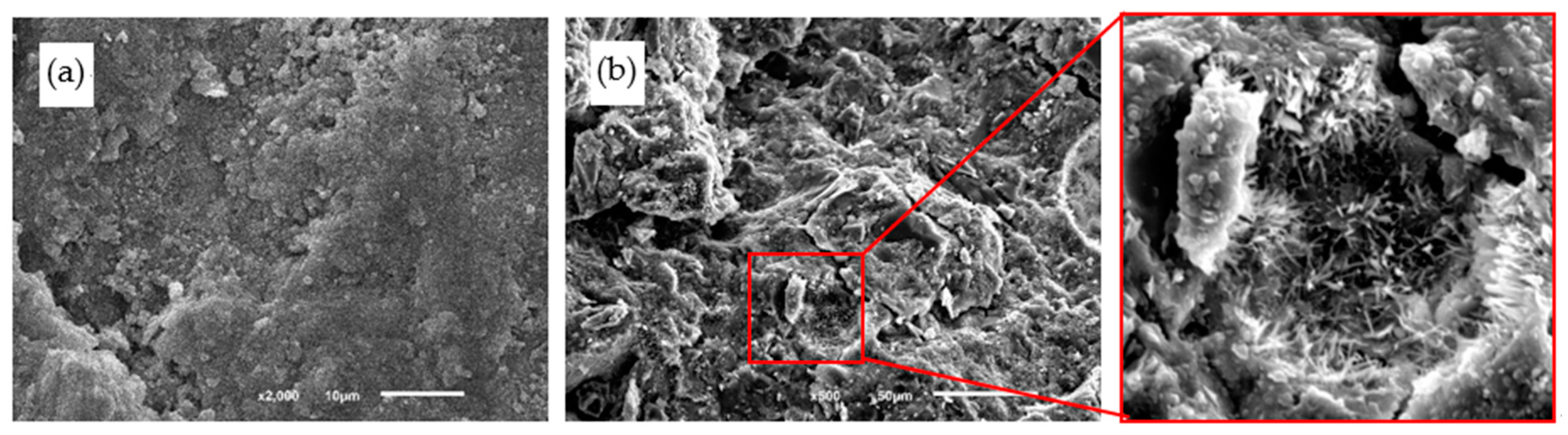
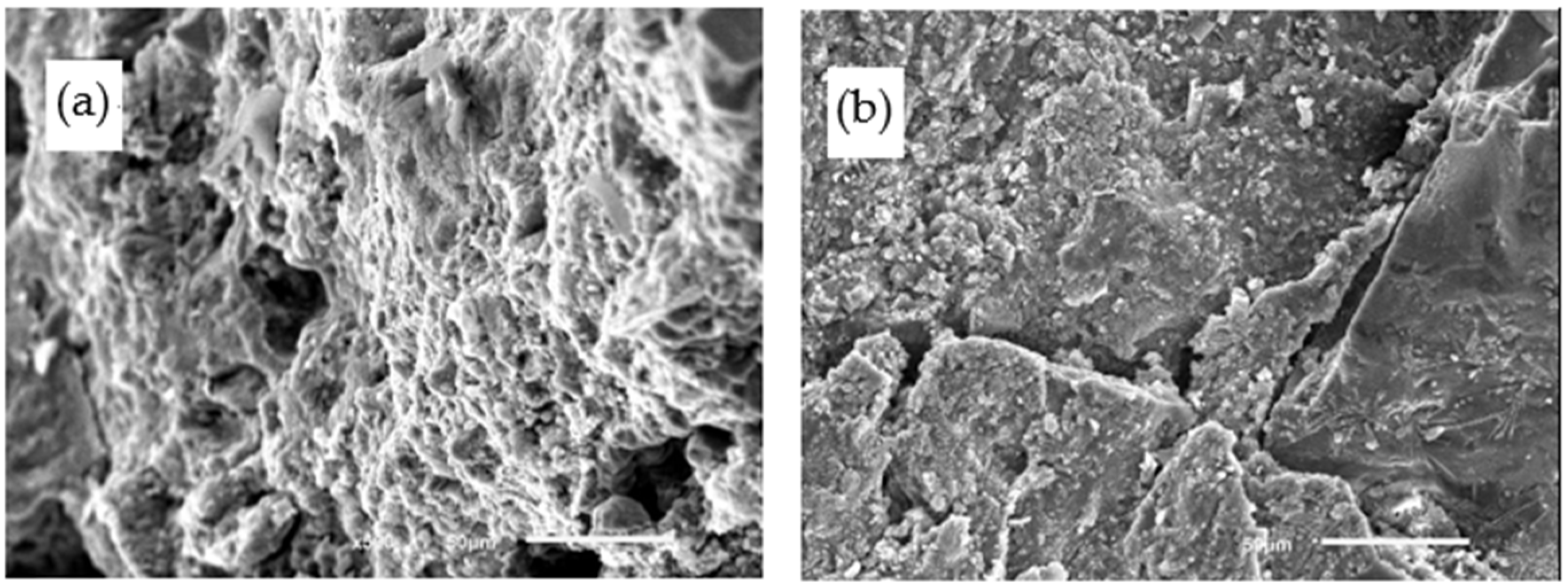
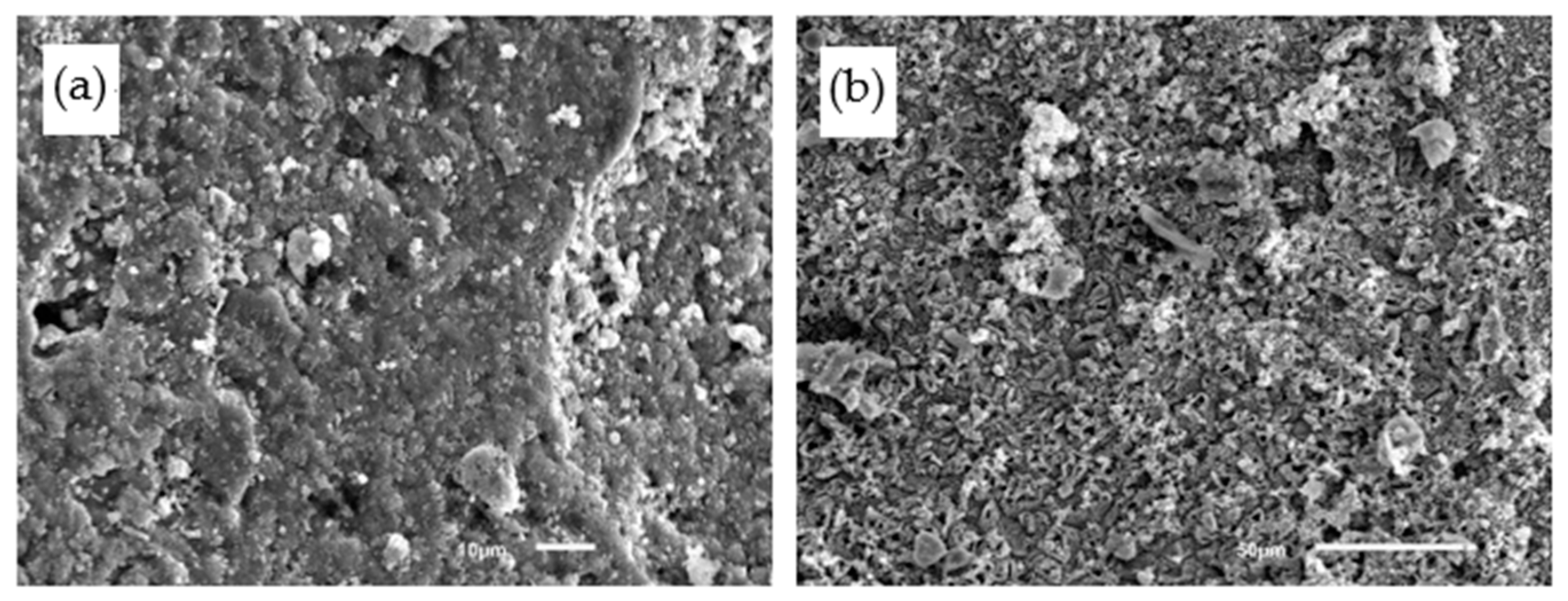
4.4. Microstructures
5. Conclusions
- (1)
- The compressive strength and the flexural strength of control PCPC are decreased by 30.7% and 40.8%, respectively.
