Hydrothermal Synthesis of Sodalite-Type N-A-S-H from Fly Ash to Remove Ammonium and Phosphorus from Water
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of SOD
2.3. Characterization Techniques
2.4. Batch Experiments
2.5. Saturated Adsorbent Desorption Regeneration Experiment
3. Results and Discussion
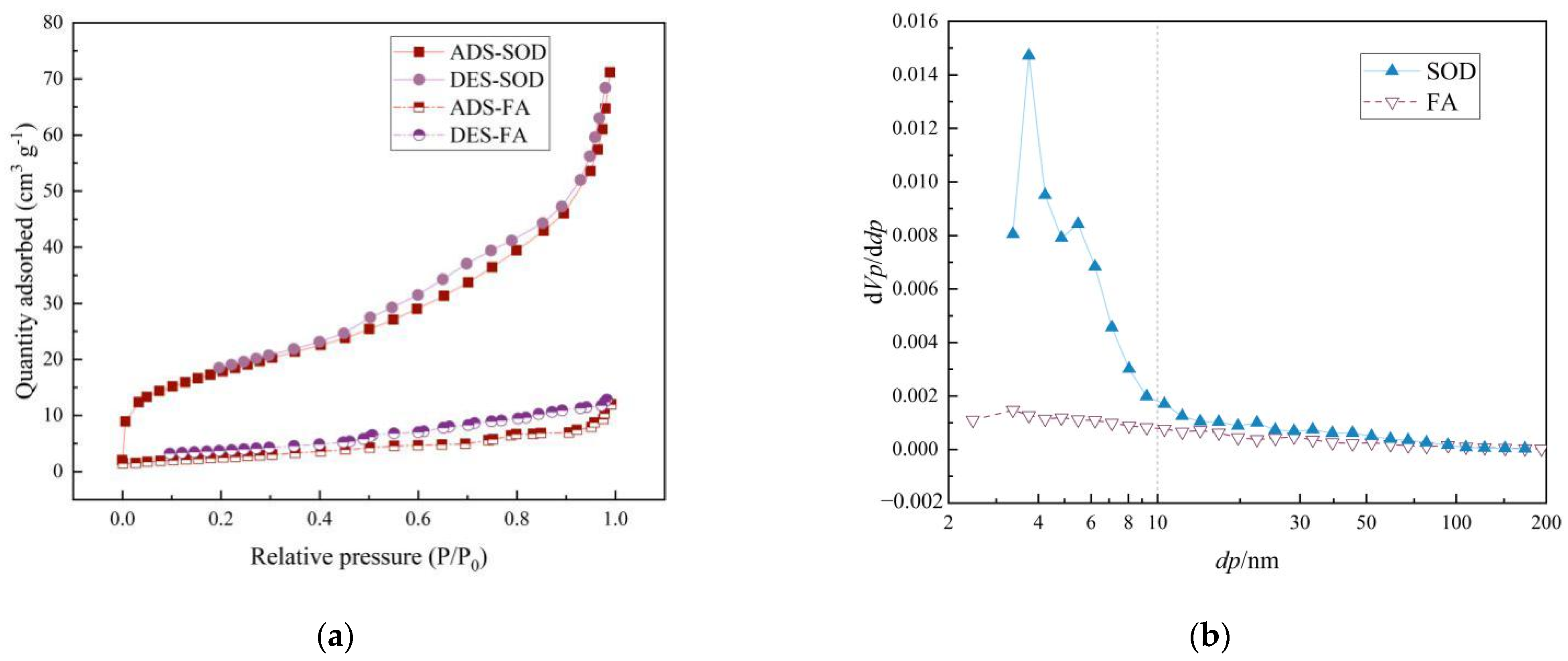
3.1. Characterization of FA and SOD
3.2. Effect of Dosage
3.3. Effect of Adsorption Time
3.4. Effect of pH
3.5. Effect of Initial Concentration
Adsorption Kinetics
3.6. Adsorption Isotherm
3.6.1. Langmuir Isotherm
3.6.2. Freundlich Isotherm
3.7. Adsorption Thermodynamics
3.8. Desorption and Regeneration Experiment
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhu, M.; Zhu, G.; Zhao, L.; Yao, X. Influence of algal bloom degradation on nutrient release at the sediment–water interface in Lake Taihu, China. Environ. Sci. Pollut. Res. 2012, 20, 1803–1811. [Google Scholar] [CrossRef] [PubMed]
- Zamparas, M.; Zacharias, I. Restoration of eutrophic freshwater by managing internal nutrient loads. A review. Sci. Total. Environ. 2014, 496, 551–562. [Google Scholar] [CrossRef] [PubMed]
- Cordell, D.; Rosemarin, A.; Schröder, J.J.; Smit, A.L. Towards global phosphorus security: A systems framework for phosphorus recovery and reuse options. Chemosphere 2011, 84, 747–758. [Google Scholar] [CrossRef]
- Yenigün, O.; Demirel, B. Ammonia inhibition in anaerobic digestion: A review. Process Biochem. 2013, 48, 901–911. [Google Scholar] [CrossRef]
- Song, Y.-H.; Qiu, G.-L.; Yuan, P.; Cui, X.-Y.; Peng, J.-F.; Zeng, P.; Duan, L.; Xiang, L.-C.; Qian, F. Nutrients removal and recovery from anaerobically digested swine wastewater by struvite crystallization without chemical additions. J. Hazard. Mater. 2011, 190, 140–149. [Google Scholar] [CrossRef]
- Du, R.; Cao, S.; Peng, Y.; Zhang, H.; Wang, S. Combined Partial Denitrification (PD)-Anammox: A method for high nitrate wastewater treatment. Environ. Int. 2019, 126, 707–716. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Lin, H.; Dong, Y.; Wang, L. Preferable adsorption of phosphate using lanthanum-incorporated porous zeolite: Characteristics and mechanism. Appl. Surf. Sci. 2017, 426, 995–1004. [Google Scholar] [CrossRef]
