Chitosan-Coated Halloysite Nanotubes As Vehicle for Controlled Drug Delivery to MCF-7 Cancer Cells In Vitro
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
2.2. Drug Loading
2.3. Nitrogen Adsorption of Drug-Loaded Halloysite Nanotubes (DLHNTs)
2.4. Coated Halloysite Nanotubes (HNTs) Using Chitosan
2.5. Characterization
2.5.1. Thermogravimetric Analysis (TGA) of HNTs, DLHNTs and DLHNTs-CH
2.5.2. Fourier Transform Infrared Spectroscopy (FTIR) of HNTs, DLHNTs, and DLHNTs-CH
2.5.3. Scanning Electron Microscopy (SEM) Analysis of HNTs, DLHNTs, and DLHNTs-CH
2.5.4. Transmission Electron Microscope (TEM) of HNTs, DLHNTs, and DLHNTs-CH
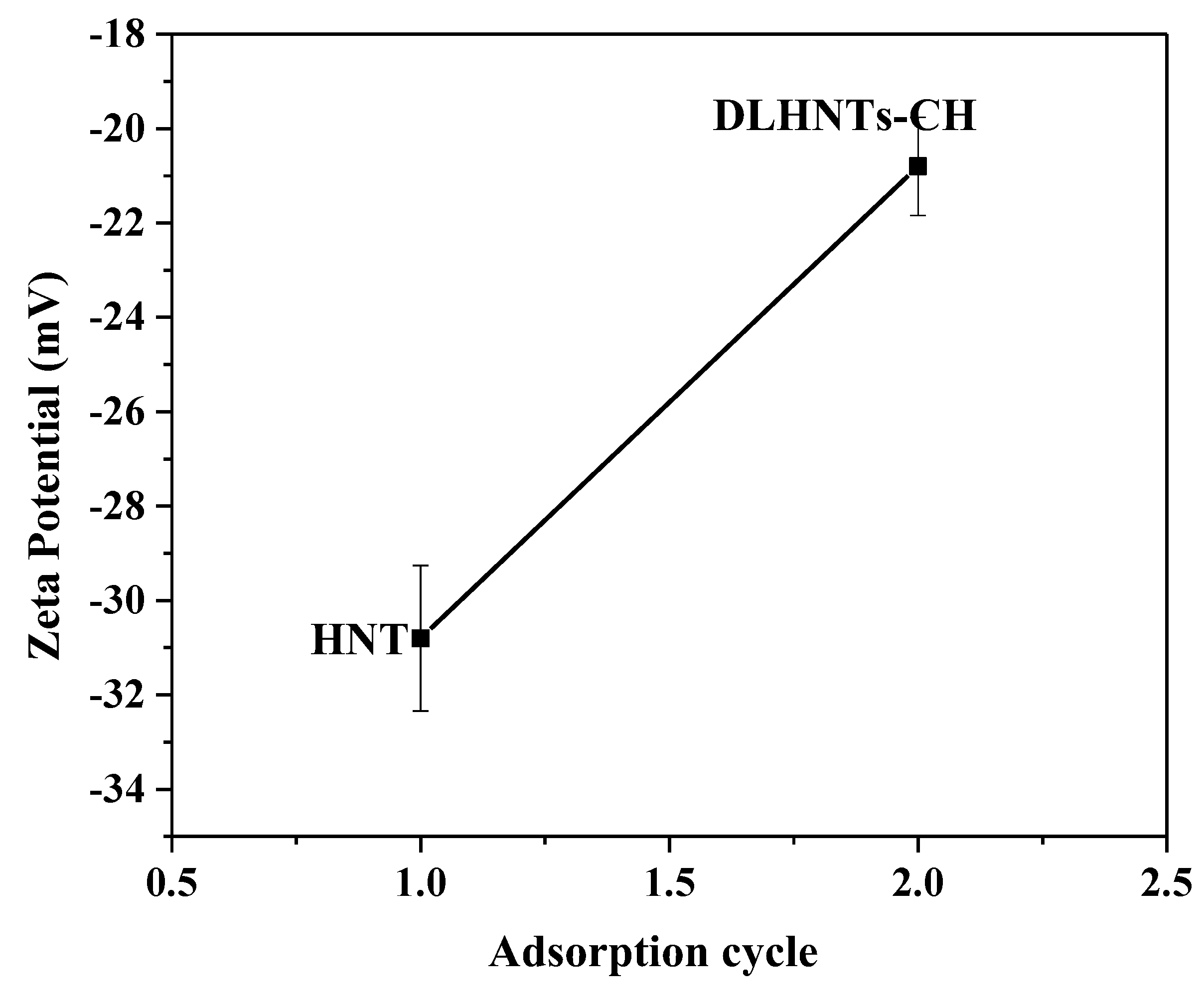
2.5.5. Zeta Potential Analysis of HNTs and DLHNTs-CH
2.6. In Vitro Curcumin Release from DLHNTs and DLHNTs-CH
2.7. Trypan Blue Exclusion Assay Studies
3. Results and Discussion
3.1. HNT Curcumin Loading and Entrapment
3.2. Nitrogen Adsorption of HNTs and DLDHNTs
3.3. Zeta Potential Measurements of HNTs and DLHNTs-CH
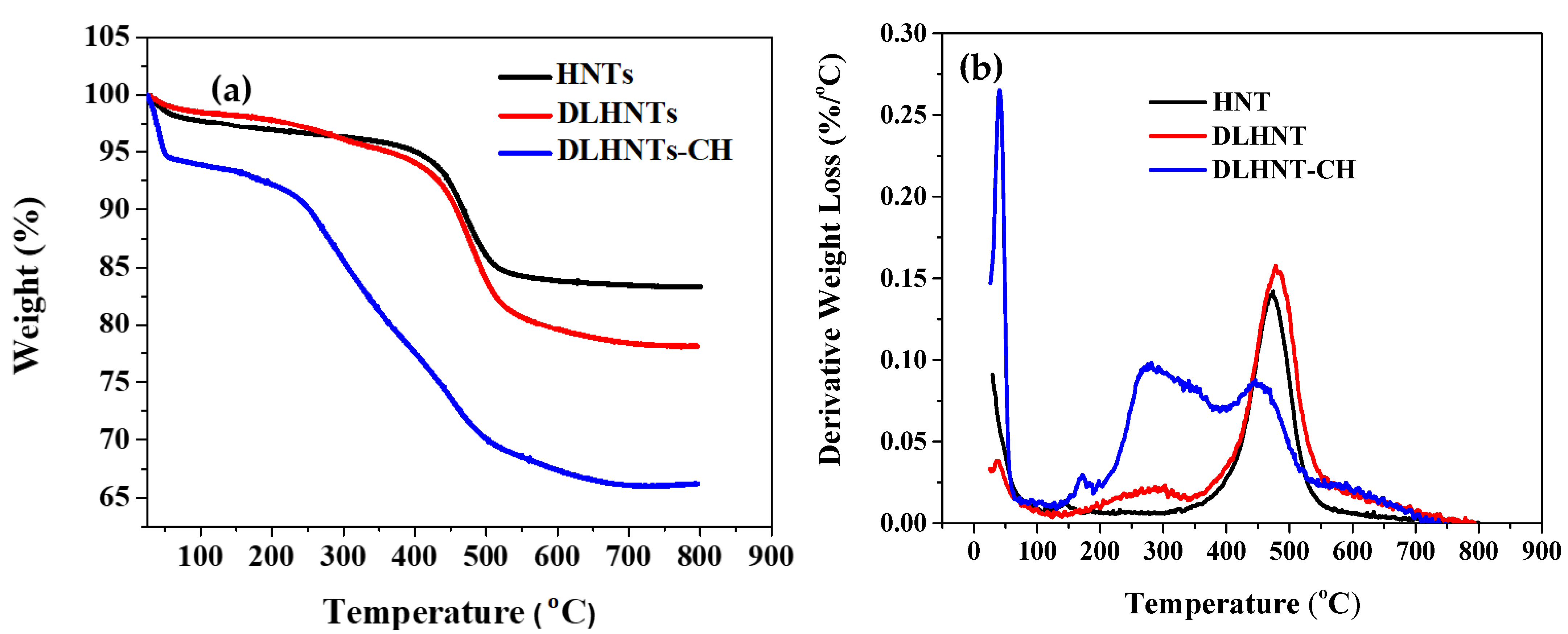
3.4. Thermogravimetric Analysis (TGA) of HNTs, DLHNTs, and DLHNTs-CH
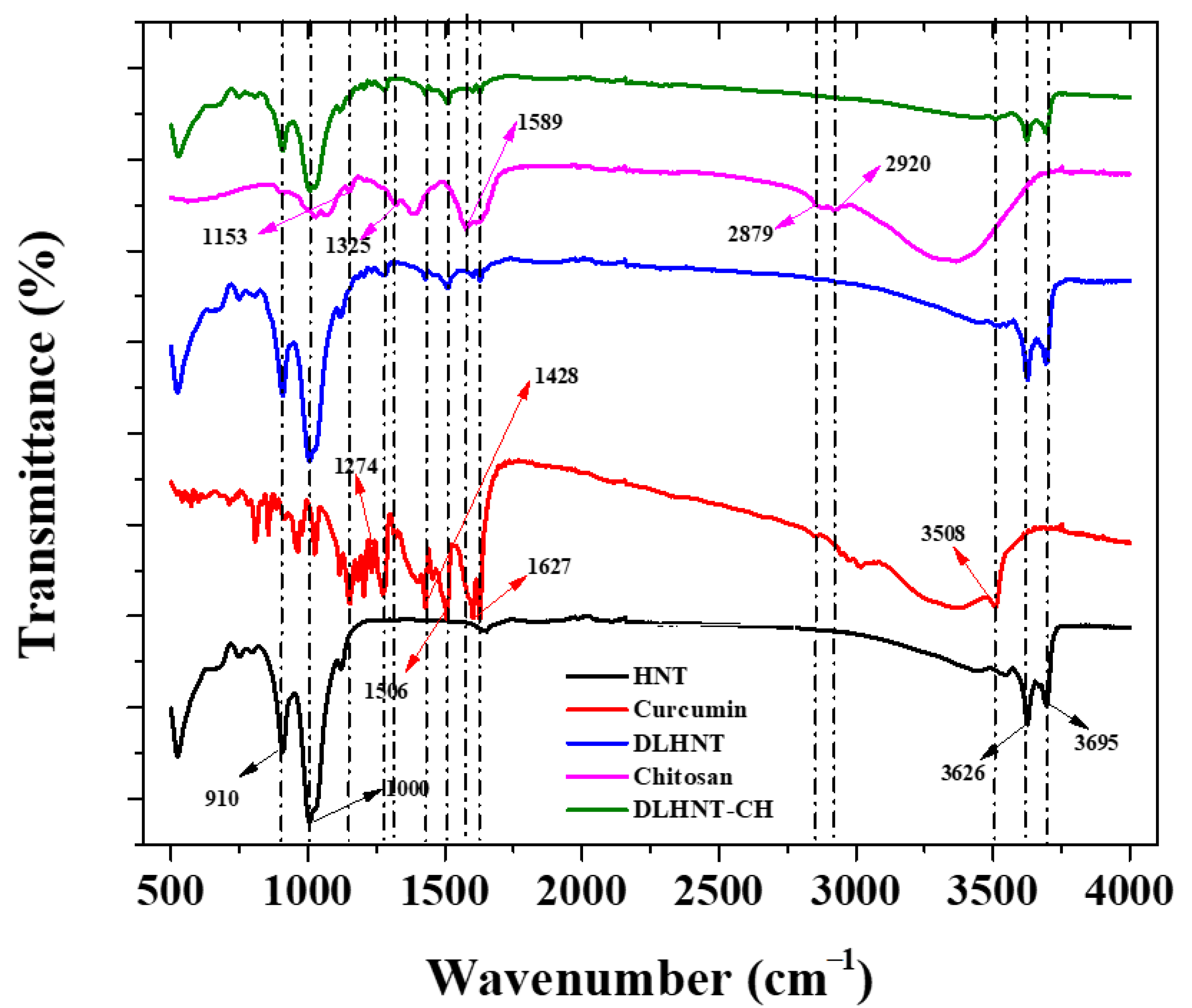
3.5. Fourier Transform Infrared Spectroscopy (FTIR)
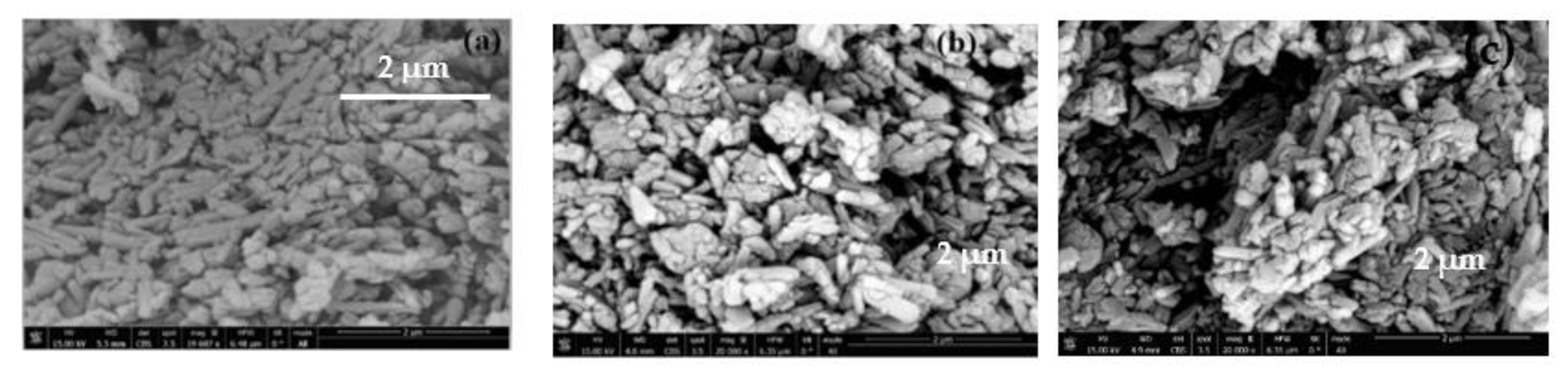
3.6. Scanning Electron Microscopy (SEM) Analysis
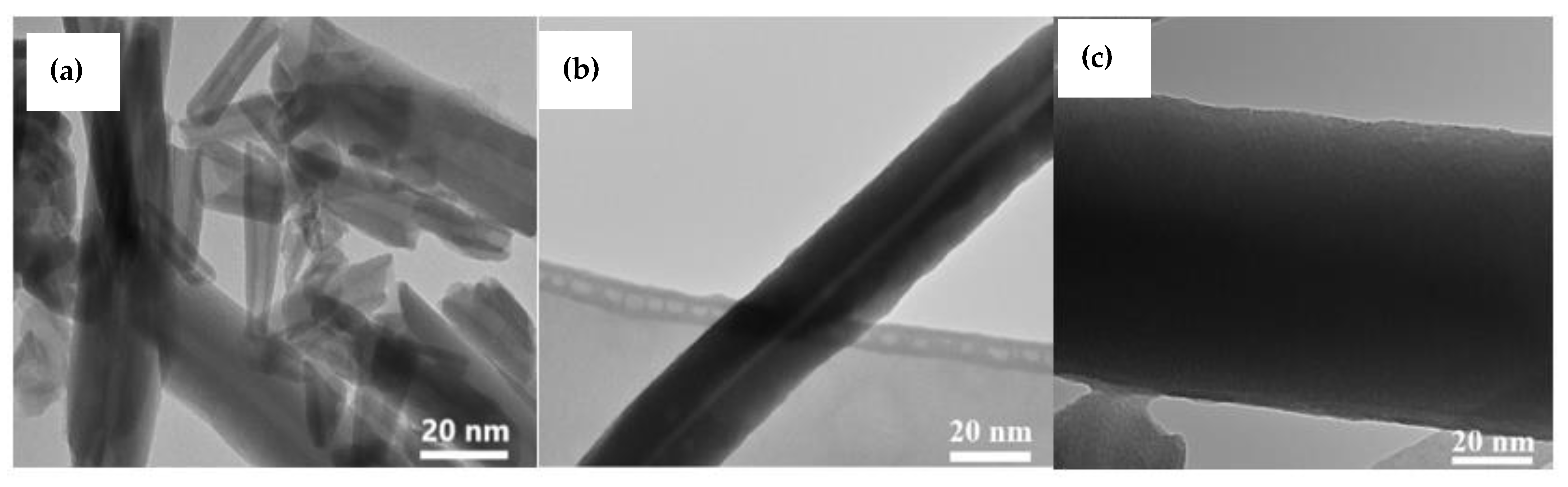
3.7. Transmission Electron Microscope (TEM)
3.8. In Vitro Curcumin Release
3.9. Curcumin Release Kinetic Profiles
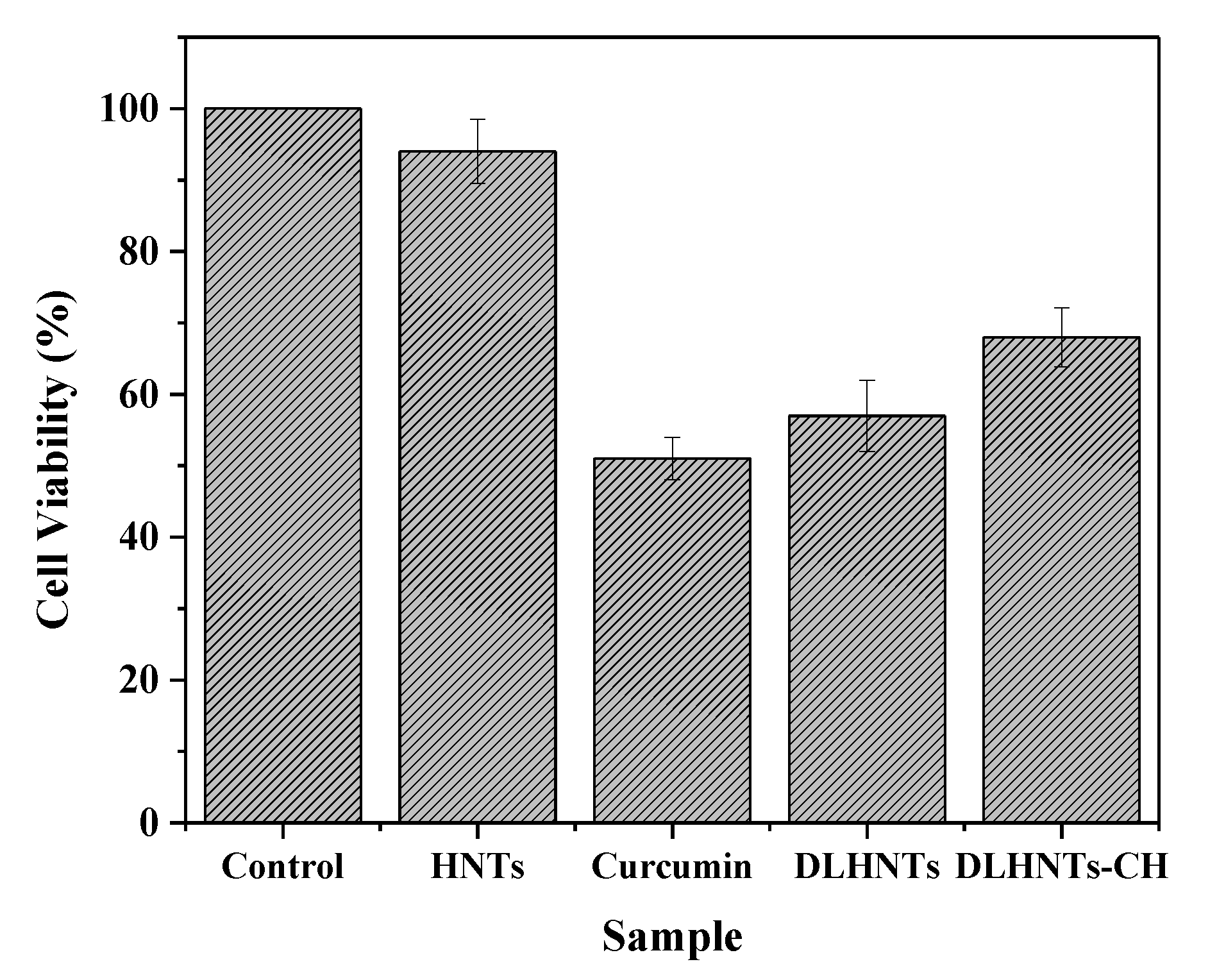
3.10. MTT Assay
3.11. Trypan Blue Exclusion Assay
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wild, C.P.; Stewart, B.W.; Wild, C. World Cancer Report 2014; World Health Organization: Geneva, Switzerland, 2014. [Google Scholar]
