Influence of Low-Pressure Treatment on the Morphological and Compositional Stability of Microscopic Ettringite
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Synthesis of Ettringite
2.2.2. Influence of Low Pressure
2.2.3. X-ray Diffraction and Pawley fit
2.2.4. Thermogravimetric Analysis
2.2.5. Quick Assessment of Mass Loss
2.2.6. Environmental Scanning Electron Microscopy
2.2.7. Scanning Electron Microscopy
2.2.8. Raman Spectroscopy
2.2.9. Transmission Electron Microscopy and Energy-Dispersive X-ray Spectroscopy
2.2.10. Selected Area Electron Diffraction
3. Results and Discussion
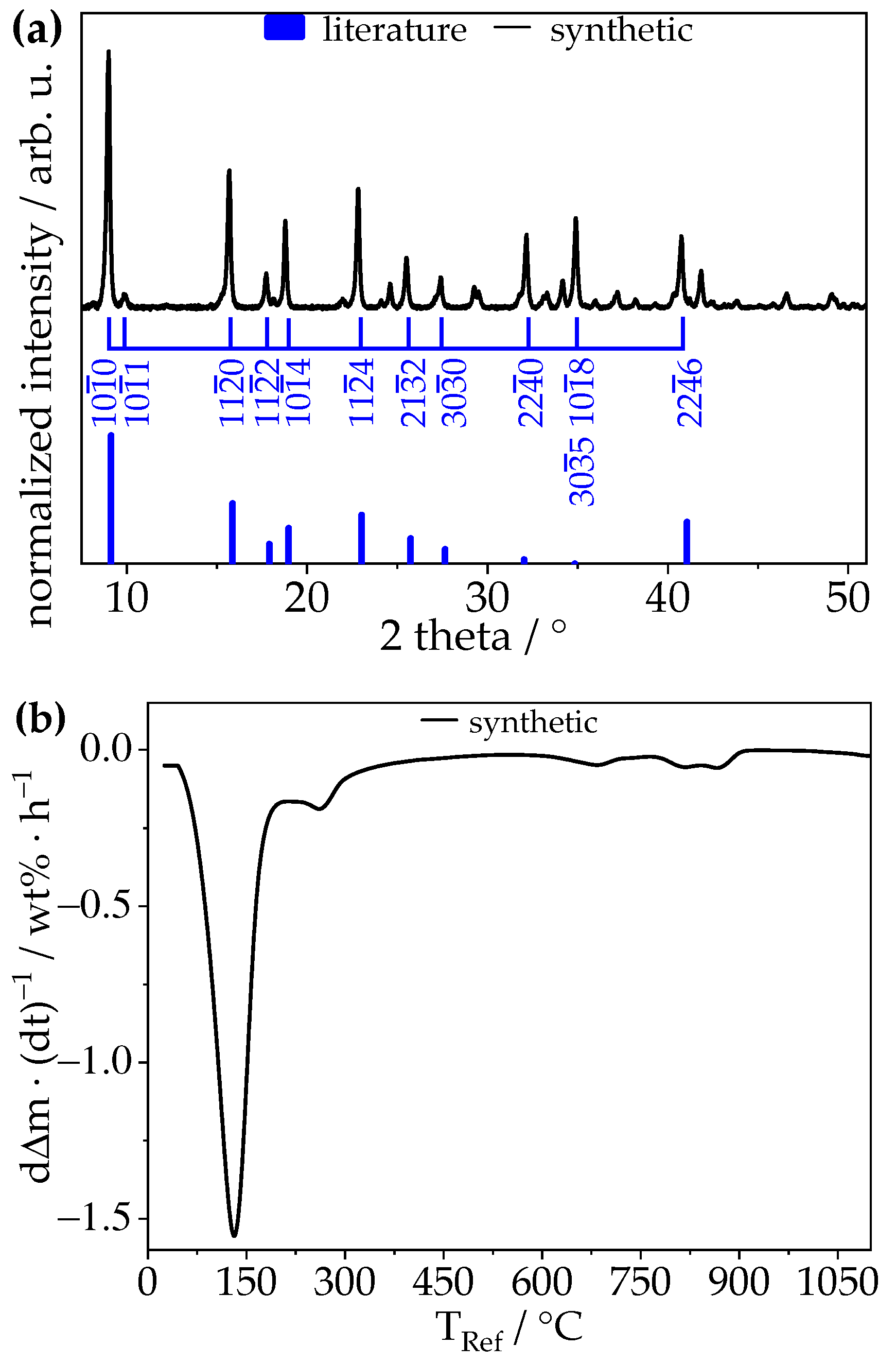
3.1. Synthetic Ettringite
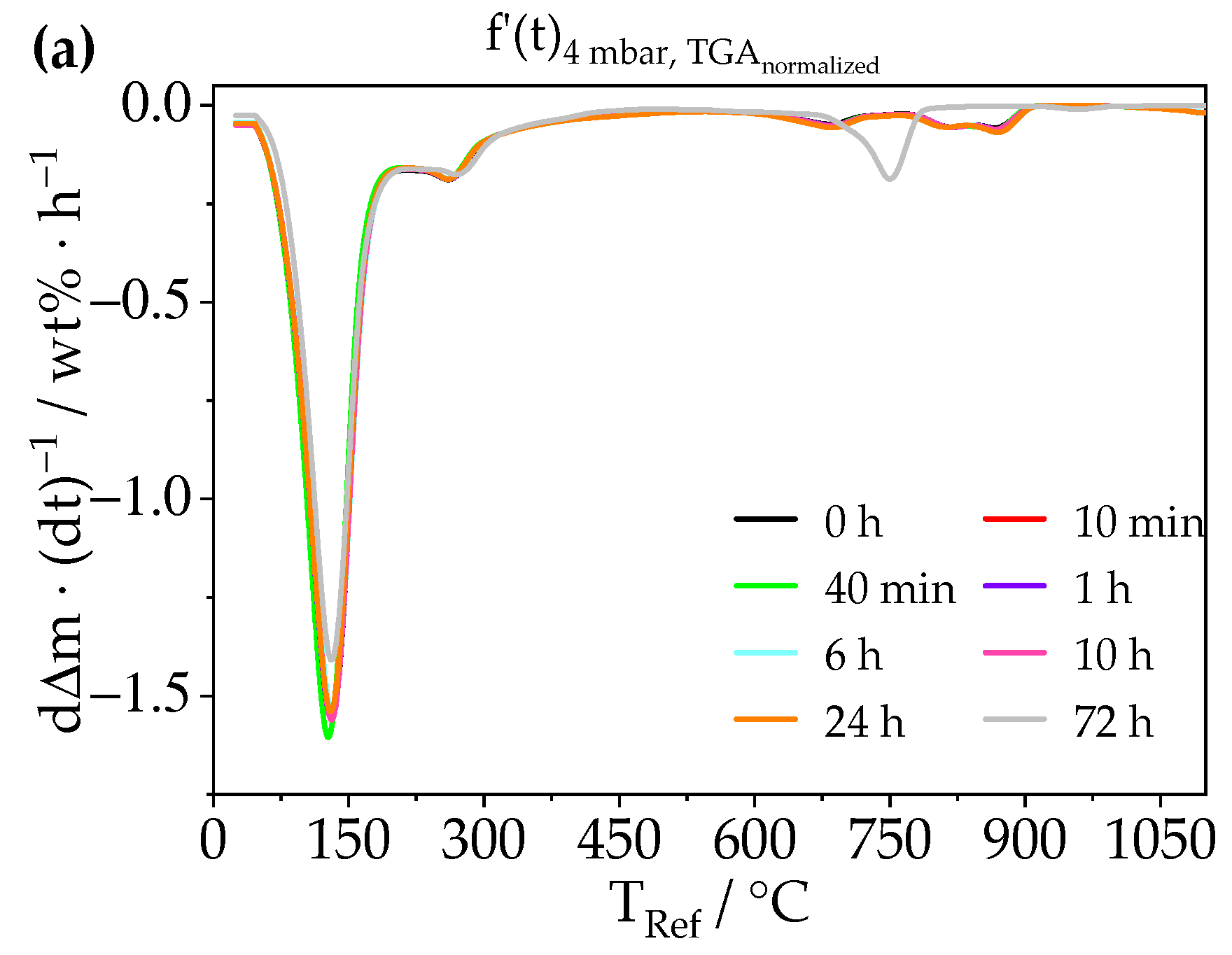
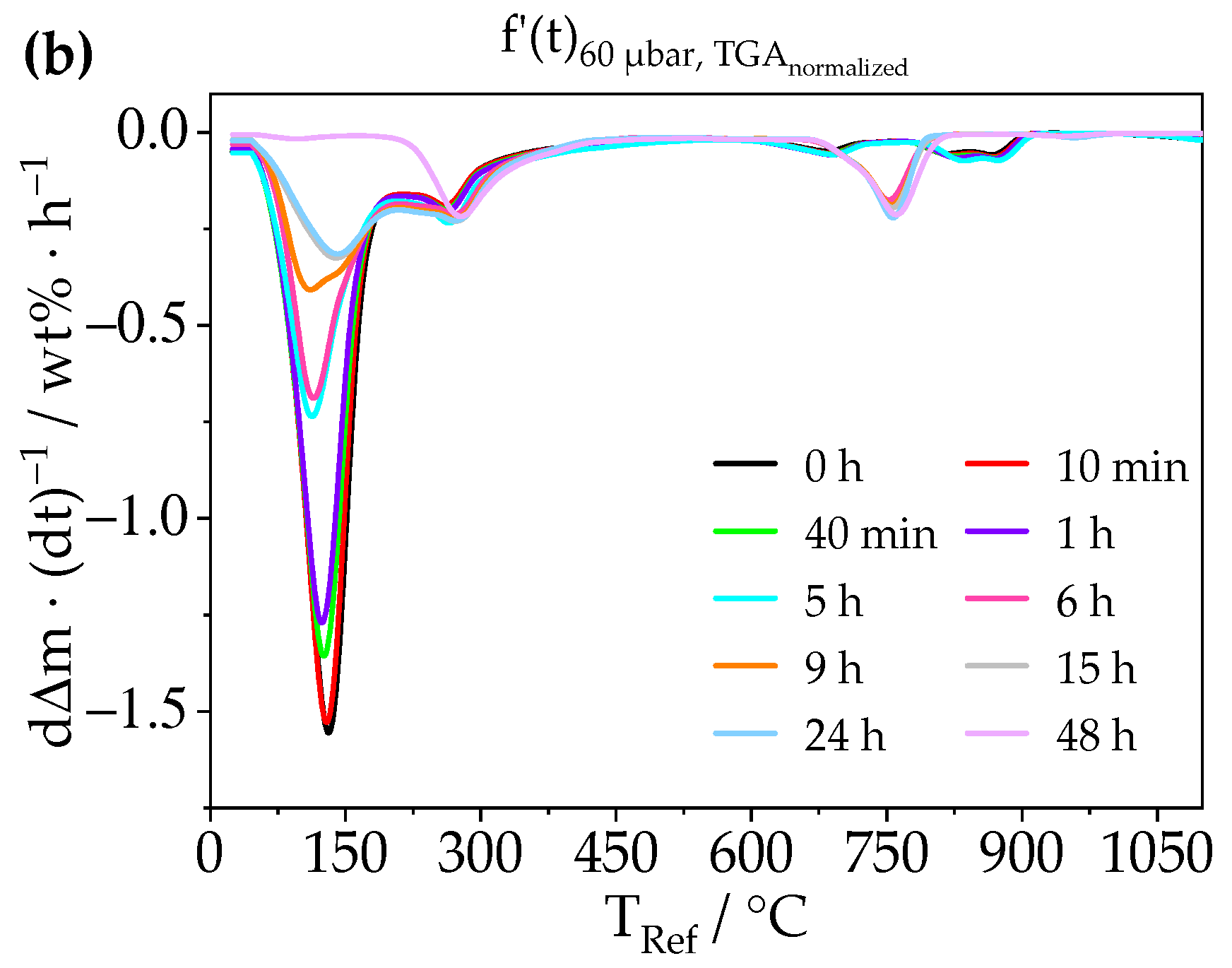
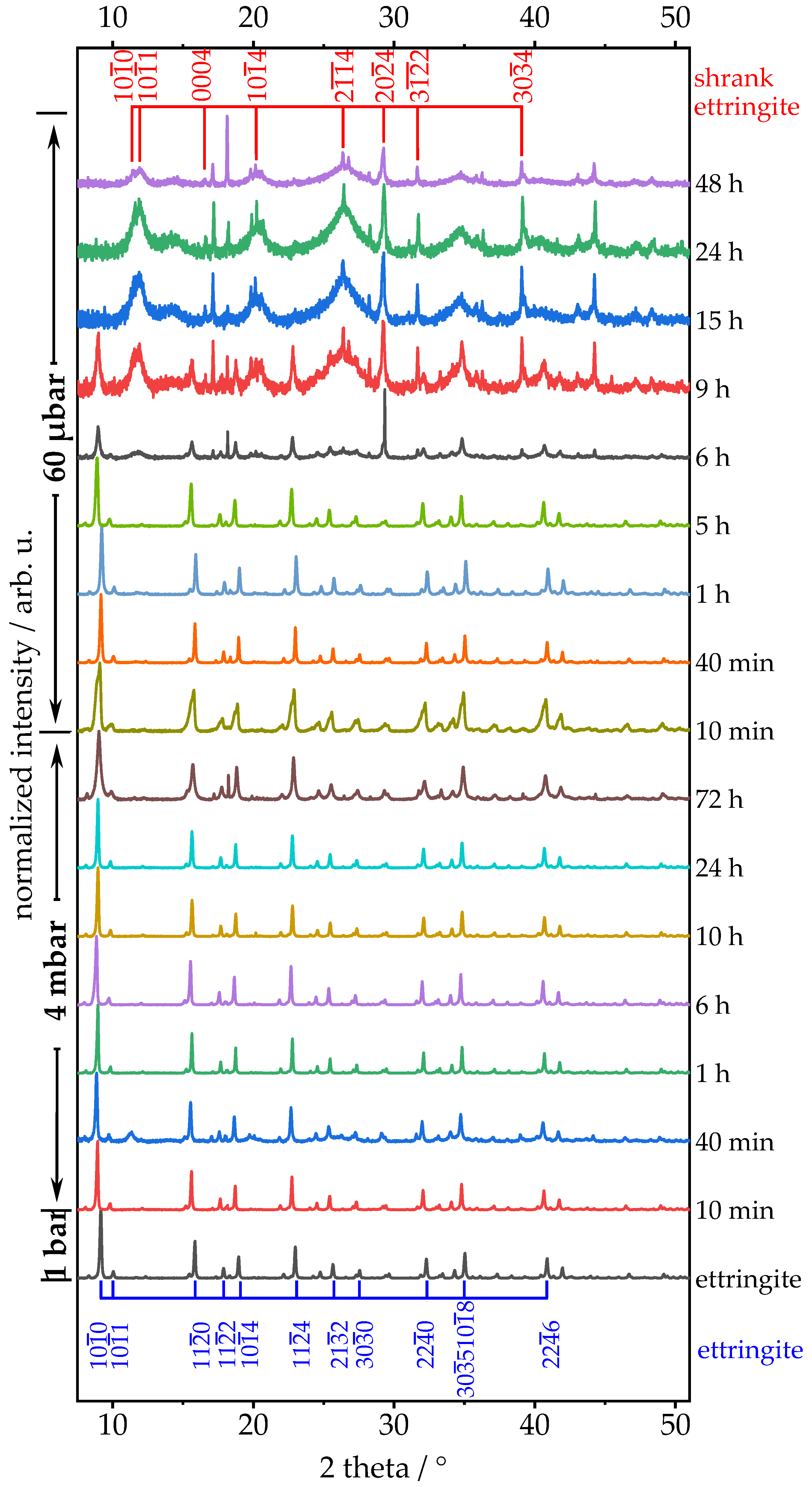
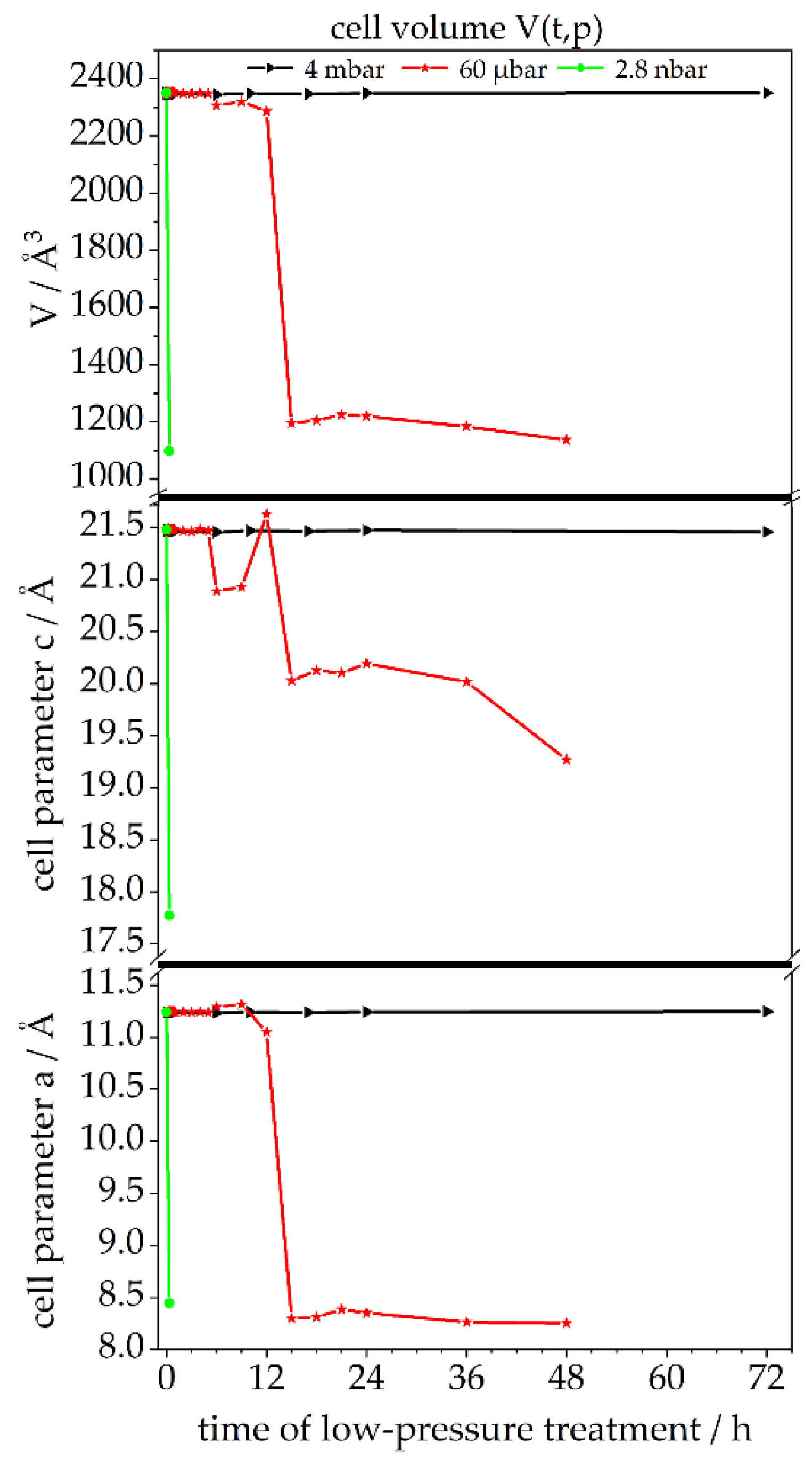