- Huang, J.; Kankanamge, N.R.; Chow, C.; Welsh, D.T.; Li, T.; Teasdale, P.R. Removing ammonium from water and wastewater using cost-effective adsorbents: A review. J. Environ. Sci. 2018, 63, 174–197. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Zhang, Y.; Li, D. Adsorptive removal of phosphate from water using mesoporous materials: A review. J. Environ. Manag. 2017, 193, 470–482. [Google Scholar] [CrossRef]
- Alshameri, A.; He, H.; Zhu, J.; Xi, Y.; Zhu, R.; Ma, L.; Tao, Q. Adsorption of ammonium by different natural clay minerals: Characterization, kinetics and adsorption isotherms. Appl. Clay Sci. 2018, 159, 83–93. [Google Scholar] [CrossRef]
- Abukhadra, M.R.; Mostafa, M. Effective decontamination of phosphate and ammonium utilizing novel muscovite/phillipsite composite; equilibrium investigation and realistic application. Sci. Total Environ. 2019, 667, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Yao, Z.T.; Ji, X.S.; Sarker, P.K.; Tang, J.H.; Ge, L.Q.; Xia, M.S.; Xi, Y.Q. A comprehensive review on the applications of coal fly ash. Earth Sci. Rev. 2015, 141, 105–121. [Google Scholar] [CrossRef] [Green Version]
- Blissett, R.S.; Rowson, N.A. A review of the multi-component utilisation of coal fly ash. Fuel 2012, 97, 1–23. [Google Scholar] [CrossRef]
- Belviso, C. State-of-the-art applications of fly ash from coal and biomass: A focus on zeolite synthesis processes and issues. Prog. Energy Combust. 2018, 65, 109–135. [Google Scholar] [CrossRef]
- Holler, H.; Wirsching, U. Zeolite formation from fly ash. Forschr. Mineral. 1985, 63, 21–27. [Google Scholar]
- Ji, X.D.; Zhang, M.L.; Ke, Y.Y.; Song, Y.C. Simultaneous immobilization of ammonium and phosphate from aqueous solution using zeolites synthesized from fly ashes. Water Sci. Technol. 2013, 67, 1324–1331. [Google Scholar] [CrossRef] [PubMed]