- (2)
- The final compressive and flexural strength of 4% SF PCPC are 6.9% and 25.4% greater than that of the control PCPC, respectively.
- (3)
- The final compressive and flexural strengths of 8% FAG are higher 30.3% and 72.3% than that of the control PCPC, respectively.
- (4)
- The wear loss of PCPC is decreased by 58.8% and 81.9% when 4% SF and 8% FAG are used to replace part of cement and coarse aggregates, respectively.
- (5)
- The addition of 4% SF and 8% FAG improves the acid rain resistance of PCPC. 4% SF increases the water permeability, but FAG reduces the water permeability of PCPC.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bassuoni, M.T.; Sonebi, M. Pervious concrete: A sustainable drainage solution. Concrete 2010, 44, 14–16. [Google Scholar]
- Haselbach, L.; Boyer, M.; Kevern, J.; Schaefer, V. Cyclic heat island impacts on traditional versus pervious concrete pavement systems. J. Transp. Res. Board. 2011, 2240, 107–115. [Google Scholar] [CrossRef]
- Kevern, J.; Haselbach, L.; Schaefer, V. Hot weather comparative heat balances in pervious concrete and impervious concrete pavement systems. J. Heat Isl. Inst. Int. 2012, 7, 231–237. [Google Scholar]
- Marolf, A.; Neithalath, N.; Sell, E.; Wegner, K.; Weiss, J.; Olek, J. Influence of aggregate size and gradation on acoustic absorption of enhanced porosity concrete. ACI Mater. J. 2004, 101, 82–91. [Google Scholar]
- Kevern, J.; Wang, K.; Suleiman, M.T.; Schaefer, V. Pervious Concrete Construction Methods and Quality Control. In Proceedings of the NRMCA Concrete Technology Forum, Nashville, TN, USA, 23–24 May 2006. [Google Scholar]
- Mehmet, G.; Erhan, G.; Ganjeena, K.; Süleyman, İ. Abrasion and freezing–thawing resistance of pervious concretes containing waste rubbers. Constr. Build. Mater. 2014, 73, 19–24. [Google Scholar]
- Jing, Y.; Guo, L.J. Experimental study on properties of pervious concrete pavement materials. Cem. Concr. Res. 2003, 33, 381–386. [Google Scholar]
- Narayanan, N.; Milani, S.S.; Omkar, D. Characterizing pore volume, sizes, and connectivity in pervious concretes for permeability prediction. Mater. Charact. 2010, 61, 802–813. [Google Scholar]
- Wu, H.; Huang, B.; Shu, X.; Dong, Q. Laboratory evaluation of abrasion resistance of Portland cement pervious concrete. Mater. Civ. Eng. 2011, 23, 697–702. [Google Scholar] [CrossRef]
- Chen, M.C.; Wang, K.; Xie, L. Deterioration mechanism of cementitious materials under acid rain attack. Eng. Fail. Anal. 2013, 27, 272–285. [Google Scholar] [CrossRef]
- Wang, T.J.; Jin, L.S.; Li, Z.K.; Lam, K.S. A modeling study on acid rain and recommended emission control strategies in China. Atmos. Environ. 2000, 34, 4467–4477. [Google Scholar] [CrossRef]
- Talero Morales, R.; Triviño Vázquez, F.; Palacios de María, J.; Díaz García, F.F. La aluminosis del cemento aluminoso o un término nuevo para una clásica enfermedad. Mater. Constr. 1989, 216, 37–51. [Google Scholar] [CrossRef][Green Version]
- Zhou, C.L.; Zhu, Z.M.; Zhu, A.J.; Zhou, L.; Fan, Y.; Lang, L. Deterioration of mode II fracture toughness, compressive strength and elastic modulus of concrete under the environment of acid rain and cyclic wetting-drying. Constr. Build. Mater. 2019, 228, 116809. [Google Scholar] [CrossRef]
- Xie, S.D.; Qi, L.; Zhou, D. Investigation of the effects of acid rain on the deterioration of cement concrete using accelerated tests established in laboratory. Atmos. Environ. 2004, 38, 4457–4466. [Google Scholar] [CrossRef]
- Su, Q. Neutralization of Artificial Sand Concrete Caused by Acid Rain. In Proceedings of the 2nd International Conference on Applied Mathematics, Simulation and Modelling, Phuket, Thailand, 6–7 August 2017; Inc. DEStech Publications: Lancaster, PA, USA; pp. 479–484. [Google Scholar]