- WHO. WHO Report on Cancer: Setting Priorities, Investing Wisely and Providing Care for All; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- Howell, A.; Anderson, A.S.; Clarke, R.B.; Duffy, S.W.; Evans, D.G.; Garcia-Closas, M.; Gescher, A.J.; Key, T.J.; Saxton, J.M.; Harvie, M.N. Risk determination and prevention of breast cancer. Breast Cancer Res. 2014, 16, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Bonsu, A.B.; Ncama, P.B. Clinicians’ experiences and perspectives of breast cancer and possible integration of breast cancer prevention and early detection into palliative care. Int. J. Afr. Nurs. Sci. 2019, 11, 100162. [Google Scholar] [CrossRef]
- Cooper, G.M. Elements of Human Cancer; Jones & Bartlett Learning: Burlington, MA, USA, 1992. [Google Scholar]
- de Martel, C.; Ferlay, J.; Franceschi, S.; Vignat, J.; Bray, F.; Forman, D.; Plummer, M. Global burden of cancers attributable to infections in 2008: A review and synthetic analysis. Lancet Oncol. 2012, 13, 607–615. [Google Scholar] [CrossRef]
- Duncan, R. Polymer conjugates as anticancer nanomedicines. Nat. Rev. Cancer 2006, 6, 688–701. [Google Scholar] [CrossRef]
- Deshpande, A.A.; Rhodes, C.T.; Shah, N.H.; Malick, A.W. Controlled-release drug delivery systems for prolonged gastric residence: An overview. Drug Dev. Ind. Pharm. 1996, 22, 531–539. [Google Scholar] [CrossRef]
- Bediako, E.G.; Nyankson, E.; Dodoo-Arhin, D.; Agyei-Tuffour, B.; Łukowiec, D.; Tomiczek, B.; Yaya, A.; Efavi, J.K. Modified halloysite nanoclay as a vehicle for sustained drug delivery. Heliyon 2018, 4, e00689. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luten, J.; van Steenis, J.; van Someren, R.; Kemmink, J.; Schuurmans-Nieuwenbroek, N.; Koning, G.; Crommelin, D.; van Nostrum, C.; Hennink, W. Water-soluble biodegradable cationic polyphosphazenes for gene delivery. J. Control. Release 2003, 89, 483–497. [Google Scholar] [CrossRef]