- You, X.; Valderrama, C.; Cortina, J.L. Simultaneous recovery of ammonium and phosphate from simulated treated wastewater effluents by activated calcium and magnesium zeolites. J. Chem. Technol. Biotechnol. 2017, 92, 2400–2409. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Xie, Q.; Chen, S.; Xing, M.; Guan, T.; Wu, D. Inactivation of phosphorus in the sediment of the Lake Taihu by lanthanum modified zeolite using laboratory studies. Environ. Pollut. 2019, 247, 9–17. [Google Scholar] [CrossRef]
- Ramanathan, S.; Gopinath, S.C.; Arshad, M.M.; Poopalan, P. Nanostruc tured Aluminosilicate from Fly Ash: Potential Approach in Waste Utilization for Industrial and Medical Applications. J. Clean. Prod. 2020, 253, 119923. [Google Scholar] [CrossRef]
- Pan, X.; Jiang, T.; Hou, X.; Wu, Y. Precipitation activity of sodium aluminosilicate hydrate during Bayer process. Chin. J. Nonferrous Met. 2017, 27, 1748–1755. [Google Scholar]
- Li, X.; Liu, J.; Wang, Y.; Zeng, L. Phase transformation of sodium hydrate alumino-silicate in alumina sintering process. Chin. J. Nonferrous Met. 2018, 28, 1225–1232. [Google Scholar]
- Williamson, T.; Katz, L.E.; Han, J.; Dobbs, H.A.; Chmelka, B.F.; Sant, G.; Juenger, M.C.G. Relationship between aqueous chemistry and composition, structure, and solubility of sodium aluminosilicate hydrates. J. Am. Ceram. Soc. 2019, 103, 2160–2172. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, M.; Xing, N.; Zou, J. Performance of nitrogen and phosphorus removal in petrochemical wastewater by fly ash synthetic zeolite. Chem. Eng. 2018, 46, 7–10. [Google Scholar]
- Vaughan, M.C.H.; Bowden, W.B.; Shanley, J.B.; Vermilyea, A.; Wemple, B.; Schroth, A.W. Using in situ UV-Visible spectrophotometer sensors to quantify riverine phosphorus partitioning and concentration at a high frequency. Limnol. Oceanogr. Methods 2018, 16, 840–855. [Google Scholar] [CrossRef]
- HJ636-2012. Water Quality—Determination of Total Nitrogen—Alkaline Potassium per Sulfate Digestion UV Spectrophotometric Method. Available online: https://max.book118.com/html/2019/0111/8076070007002001.shtm (accessed on 23 December 2019).
- GB11893-89. Water Quality—Determination of Total Phosphorus—Ammonium Molybdate Spectrophotometric Method. Available online: http://www.mee.gov.cn/image20010518/3655.pdf (accessed on 23 December 2019).