- Zhou, C.L.; Zhu, Z.M.; Wang, Z.H.; Qiu, H. Deterioration of concrete fracture toughness and elastic modulus under simulated acid-sulfate environment. Constr. Build. Mater. 2018, 176, 490–499. [Google Scholar] [CrossRef]
- Fan, Y.F.; Luan, H.Y. Pore structure in concrete exposed to acid deposit. Constr. Build. Mater. 2013, 49, 407–416. [Google Scholar] [CrossRef]
- Wang, K.; Yu, L.N.; Chen, M.C. Deterioration of Concrete Due to Acid Rain Attack in North China. In Construction and Urban Planning; Huang, Y., Bao, T., Wang, H., Eds.; Trans Tech Publications, Ltd.: Bach, Switzerland, 2013; pp. 1676–1679. [Google Scholar]
- Kanazu, T.; Matsumura, T.; Nishiuchi, T.; Yamamoto, T. Effect of simulated acid rain on deterioration of concrete. Water Air Soil Pollut. 2001, 130, 1481–1486. [Google Scholar] [CrossRef]
- Dias, C.M.R.; Cincotto, M.A.; Savastano, H.; John, V.M. Long-term aging of fiber-cement corrugated sheets—The effect of carbonation, leaching and acid rain. Cem. Concr. Compos. 2008, 30, 255–265. [Google Scholar] [CrossRef]
- Derry, D.J.; Malati, M.A.; Pullen, J.C. Effects of atmospheric acids on Portland cements. J. Chem. Technol. Biotechnol. 2001, 76, 1049–1056. [Google Scholar] [CrossRef]
- Gay, H.; Meynet, T.; Colombani, J. Local study of the corrosion kinetics of hardened Portland cement under acid attack. Cem. Concr. Res. 2016, 90, 36–42. [Google Scholar] [CrossRef]
- Guo, H.F.; Song, Z.G.; Yang, S.Y. Corrosion of Permeable Concrete under Simulated Acid Rain. In Novel and Non-Conventional Materials and Technologies for Sustainability; Xiao, Y., Li, Z., Wang, R., Shan, B., Ghavami, K., Eds.; Trans Tech Publications, Ltd.: Bach, Switzerland, 2012; pp. 352–356. [Google Scholar]
- Fan, Y.F.; Hu, Z.Q.; Zhang, Y.Z. Deterioration of compressive property of concrete under simulated acid rain environment. Constr. Build. Mater. 2010, 24, 1975–1983. [Google Scholar] [CrossRef]
- Zivica, V.; Krizma, M. Acidic-resistant slag cement. Mag. Concr. Res. 2013, 65, 1073–1080. [Google Scholar] [CrossRef]
- Estokova, A.; Kovalcikova, M. Application of Silica Fume as Waste Material in Cement Composites Exposed to Hydrochloric Acid. In Proceedings of the Geoconference on Ecology, Economics, Education and Legislation, Albena, Bulgaria, 19–25 June 2014; Volume I, pp. 23–30. [Google Scholar]
- Mahdikhani, M.; Bamshad, O.; Shirvani, M.F. Mechanical properties and durability of concrete specimens containing nano silica in sulfuric acid rain condition. Constr. Build. Mater. 2018, 167, 929–935. [Google Scholar] [CrossRef]
- Bakharev, T.; Sanjayan, J.G.; Cheng, Y.B. Resistance of alkali-activated slag concrete to acid attack. Cem. Concr. Res. 2003, 33, 1607–1611. [Google Scholar] [CrossRef]
- Lu, C.F.; Wang, W.; Zhou, Q.S.; Wei, S.H.; Wang, C. Mechanical behavior degradation of recycled aggregate concrete after simulated acid rain spraying. J. Clean. Prod. 2020, 262, 121237. [Google Scholar] [CrossRef]
- Preetham, R.; Krishna, R.H.; Chandraprabha, M.N.; Thomas, T. Vehicular soot for improvement of chemical stability of cement composites towards acid rain and sewage like atmospheres. Constr. Build. Mater. 2020, 248, 118604. [Google Scholar] [CrossRef]
- Sersale, R.; Frigione, G.; Bonavita, L. Acid deposition and concrete attack: Main influences. Cem. Concr. Res. 1998, 28, 19–24. [Google Scholar] [CrossRef]
- National Standard of the People’s Republic of China. Technical Specification for Pervious Cement Concrete Pavement; CJJ/T135-2009; Ministry of Housing and Urban-Rural Development of P.R.: Beijing, China, 2012.