3.2. Treatment of Ettringite with Low-Pressure
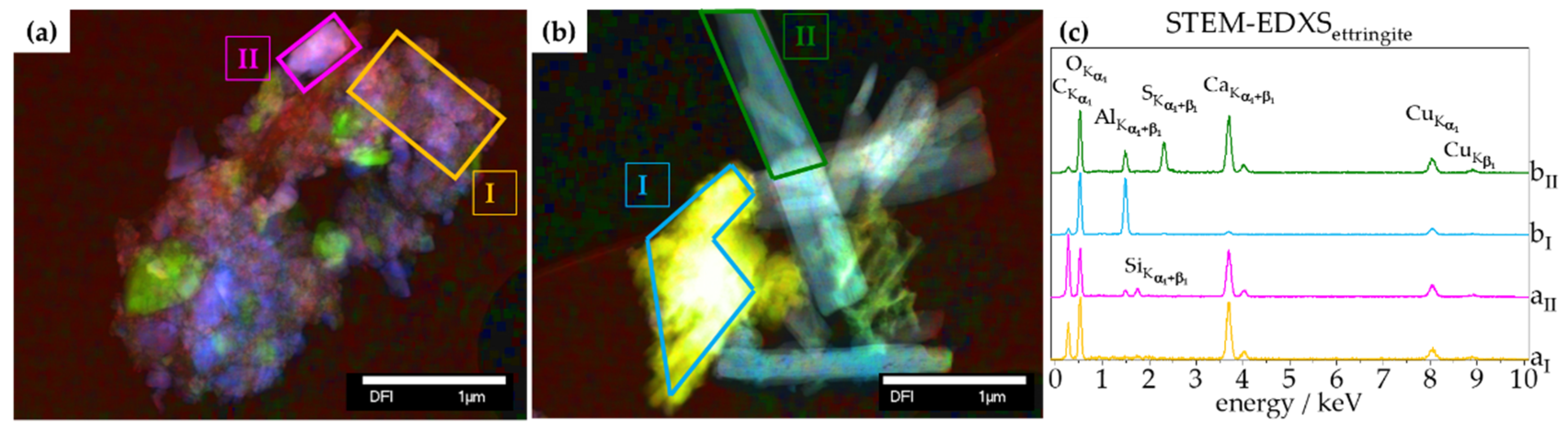
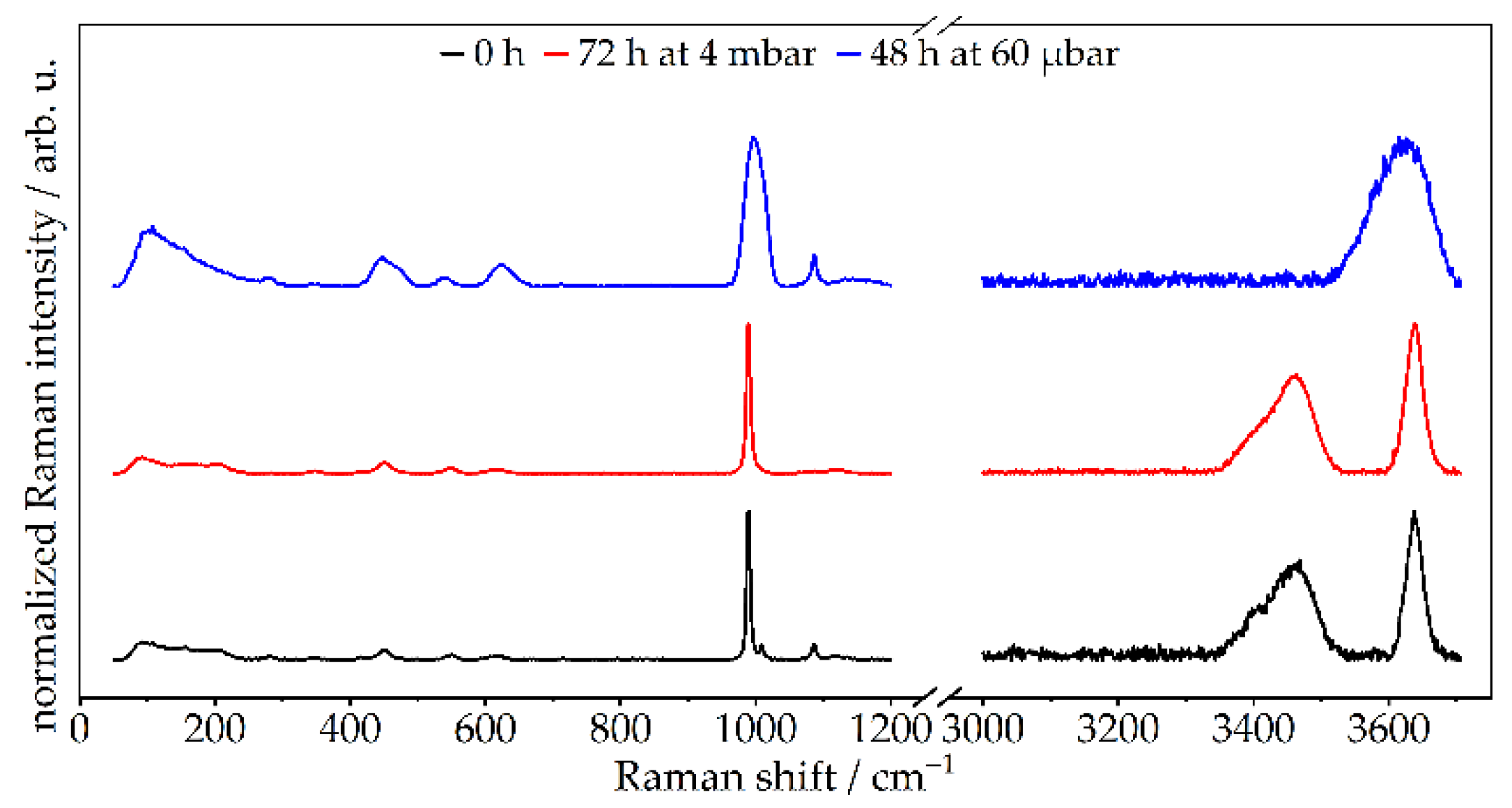
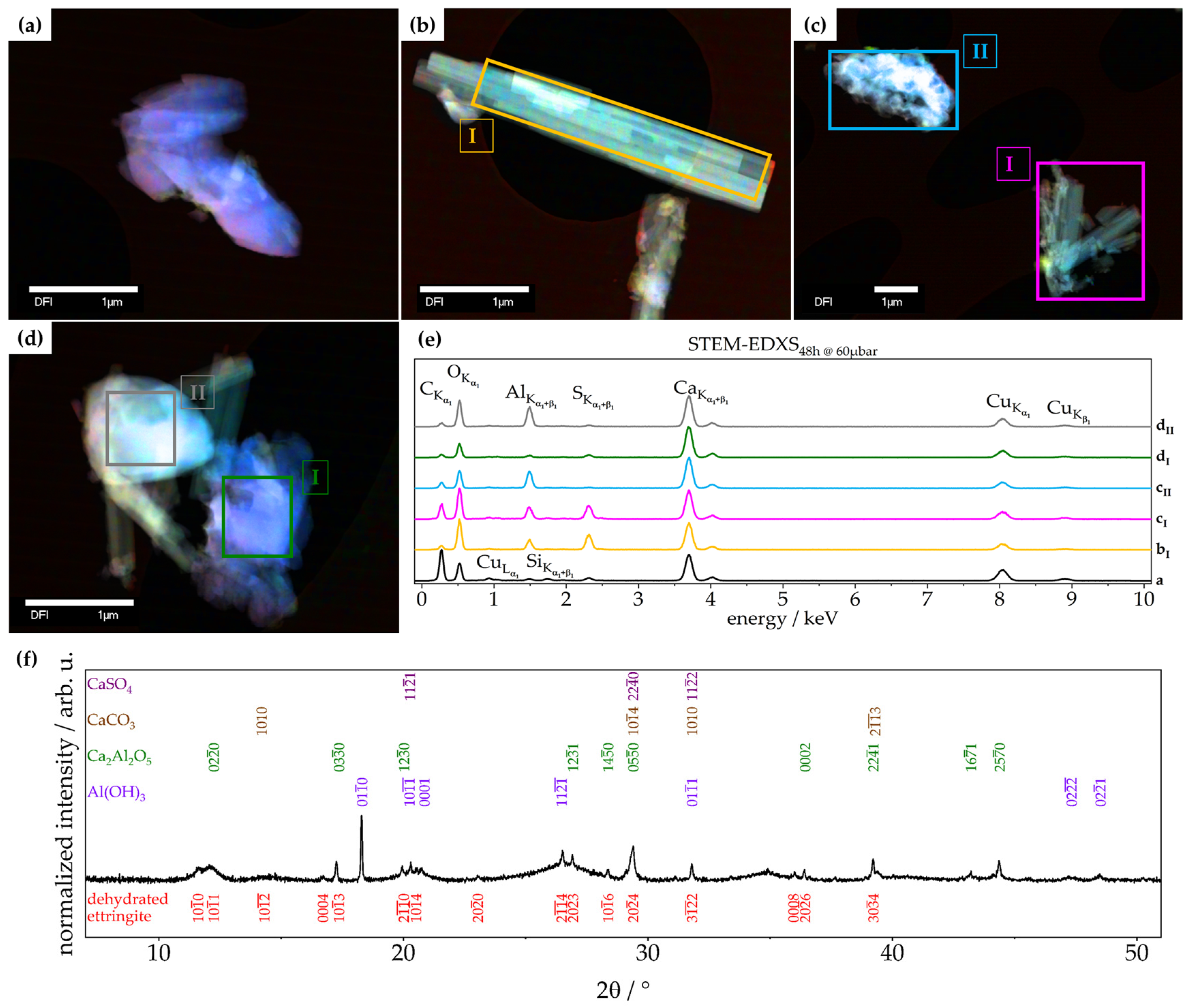
3.2.1. Chemical Composition—TGA, XRD, Raman, STEM-EDXS
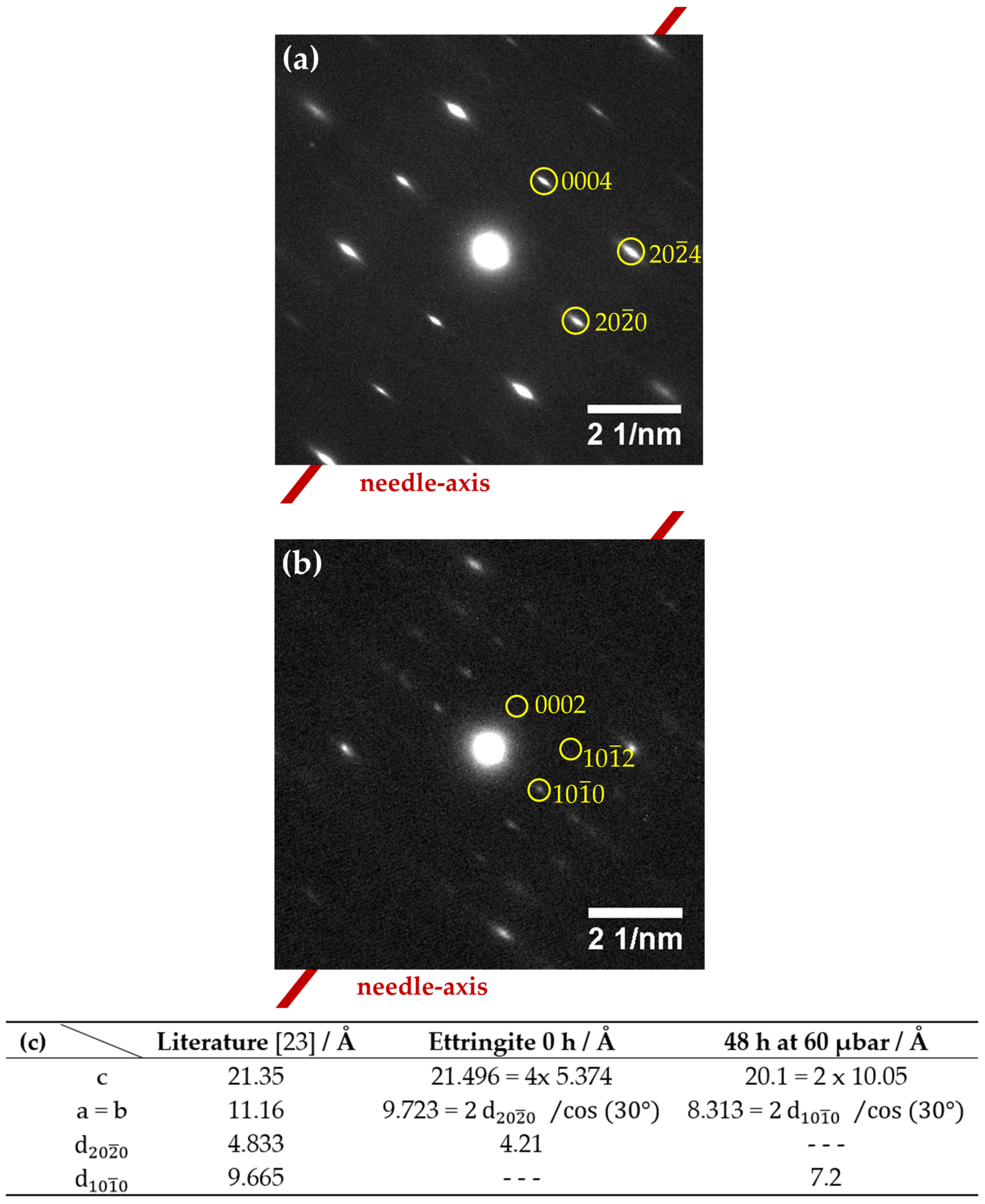
3.2.2. Crystallographic Assessment—SAED, Pawley fit
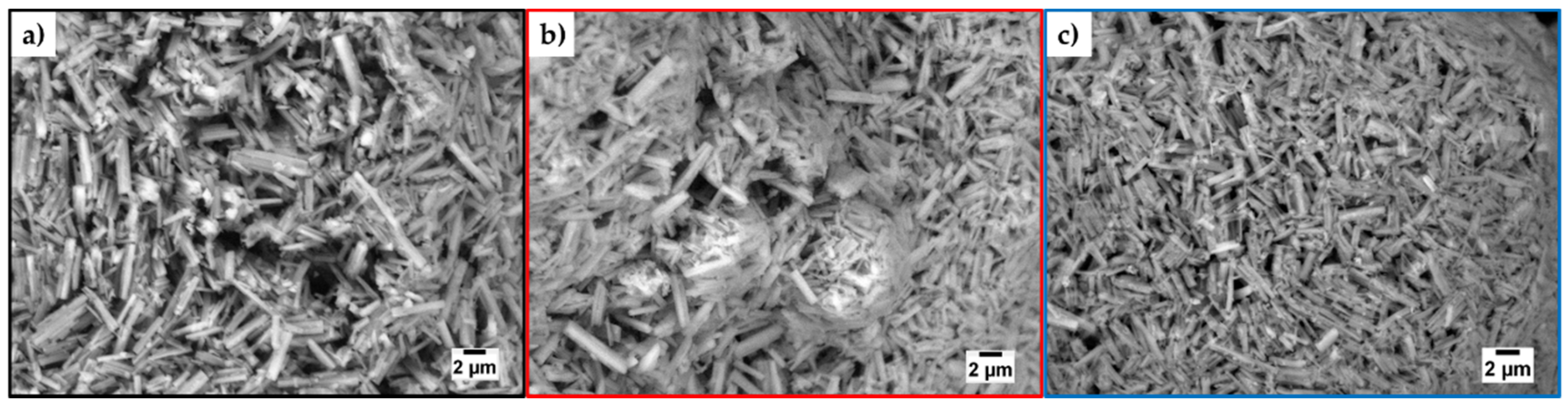
3.2.3. Morphology—SEM, ESEM
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Moore, A.; Taylor, H.F.W. Crystal Structure of Ettringite. Nature 1968, 218, 1048–1049. [Google Scholar] [CrossRef]
- Barger, G.S.; Bayles, J.; Blair, B.; Brown, D.; Chen, H.; Conway, T.; Hawkins, P. Ettringite Formation and the Performance of Concrete; Portland Cement Association: Skokie, IL, USA, 2001; pp. 1–16. [Google Scholar]