- Liu, M.; Chang, Y.; Yang, J.; You, Y.; He, R.; Chen, T.; Zhou, C. Functionalized halloysite nanotube by chitosan grafting for drug delivery of curcumin to achieve enhanced anticancer efficacy. J. Mater. Chem. B 2016, 4, 2253–2263. [Google Scholar] [CrossRef]
- Rawtani, D.; Agrawal, Y. Multifarious applications of halloysite nanotubes: A review. Rev. Adv. Mater. Sci. 2012, 30, 282–295. [Google Scholar]
- Vergaro, V.; Lvov, Y.M.; Leporatti, S. Halloysite clay nanotubes for resveratrol delivery to cancer cells. Macromol. Biosci. 2012, 12, 1265–1271. [Google Scholar] [CrossRef]
- Allen, T.M.; Cullis, P.R. Drug delivery systems: Entering the mainstream. Science 2004, 303, 1818–1822. [Google Scholar] [CrossRef] [Green Version]
- Iliescu, R.I.; Andronescu, E.; Ghitulica, C.D.; Voicu, G.; Ficai, A.; Hoteteu, M. Montmorillonite–alginate nanocomposite as a drug delivery system–incorporation and in vitro re-lease of irinotecan. Int. J. Pharm. 2014, 463, 184–192. [Google Scholar] [CrossRef] [PubMed]
- Arruebo, M. Drug delivery from structured porous inorganic materials. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2011, 4, 16–30. [Google Scholar] [CrossRef]
- Kamoun, E.A.; Kenawy, E.-R.S.; Chen, X. A review on polymeric hydrogel membranes for wound dressing applications: PVA-based hydrogel dressings. J. Adv. Res. 2017, 8, 217–233. [Google Scholar] [CrossRef]
- Prencipe, G.; Tabakman, S.M.; Welsher, K.; Liu, Z.; Goodwin, A.P.; Zhang, L.; Henry, J.; Dai, H. PEG branched polymer for functionalization of nanomaterials with ultralong blood circulation. J. Am. Chem. Soc. 2009, 131, 4783–4787. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alexis, F.; Pridgen, E.; Molnar, L.K.; Farokhzad, O.C. Factors affecting the clearance and biodistribution of polymeric nanoparticles. Mol. Pharm. 2008, 5, 505–515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, M.; Wang, A.; Muhammad, F.; Qi, W.; Ren, H.; Guo, Y.; Zhu, G. Halloysite nanotubes, a multifunctional nanovehicle for anticancer drug delivery. Chin. J. Chem. 2012, 30, 2115–2120. [Google Scholar] [CrossRef]
- Price, R.; Gaber, B.G.; Lvov, Y. In-vitro release characteristics of tetracycline HCl, khellin and nicotinamide adenine dine-culeotide from halloysite: A cylindrical mineral. J. Microencapsul. 2001, 18, 713–722. [Google Scholar]
- Lvov, Y.M.; Shchukin, D.G.; Möhwald, H.; Price, R.R. Halloysite clay nanotubes for controlled release of protective agents. ACS Nano 2008, 2, 814–820. [Google Scholar] [CrossRef]
- Yoo, H.S.; Park, T.G. Folate-receptor-targeted delivery of doxorubicin nano-aggregates stabilized by doxorubicin–PEG–folate conjugate. J. Control. Release 2004, 100, 247–256. [Google Scholar] [CrossRef]
- Nyankson, E.; Kumar, R.V. Removal of water-soluble dyes and pharmaceutical wastes by combining the photocatalytic properties of Ag3PO4 with the adsorption properties of halloysite nanotubes. Mater. Today Adv. 2019, 4, 100025. [Google Scholar] [CrossRef]
- Nyankson, E.; Agyei-Tuffour, B.; Adjasoo, J.; Ebenezer, A.; Dodoo-Arhin, D.; Yaya, A.; Mensah, B.; Efavi, J.K. Synthesis and application of fe-doped TiO2-halloysite nanotubes composite and their potential application in water treatment. Adv. Mater. Sci. Eng. 2019, 2019, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Owoseni, O.; Nyankson, E.; Zhang, Y.; Adams, S.J.; He, J.; McPherson, G.L.; Bose, A.; Gupta, R.B.; John, V.T. Release of surfactant cargo from interfacially-active halloysite clay nanotubes for oil spill remediation. Langmuir 2014, 30, 13533–13541. [Google Scholar] [CrossRef] [PubMed]