- National Standard of the People’s Republic of China. Standard for Test Method of Mechanical Properties on Ordinary Concrete; GB/T 50081-2019; Ministry of Housing and Urban-Rural Development of P.R.: Beijing, China, 2012.
- National Standard of the People’s Republic of China. Test Methods of Cement and Concrete for Highway Engineering; JTG E3420-2020; Ministry of Transport of the P.R.: Beijing, China, 2020.
- Sotiriadis, K.; Nikolopoulou, E.; Tsivilis, S. Sulfate resistance of limestone cement concrete exposed to combined chloride and sulfate environment at low temperature. Cem. Concr. Compos. 2012, 34, 903–910. [Google Scholar] [CrossRef]
- Kejin, W.; Daniel, E.N.; Wilfrid, A.N. Damaging effects of deicing chemicals on concrete materials. Cem. Concr. Compos. 2006, 28, 173–188. [Google Scholar]
- Hammad, A.K.; Arnaud, C.; Mohammad, S.H.K.; Aziz, H.M. Durability of calcium aluminate and sulphate resistant Portland cement based mortars in aggressive sewer environment and sulphuric acid. Cem. Concr. Res. 2019, 124, 105852. [Google Scholar]
- Anaïs, G.; Patrick, D.; Marielle, G.M.; Thierry, C. Modelling of the sulfuric acid attack on different types of cementitious materials. Cem. Concr. Res. 2018, 105, 126–133. [Google Scholar]
- Chen, J.J.; Fung, W.W.S.; Kwan, A.K.H. Effects of CSF on strength, rheology and cohesiveness of cement paste. Constr. Build. Mater. 2012, 35, 979–987. [Google Scholar] [CrossRef]
- Taylor, H.F.W. Cement Chemistry; Thomas Telford Publishing: London, UK, 1997. [Google Scholar]
- Bassuoni, M.T.; Nehdi, M.L. Resistance of self-consolidating concrete to sulfuric acid attack with consecutive pH reduction. Cem. Concr. Res. 2007, 37, 1070–1084. [Google Scholar] [CrossRef]
- Davis, J.L.; Nica, D.; Shields, K.; Roberts, D.J. Analysis of concrete from corroded sewer pipe. Int. Biodeterior. Biodegr. 1998, 42, 75–84. [Google Scholar] [CrossRef]
- Mori, T.; Nonaka, T.; Tazaki, K.; Koga, M.; Hikosaka, Y.; Noda, S. Interactions of nutrients, moisture and pH on microbial corrosion of concrete sewer pipes. Water Res. 1992, 26, 29–37. [Google Scholar] [CrossRef]
- Zhang, J.; Gao, Y.; Han, Y.D.; Sun, W. Shrinkage and Interior Humidity of Concrete under Dry–Wet Cycles. Dry. Technol. 2012, 30, 583–596. [Google Scholar] [CrossRef]
- Bertron, A.; Duchesne, J.; Escadeillas, G. Accelerated tests of hardened cement pastes alteration by organic acids: Analysis of the pH effect. Cem. Concr. Res. 2005, 35, 155–166. [Google Scholar] [CrossRef]
- Oueslati, O.; Duchesne, J. The effect of SCMs and curing time on resistance of mortars subjected to organic acids. Cem. Concr. Res. 2012, 42, 205–214. [Google Scholar] [CrossRef]
- Chiara, F.F.; Karthik, H.O.; Russell, H. The influence of mineral admixtures on the rheology of cement paste and concrete. Cem. Concr. Res. 2001, 31, 245–255. [Google Scholar]