- Jakob, C.; Jansen, D.; Ukrainczyk, N.; Koenders, E.; Pott, U.; Stephan, D.; Neubauer, J. Relating Ettringite Formation and Rheological Changes during the Initial Cement Hydration: A Comparative Study Applying XRD Analysis, Rheological Measurements and Modeling. Materials 2019, 12, 2957. [Google Scholar] [CrossRef] [PubMed]
- Merlini, M.; Artioli, G.; Cerulli, T.; Cella, F.; Bravo, A. Tricalcium aluminate hydration in additivated systems. A crystallographic study by SR-XRPD. Cem. Concr. Res. 2008, 38, 477–486. [Google Scholar] [CrossRef]
- Skoblinskaya, N.N.; Krasilnikov, K.G. Changes in crystal structure of ettringite on dehydration. 1. Cem. Concr. Res. 1975, 5, 381–394. [Google Scholar] [CrossRef]
- Skoblinskaya, N.N.; Krasilnikov, K.G.; Nikitina, L.V.; Varlamov, V.P. Changes in crystal structure of ettringite on dehydration. 2. Cem. Concr. Res. 1975, 5, 419–431. [Google Scholar] [CrossRef]
- Stroh, J.; Ali, N.Z.; Maierhofer, C.; Emmerling, F. Ettringite via Mechanochemistry: A Green and Rapid Approach for Industrial Application. ACS Omega 2019, 4, 7734–7737. [Google Scholar] [CrossRef] [PubMed]
- Lehmkuhl, J.; Fendel, A.D.; Bings, H. Mineralischer Füllstoff und Baustoff-Additiv auf Basis von Calciumaluminiumsulfat und deren Herstellung und Verwendung. German Patent DE19611454, 25 September 1997. [Google Scholar]
- Moore, A.; Taylor, H.F.W. Crystal Structure of Ettringite. Acta Crystallogr. Sect. B Struct. Crystallogr. Cryst. Chem. 1970, 26, 386–393. [Google Scholar] [CrossRef]
- Taylor, H.F.W. Crystal structures of some double hydroxide minerals. Mineral. Mag. 1973, 39, 377–389. [Google Scholar] [CrossRef]