- Nyankson, E.; Olasehinde, O.; John, V.T.; Gupta, R.B. Surfactant-loaded halloysite clay nanotube dispersants for crude oil spill remediation. Ind. Eng. Chem. Res. 2015, 54, 9328–9341. [Google Scholar] [CrossRef]
- Owoseni, O.; Nyankson, E.; Zhang, Y.; Adams, S.J.; He, J.; Spinu, L.; McPherson, G.L.; Bose, A.; Gupta, R.B.; John, V.T. Interfacial adsorption and surfactant release characteristics of magnetically functionalized halloysite nanotubes for responsive emulsions. J. Colloid Interface Sci. 2016, 463, 288–298. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, M.; Zheng, Y.-G.; Tan, H.; Hsu, B.Y.-W.; Yang, Z.-C.; Wong, S.Y.; Chang, A.Y.-C.; Choolani, M.; Li, X.; et al. PEOlated micelle/silica as dual-layer protection of quantum dots for stable and targeted bioimaging. Chem. Mater. 2013, 25, 2976–2985. [Google Scholar] [CrossRef]
- He, L.; Chen, T.; You, Y.; Hu, H.; Zheng, W.; Kwong, W.-L.; Zou, T.; Che, C.-M. A cancer-targeted nanosystem for delivery of gold(III) complexes: Enhanced selectivity and apoptosis-inducing efficacy of a gold(III) porphyrin complex. Angew. Chem. 2014, 126, 12740–12744. [Google Scholar] [CrossRef]
- Zhao, F.; Grayson, W.L.; Ma, T.; Bunnell, B.; Lu, W.W. Effects of hydroxyapatite in 3-D chitosan–gelatin polymer network on human mesenchymal stem cell construct development. Biomaterials 2006, 27, 1859–1867. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.F.; Tian, Z.; Zhang, Y.; Shen, H.B.; Jia, N.Q. Functionalized halloysite nanotube-based carrier for intracellular delivery of antisense oligonucleotides. Nanoscale Res. Lett. 2011, 6, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Veerabadran, N.G.; Mongayt, D.; Torchilin, V.; Price, R.R.; Lvov, Y.M. Organized shells on clay nanotubes for controlled release of macromolecules. Macromol. Rapid Commun. 2009, 30, 99–103. [Google Scholar] [CrossRef]