- Adil, G.; Kevern, J.T.; Mann, D. Influence of silica fume on mechanical and durability of pervious concrete. Constr. Build. Mater. 2020, 247, 118453. [Google Scholar] [CrossRef]
- Alessandra, B.; Filippo, G.; Maurizio, C. Experimental study on the effects of fine sand addition on differentially compacted pervious concrete. Constr. Build. Mater. 2015, 91, 102–110. [Google Scholar]
- Zhang, W.M.; Li, H.H.; Zhang, Y.C. Effect of porosity on frost resistance of Portland cement pervious concrete. Adv. Concr. Constr. 2018, 6, 363–373. [Google Scholar]
- Zhang, Y.B.; Zhang, W.M.; Zhang, Y.C. Combined effect of fine aggregate and silica fume on properties of Portland cement pervious concrete. Adv. Concr. Constr. 2019, 8, 47–54. [Google Scholar]
- Yuwadee, Z.; Vanchai, S.; Ampol, W.; Prinya, C. Properties of pervious concrete containing recycled concrete block aggregate and recycled concrete aggregate. Constr. Build. Mater. 2016, 111, 15–21. [Google Scholar]
- Senhadji, Y.; Escadeillas, G.; Mouli, M.; Khelafi, H. Influence of natural pozzolan, silica fume and limestone fine on strength, acid resistance and microstructure of mortar. Powder Technol. 2014, 254, 314–323. [Google Scholar] [CrossRef]
- Maguesvari, M.U.; Narasimha, V.L. Studies on Characterization of Pervious Concrete for Pavement Applications. Proced. Soc. Behav. Sci. 2013, 104, 198–207. [Google Scholar] [CrossRef]
- Xu, G.L.; Shen, W.G.; Huo, X.J. Investigation on the properties of porous concrete as road base material. Constr. Build. Mater. 2018, 158, 141–148. [Google Scholar] [CrossRef]
- Fattuhi, N.I.; Hughes, B.P. The performance of cement paste and concrete subjected to sulphuric acid attack. Cem. Concr. Res. 1988, 18, 545–553. [Google Scholar] [CrossRef]
- Lei, G.; Phillip, V.; Terry, B. Evaluation of accelerated degradation test methods for cementitious composites subject to sulfuric acid attack; application to conventional and alkali-activated concretes. Cem. Concr. Compos. 2018, 87, 187–204. [Google Scholar]
- Nežerka, V.; Bílý, P.; Hrbek, V.; Fládr, J. Impact of silica fume, fly ash, and metakaolin on the thickness and strength of the ITZ in concrete. Cem. Concr. Compos. 2019, 103, 252–262. [Google Scholar] [CrossRef]
- Merchanta, I.J.; Macpheeb, D.E.; Chandlera, H.W.; Henderson, R.J. Toughening cement-based materials through the control of interfacial bonding. Cem. Concr. Res. 2001, 31, 1873–1880. [Google Scholar] [CrossRef]
- Bu, J.; Tian, Z.; Zheng, S.; Tang, Z. Effect of sand content on strength and pore structure of cement mortar. J. Wuhan Univ. Technol. Mater. Sci. Ed. 2017, 32, 382–390. [Google Scholar] [CrossRef]
- Zampini, D.; Jennings, H.M.; Shah, S.P. Characterization of the paste aggregate interfacial zone to the fracture toughness of concrete. J. Mater. Sci. 1995, 30, 3149–3154. [Google Scholar] [CrossRef]