- Vassilev, S.V.; Baxter, D.; Andersen, L.K.; Vassileva, C.G. An overview of the composition and application of biomass ash. Part 1. Phase–mineral and chemical composition and classification. Fuel 2013, 105, 40–76. [Google Scholar] [CrossRef]
- Novembre, D.; di Sabatino, B.; Gimeno, D.; Pace, C. Synthesis and characterization of Na-X, Na-A and Na-P zeolites and hydroxysodalite from metakaolinite. Clay Miner. 2011, 46, 339–354. [Google Scholar] [CrossRef]
- Franus, W.; Wdowin, M.; Franus, M. Synthesis and characterization of zeolites prepared from industrial fly ash. Environ. Monit. Assess. 2014, 186, 5721–5729. [Google Scholar] [CrossRef] [Green Version]
- García-Lodeiro, I.; Fernández-Jiménez, A.; Palomo, A.; Macphee, D.E. Effect of Calcium Additions on N-A-S-H Cementitious Gels. J. Am. Ceram. Soc. 2010, 93, 1934–1940. [Google Scholar] [CrossRef]
- Malonda Shabani, J.; Babajide, O.; Oyekola, O.; Petrik, L. Synthesis of Hydroxy Sodalite from Coal Fly Ash for Biodiesel Production from Waste-Derived Maggot Oil. Catalysts 2019, 9, 1052. [Google Scholar] [CrossRef] [Green Version]
- Watanabe, Y.; Yamada, H.; Kokusen, H.; Tanaka, J.; Moriyoshi, Y.; Komatsu, Y. Ion Exchange Behavior of Natural Zeolites in Distilled Water, Hydrochloric Acid, and Ammonium Chloride Solution. Sep. Sci. Technol. 2003, 38, 1519–1532. [Google Scholar] [CrossRef]
- Wan, C.; Ding, S.; Zhang, C.; Tan, X.; Zou, W.; Liu, X.; Yang, X. Simultaneous recovery of nitrogen and phosphorus from sludge fermentation liquid by zeolite adsorption: Mechanism and application. Sep. Purif. Technol. 2017, 180, 1–12. [Google Scholar] [CrossRef]
- Cheng, Q.; Li, H.; Xu, Y.; Chen, S.; Liao, Y.; Deng, F.; Li, J. Study on the adsorption of nitrogen and phosphorus from biogas slurry by NaCl-modified zeolite. PLoS ONE 2017, 12, e0176109. [Google Scholar] [CrossRef]
- Guo, H.; Tang, L.; Yan, B.; Wan, K.; Li, P. NaA zeolite derived from blast furnace slag: Its application for ammonium removal. Water Sci. Technol. 2017, 76, 1140–1149. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yan, C.; Zhao, J.; Zhang, Z.; Wang, H.; Zhou, S.; Wu, L. Synthesis of zeolite P1 from fly ash under solvent-free conditions for ammonium removal from water. J. Clean. Prod. 2018, 202, 11–22. [Google Scholar] [CrossRef]
- Mitrogiannis, D.; Psychoyou, M.; Baziotis, I.; Inglezakis, V.J.; Koukouzas, N.; Tsoukalas, N.; Palles, D.; Kamitsos, E.; Oikonomou, G.; Markou, G. Removal of phosphate from aqueous solutions by adsorption onto Ca(OH)2 treated natural clinoptilolite. Chem. Eng. J. 2017, 320, 510–522. [Google Scholar] [CrossRef]
- Pan, M.; Lin, X.; Xie, J.; Huang, X. Kinetic, equilibrium and thermodynamic studies for phosphate adsorption on aluminum hydroxide modified palygorskite nano-composites. RSC Adv. 2017, 7, 4492–4500. [Google Scholar] [CrossRef] [Green Version]
- Luukkonen, T.; Věžníková, K.; Tolonen, E.-T.; Runtti, H.; Yliniemi, J.; Hu, T.; Lassi, U. Removal of ammonium from municipal wastewater with powdered and granulated metakaolin geopolymer. Environ. Technol. 2017, 39, 414–423. [Google Scholar] [CrossRef] [PubMed] [Green Version]