- Bezjak, A.; Jelenić, I. Crystal Structure Investigation of Calcium Aluminium Sulphate Hydrate—Ettringite. Croat. Chem. Acta 1966, 38, 239–242. [Google Scholar]
- Shimada, Y.; Young, J.F. Structural changes during thermal dehydration of ettringite. Adv. Cem. Res. 2001, 13, 77–81. [Google Scholar] [CrossRef]
- Winnefeld, F.; Zingg, A.; Holzer, L.; Pakusch, J.; Becker, S. The Ettringite-superplasticizer interaction and its impact on ettringite distribution in cement suspensions. In Proceedings of the 9th ACI International Conference on Superplasticizers and Other Chemical Admixtures in Concrete, Sevilla, Spain, 12–14 October 2009; pp. 420.1–420.17. [Google Scholar]
- Tattersall, G.H. Workability and Quality Control of Concrete, 1st ed.; CRC Press: London, UK, 2014; ISBN 9781482267006. [Google Scholar]
- Gołaszewski, J. Influence of cement properties on rheology of fresh cement mortars without and with superplasticizer. Archit. Civ. Eng. Environ. 2008, 4, 49–66. [Google Scholar]
- Struble, L.J.; Lei, W.-G. Rheological changes associated with setting of cement paste. Adv. Cem. Based Mater. 1995, 2, 224–230. [Google Scholar] [CrossRef]
- Uchikawa, H.; Ogawa, K.; Uchida, S. Influence of character of clinker on the early hydration process and rheological property of cement paste. Cem. Concr. Res. 1985, 15, 561–572. [Google Scholar] [CrossRef]
- Pott, U.; Ehm, C.; Jakob, C.; Stephan, D. Investigation of the Early Cement Hydration with a New Penetration Test, Rheometry and In-Situ XRD. In Rheology and Processing of Construction Materials; Springer: Cham, Switzerland, 2020; pp. 246–255. [Google Scholar]
- Pawley, G.S. Unit-cell refinement from powder diffraction scans. J. Appl. Crystallogr. 1981, 14, 357–361. [Google Scholar] [CrossRef]
- Struble, L.J. Synthesis and characterization of ettringite and related phases. In 8th International Congress on the Chemistry of Cement; Abla Grafica e Editora: Rio de Janeiro, Brazil, 1986; pp. 582–588. [Google Scholar]
- Guildner, L.; Johnson, D.; Jones, F.E. Vapor pressure of water at its triple point. J. Res. Natl. Bur. Stand. Sect. A Phys. Chem. 1976, 80A, 505. [Google Scholar] [CrossRef] [PubMed]
- The International Association for the Properties of Water and Steam. IAPWS G5-01, Guideline on the Use of Fundamental Physical Constants and Basic Constants of Water. Available online: http://www.iapws.org/relguide/fundam.pdf (accessed on 20 March 2021).