- Amparano, F.E.; Xi, Y.; Roh, Y.S. Experimental study on the effect of aggregate content on fracture behavior of concrete. Eng. Fract. Mech. 2000, 67, 65–84. [Google Scholar] [CrossRef]
- Belkacem, B.; Madani, B.; Khadra, B.; Michele, Q. Effect of the type of sand on the fracture and mechanical properties of sand concrete. Adv. Concrete Constr. 2014, 2, 13–27. [Google Scholar]
- Wu, D.; Liu, X.; Wu, X.Q. Effect of forming method and sand ratio on performance of pervious concrete. Concrete 2009, 5, 100–102. [Google Scholar]
- Zaetang, Y.; Wongsa, A.; Sata, V.; Chindaprasirt, P. Influence of mineral additives on the properties of pervious concrete. Ind. J. Eng. Mater. Sci. 2017, 24, 507–515. [Google Scholar]
- Chougan, M.; Ghaffar, S.H.; Sikora, P.; Chung, S.Y.; Swash, M.R. Investigation of additive incorporation on rheological, microstructural and mechanical properties of 3d printable alkali-activated materials. Mater. Des. 2021, 202, 109574. [Google Scholar] [CrossRef]
- Lamastra, F.R.; Chougan, M.; Marotta, E.; Ciattini, S.; Bianco, A. Toward a better understanding of multifunctional cement-based materials: The impact of graphite nanoplatelets (GNPs). Ceram. Int. 2021. [Google Scholar] [CrossRef]

| SiO2 | Al2O3 | Fe2O3 | CaO | MgO | SO3 | Na2O | K2O | TiO2 |
|---|---|---|---|---|---|---|---|---|
| 23.1 | 7.11 | 3.69 | 57.57 | 2.20 | 2.67 | 2.16 | 0.73 | 0.33 |
| Water (kg/m3) | Cement (kg/m3) | Coarse Aggregate (kg/m3) | Fine Aggregate (kg/m3) | Silica Fume (kg/m3) | SP (kg/m3) | Void Ratio (%) | |
|---|---|---|---|---|---|---|---|
| Control PCPC | 116 | 415 | 1453 | 0 | 0 | 4.15 | 18.5 |
| 4%SF PCPC | 116 | 398.4 | 1453 | 0 | 16.6 | 4.15 | 20.1 |
| 8%FAG PCPC | 116 | 415 | 1336.8 | 116.2 | 0 | 4.15 | 15.3 |
| Cube 100 mm × 100 mm × 100 mm | Prism 100 mm × 100 mm × 400 mm | Cube 150 mm × 150 mm × 150 mm | |
|---|---|---|---|
| Control PCPC | 27 | 27 | 3 |
| 4%SF PCPC | 27 | 15 | 3 |
| 8%FAG PCPC | 15 | 15 | 3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, L.; Lai, Y.; Islam Pramanic, M.R.; Zhang, W. Deterioration of Portland Cement Pervious Concrete in Sponge Cities Subjected to Acid Rain. Materials 2021, 14, 2670. https://doi.org/10.3390/ma14102670
Gao L, Lai Y, Islam Pramanic MR, Zhang W. Deterioration of Portland Cement Pervious Concrete in Sponge Cities Subjected to Acid Rain. Materials. 2021; 14(10):2670. https://doi.org/10.3390/ma14102670
Chicago/Turabian StyleGao, Longxin, Yong Lai, Mohammad Rashadul Islam Pramanic, and Wuman Zhang. 2021. "Deterioration of Portland Cement Pervious Concrete in Sponge Cities Subjected to Acid Rain" Materials 14, no. 10: 2670. https://doi.org/10.3390/ma14102670
APA StyleGao, L., Lai, Y., Islam Pramanic, M. R., & Zhang, W. (2021). Deterioration of Portland Cement Pervious Concrete in Sponge Cities Subjected to Acid Rain. Materials, 14(10), 2670. https://doi.org/10.3390/ma14102670