- Vergaro, V.; Abdullayev, E.; Lvov, Y.M.; Zeitoun, A.; Cingolani, R.; Rinaldi, R.; Leporatti, S. Cytocompatibility and uptake of halloysite clay nanotubes. Biomacromolecules 2010, 11, 820–826. [Google Scholar] [CrossRef]
- White, R.D.; Bavykin, D.V.; Walsh, F.C. The stability of halloysite nanotubes in acidic and alkaline aqueous suspensions. Nanotechnology 2012, 23, 065705. [Google Scholar] [CrossRef] [PubMed]
- Del Rey-Bueno, F.; Romero-Carballo, J.; Villafranca-Sánchez, E.; Garcia-Rodriguez, A.; Sebastian-Pardo, E. Adsorption of ammonia over halloysite activated at different temperatures. Mater. Chem. Phys. 1989, 21, 67–84. [Google Scholar] [CrossRef]
- Molaei, A.; Amadeh, A.; Yari, M.; Afshar, M.R. Structure, apatite inducing ability, and corrosion behavior of chitosan/halloysite nanotube coatings prepared by electrophoretic deposition on titanium substrate. Mater. Sci. Eng. C 2016, 59, 740–747. [Google Scholar] [CrossRef] [PubMed]
- Bordeepong, S.; Bhongsuwan, D.; Pungrassami, T.; Bhongsuwan, T. Characterization of halloysite from Thung Yai District, Nakhon Si Thammarat Province, in Southern Thailand. Songklanakarin J. Sci. Technol. 2011, 33, 599–607. [Google Scholar]
- Yuan, P.; Southon, P.D.; Liu, Z.; Green, M.E.R.; Hook, J.M.; Antill, S.J.; Kepert, C.J. Functionalization of halloysite clay nanotubes by grafting with γ-aminopropyltriethoxysilane. J. Phys. Chem. C 2008, 112, 15742–15751. [Google Scholar] [CrossRef]
- Elhefian, E.A.; Nasef, M.M.; Yahaya, A.H. Preparation and characterization of chitosan/agar blended films: Part 2. Thermal, mechanical, and surface properties. J. Chem. 2012, 9, 510–516. [Google Scholar] [CrossRef]