- Hartman, M.R.; Berliner, R. Investigation of the structure of ettringite by time-of-flight neutron powder diffraction techniques. Cem. Concr. Res. 2006, 36, 364–370. [Google Scholar] [CrossRef]
- Renaudin, G.; Filinchuk, Y.; Neubauer, J.; Goetz-Neunhoeffer, F. A comparative structural study of wet and dried ettringite. Cem. Concr. Res. 2010, 40, 370–375. [Google Scholar] [CrossRef]
- Hall, C.; Barnes, P.; Billimore, A.D.; Jupe, A.C.; Turrillas, X. Thermal decomposition of ettringite Ca6[Al(OH)6]2(SO4)3·26H2O. J. Chem. Soc. Faraday Trans. 1996, 92, 2125–2129. [Google Scholar] [CrossRef]
- European Commission; Joint Research Centre; Institute for Health and Consumer Protection; European Chemicals Bureau. IUCLID-CD-ROM, 2000th ed.; European Commission: Ispra, Italy, 2000. [Google Scholar]
- Roth, L.; Weller, U. Gefährliche Chemische Reaktionen: Stoffinformationen, Reaktionstabellen, Unfallberichte/Roth; Weller; 1; Ecomed-Storck GmbH: Landsberg am Lech, Germany, 1982; ISBN 360964530X. [Google Scholar]
- Taylor, H.F.W. Cement Chemistry; Thomas Telford Publishing: London, UK, 1997; ISBN 0-7277-3945-X. [Google Scholar]
- Lea, F.M. Lea’s Chemistry of Cement and Concrete, 3rd ed.; Elsevier: London, UK, 1998; ISBN 9780750662567. [Google Scholar]
- Ghorab, H.Y.; Kishar, E.A. Studies on the stability of the calcium sulfoaluminate hydrates. Part 1: Effect of temperature on the stability of ettringite in pure water. Cem. Concr. Res. 1985, 15, 93–99. [Google Scholar] [CrossRef]
- Grounds, T.; Midgley, H.G.; Nowell, D. The use of thermal methods to estimate the state of hydration of calciumtrisulphoaluminate hydrate 3CaO·Al2O3·3CaSO4·nH2O. Thermochim. Acta 1985, 85, 215–218. [Google Scholar] [CrossRef]
- Stepkowska, E.T.; Blanes, J.M.; Real, C.; Perez-Rodriguez, J.L. Hydration products in two aged cement pastes. J. Therm. Anal. Calorim. 2005, 82, 731–739. [Google Scholar] [CrossRef]
- Villain, G.; Thiery, M.; Platret, G. Measurement methods of carbonation profiles in concrete: Thermogravimetry, chemical analysis and gammadensimetry. Cem. Concr. Res. 2007, 37, 1182–1192. [Google Scholar] [CrossRef]
- Renaudin, G.; Segni, R.; Mentel, D.; Nedelec, J.M.; Leroux, F.; Taviot-Gueho, C. A Raman study of the sulfated cement hydrates: Ettringite and monosulfoaluminate. J. Adv. Concr. Technol. 2007, 5, 299–312. [Google Scholar] [CrossRef]
- Effenberger, H.; Mereiter, K.; Zemann, J. Crystal structure refinements of magnesite, calcite, rhodochrosite, siderite, smithonite, and dolomite, with discussion of some aspects of the stereochemistry of calcite type carbonates. Z. Krist. Cryst. Mater. 1981, 156, 233–243. [Google Scholar] [CrossRef]
- Aggarwal, P.; Gard, J.; Glasser, F.; Biggar, G. Synthesis and properties of dicalcium aluminate, 2CaO·Al2O3. Cem. Concr. Res. 1972, 2, 291–297. [Google Scholar] [CrossRef]
- Clark, G.R.; Rodgers, K.A.; Henderson, G.S. The crystal chemistry of doyleite, Al(OH)3. Z. Krist. Cryst. Mater. 1998, 213, 96–100. [Google Scholar] [CrossRef]
- Bezou, C.; Nonat, A.; Mutin, J.-C.; Christensen, A.N.; Lehmann, M.S. Investigation of the Crystal Structure of γ-CaSO4, CaSO4·0.5 H2O, and CaSO4·0.6 H2O by Powder Diffraction Methods. J. Solid State Chem. 1995, 117, 165–176. [Google Scholar] [CrossRef]
- Ross, F.M. (Ed.) Liquid Cell Electron Microscopy; Cambridge University Press: Cambridge, UK, 2016; ISBN 9781316337455. [Google Scholar] [CrossRef]
- Toby, B.H. R factors in Rietveld analysis: How good is good enough? Powder Diffr. 2006, 21, 67–70. [Google Scholar] [CrossRef]
- DFG SPP 2005—Priority Programm Opus Fluidum Futurum—Rheology of Reactive, Multiscale, Multiphase Construction Materials. Available online: https://www.spp2005.de (accessed on 29 October 2019).


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kißling, P.A.; Lübkemann, F.; von Bronk, T.; Cotardo, D.; Lei, L.; Feldhoff, A.; Lohaus, L.; Haist, M.; Bigall, N.C. Influence of Low-Pressure Treatment on the Morphological and Compositional Stability of Microscopic Ettringite. Materials 2021, 14, 2720. https://doi.org/10.3390/ma14112720
Kißling PA, Lübkemann F, von Bronk T, Cotardo D, Lei L, Feldhoff A, Lohaus L, Haist M, Bigall NC. Influence of Low-Pressure Treatment on the Morphological and Compositional Stability of Microscopic Ettringite. Materials. 2021; 14(11):2720. https://doi.org/10.3390/ma14112720
Chicago/Turabian StyleKißling, Patrick A., Franziska Lübkemann, Tabea von Bronk, Dario Cotardo, Lei Lei, Armin Feldhoff, Ludger Lohaus, Michael Haist, and Nadja C. Bigall. 2021. "Influence of Low-Pressure Treatment on the Morphological and Compositional Stability of Microscopic Ettringite" Materials 14, no. 11: 2720. https://doi.org/10.3390/ma14112720
APA StyleKißling, P. A., Lübkemann, F., von Bronk, T., Cotardo, D., Lei, L., Feldhoff, A., Lohaus, L., Haist, M., & Bigall, N. C. (2021). Influence of Low-Pressure Treatment on the Morphological and Compositional Stability of Microscopic Ettringite. Materials, 14(11), 2720. https://doi.org/10.3390/ma14112720