| Sample | Chemical Composition (wt%) | ||||||
|---|---|---|---|---|---|---|---|
| SiO2 | Al2O3 | CaO | Fe2O3 | MgO | Na2O | LOI | |
| FA | 41.31 | 34.30 | 9.41 | 7.00 | 1.54 | 0.31 | 1.33 |
| SOD | 31.11 | 30.87 | 7.15 | 5.74 | 1.06 | 11.21 | -- |
| Sample | Chemical Composition (wt%) | |||||||
|---|---|---|---|---|---|---|---|---|
| SiO2 | Al2O3 | CaO | Na2O | K2O | MgO | Fe2O3 | LOI | |
| 1 (CN = 25 mg/L) | 32.09 | 31.53 | 7.85 | 10.59 | 0.28 | 1.06 | 5.74 | -- |
| 2 (CN = 50 mg/L) | 32.52 | 31.55 | 7.96 | 9.38 | 0.29 | 1.14 | 5.82 | -- |
| 3 (CN = 100 mg/L) | 32.73 | 32.04 | 7.97 | 9.34 | 0.30 | 1.14 | 5.86 | -- |
| 4 (CN = 250 mg/L) | 32.55 | 31.96 | 7.96 | 9.30 | 0.29 | 1.15 | 5.89 | -- |
| 5 (CN = 500 mg/L) | 32.61 | 31.83 | 8.05 | 9.22 | 0.30 | 1.16 | 5.88 | -- |
| Adsorbate | Qe (mg/g) | Lagergren First-Order | Ho’s Pseudo-Second-Order | ||||
|---|---|---|---|---|---|---|---|
| R2 | K1 (min−1) | Qe,1 (mg/g) | R2 | K2 [g/(mg min)] | Qe,2 (mg/g) | ||
| Ammonium | 9.15 | 0.9604 | 0.0405 | 4.9499 | 0.9989 | 2.5056 | 9.3832 |
| Phosphate | 2.14 | 0.8107 | 0.0241 | 0.5147 | 0.9993 | 1.5163 | 2.1463 |
| Adsorbate | Temp (°C) | Langmuir Parameters | Freundlich Parameters | ||||
|---|---|---|---|---|---|---|---|
| Qm (mg/g) | b (L/mg) | R2 | Kf (mg·g−1) | nf | R2 | ||
| Ammonium | 25 | 17.2891 | 0.0462 | 0.9935 | 0.2491 | 0.9274 | 0.9995 |
| 35 | 28.6944 | 0.0858 | 0.9928 | 0.2622 | 0.9228 | 0.9989 | |
| 45 | 30.3122 | 0.0979 | 0.9938 | 0.2785 | 0.9163 | 0.9994 | |
| Phosphate | 25 | 6.8573 | 0.0180 | 0.9972 | 0.2491 | 0.9260 | 0.9991 |
| 35 | 10.3950 | 0.0610 | 0.9878 | 0.2516 | 0.9676 | 0.9998 | |
| 45 | 12.2911 | 0.0683 | 0.9946 | 0.2725 | 0.9527 | 0.9995 | |
| Adsorbent | ∆G (KJ·mol−1) | ∆H (KJ·mol−1) | ∆S (J·mol−1·K−1) | ||
|---|---|---|---|---|---|
| 298 K | 308 K | 318 K | |||
| Ammonium | −33.64 | −38.77 | −43.91 | 119.48 | 531.81 |
| Phosphate | −13.89 | −16.13 | −18.38 | 52.98 | 224.39 |
| Times | The Desorption Rate (%) | |
|---|---|---|
| Ammonium | Phosphate | |
| 1 | 0.2 | 0.1 |
| 2 | 0.2 | 0 |
| 3 | 0.1 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lv, P.; Meng, R.; Mao, Z.; Deng, M. Hydrothermal Synthesis of Sodalite-Type N-A-S-H from Fly Ash to Remove Ammonium and Phosphorus from Water. Materials 2021, 14, 2741. https://doi.org/10.3390/ma14112741
Lv P, Meng R, Mao Z, Deng M. Hydrothermal Synthesis of Sodalite-Type N-A-S-H from Fly Ash to Remove Ammonium and Phosphorus from Water. Materials. 2021; 14(11):2741. https://doi.org/10.3390/ma14112741
Chicago/Turabian StyleLv, Pengcheng, Ruihong Meng, Zhongyang Mao, and Min Deng. 2021. "Hydrothermal Synthesis of Sodalite-Type N-A-S-H from Fly Ash to Remove Ammonium and Phosphorus from Water" Materials 14, no. 11: 2741. https://doi.org/10.3390/ma14112741
APA StyleLv, P., Meng, R., Mao, Z., & Deng, M. (2021). Hydrothermal Synthesis of Sodalite-Type N-A-S-H from Fly Ash to Remove Ammonium and Phosphorus from Water. Materials, 14(11), 2741. https://doi.org/10.3390/ma14112741