- Szczepanik, B.; Słomkiewicz, P.; Garnuszek, M.; Czech, K.; Banaś, D.; Kubala-Kukuś, A.; Stabrawa, I. The effect of chemical modification on the physico-chemical characteristics of halloysite: FTIR, XRF, and XRD studies. J. Mol. Struct. 2015, 1084, 16–22. [Google Scholar] [CrossRef]
- Barman, M.; Mahmood, S.; Augustine, R.; Hasan, A.; Thomas, S.; Ghosal, K. Natural halloysite nanotubes/chitosan based bio-nanocomposite for delivering norfloxacin, an anti-microbial agent in sustained release manner. Int. J. Biol. Macromol. 2020, 162, 1849–1861. [Google Scholar] [CrossRef]
- Chen, X.; Zou, L.-Q.; Niu, J.; Liu, W.; Peng, S.-F.; Liu, C.-M. The stability, sustained release and cellular antioxidant activity of curcumin nanolposomes. Molecules 2015, 20, 14293–14311. [Google Scholar] [CrossRef] [Green Version]
- Senol-Arslan, D. Isotherms, kinetics and thermodynamics of ph(II) adsorption by crosslinked chitosan/sepiolite composite. Polym. Bull. 2021, 1–18. [Google Scholar] [CrossRef]
- Jing, H.; Higaki, Y.; Ma, W.; Wu, H.; Yah, W.O.; Otsuka, H.; Lvov, Y.M.; Takahara, A. Internally modified halloysite nanotubes as inorganic nanocontainers for a flame retardant. Chem. Lett. 2013, 42, 121–123. [Google Scholar] [CrossRef] [Green Version]
- Shaikh, H.K.; Kshirsagar, R.; Patil, S. Mathematical models for drug release characterization: A review. World J. Pharm. Pharm. Sci. 2015, 4, 324–338. [Google Scholar]
- Shehzad, A.; Lee, J.; Lee, Y.S. Curcumin in various cancers. BioFactors 2013, 39, 56–68. [Google Scholar] [CrossRef] [PubMed]
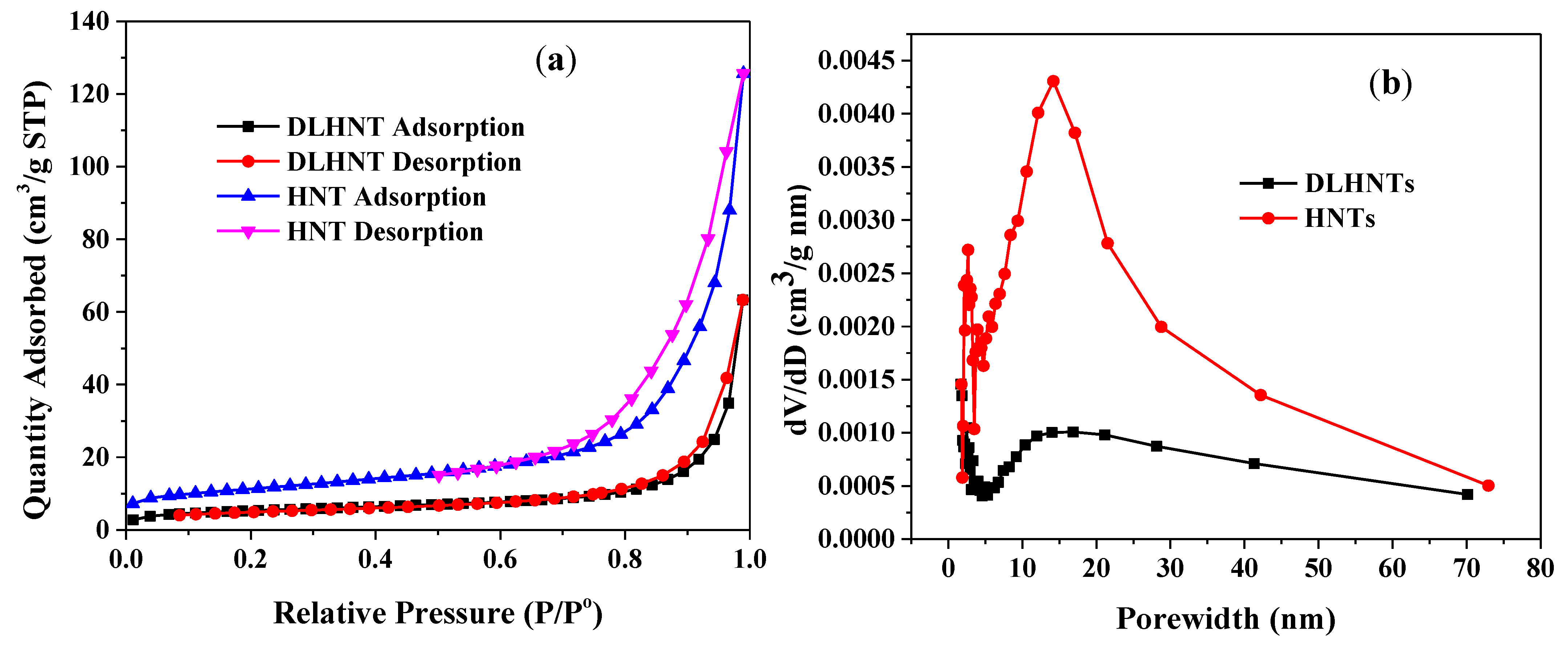




| Material | Surface Area (m2/g) | Pore Volume (cm3/g) |
|---|---|---|
| HNT | 38.95 | 0.11 |
| DLHNT | 18.21 | 0.04 |
| Sample | Temperature (°C) | Weight Loss (%) |
|---|---|---|
| HNTs | 463 | ~10 |
| DLHNTs | 283 | ~3.5 |
| 464 | ~10.8 | |
| DLHNTs-CH | 42 | ~3.5 |
| 282 | ~12.7 | |
| 463 | ~27.4 |
| Model | Parameter | DLHNTs | DLHNTs-CH |
|---|---|---|---|
| Zero-Order C = Kot | R2 | 0.632 | 0.752 |
| Ko | 0.17 | 0.14 | |
| First-Order Log Co − Log Ct = K1t/2.303 | R2 | 0.658 | 0.731 |
| K1 | −8.03 × 10−5 | −3.24 × 10−4 | |
| Higuchi Log Q = Log KH + 1/2 Log t | R2 | 0.752 | 0.603 |
| KH | 6.23 | 2.44 | |
| Korsmeyer-Peppas Log (Mt) = Log Kkp + n Log t | R2 | 0.819 | 0.946 |
| Kkp | −0.45 | −1.04 | |
| n | 0.031 | 0.249 | |
| Hixson–Crowell model Q01/3 − Qt1/3 = KHCt | R2 | 0.645 | 0.642 |
| KHC | 3.11× 10−4 | 0.002 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nyankson, E.; Aboagye, S.O.; Efavi, J.K.; Agyei-Tuffour, B.; Paemka, L.; Asimeng, B.O.; Balapangu, S.; Arthur, P.K.; Tiburu, E.K. Chitosan-Coated Halloysite Nanotubes As Vehicle for Controlled Drug Delivery to MCF-7 Cancer Cells In Vitro. Materials 2021, 14, 2837. https://doi.org/10.3390/ma14112837
Nyankson E, Aboagye SO, Efavi JK, Agyei-Tuffour B, Paemka L, Asimeng BO, Balapangu S, Arthur PK, Tiburu EK. Chitosan-Coated Halloysite Nanotubes As Vehicle for Controlled Drug Delivery to MCF-7 Cancer Cells In Vitro. Materials. 2021; 14(11):2837. https://doi.org/10.3390/ma14112837
Chicago/Turabian StyleNyankson, Emmanuel, Shadrack O. Aboagye, Johnson Kwame Efavi, Benjamin Agyei-Tuffour, Lily Paemka, Bernard O. Asimeng, Srinivasan Balapangu, Patrick K. Arthur, and Elvis K. Tiburu. 2021. "Chitosan-Coated Halloysite Nanotubes As Vehicle for Controlled Drug Delivery to MCF-7 Cancer Cells In Vitro" Materials 14, no. 11: 2837. https://doi.org/10.3390/ma14112837







