DABCO Derived Nitrogen-Doped Carbon Nanotubes for Oxygen Reduction Reaction (ORR) and Removal of Hexavalent Chromium from Contaminated Water
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
2.2. Methods
2.3. In-Situ Synthesis of Graphene-CNT Hybrids
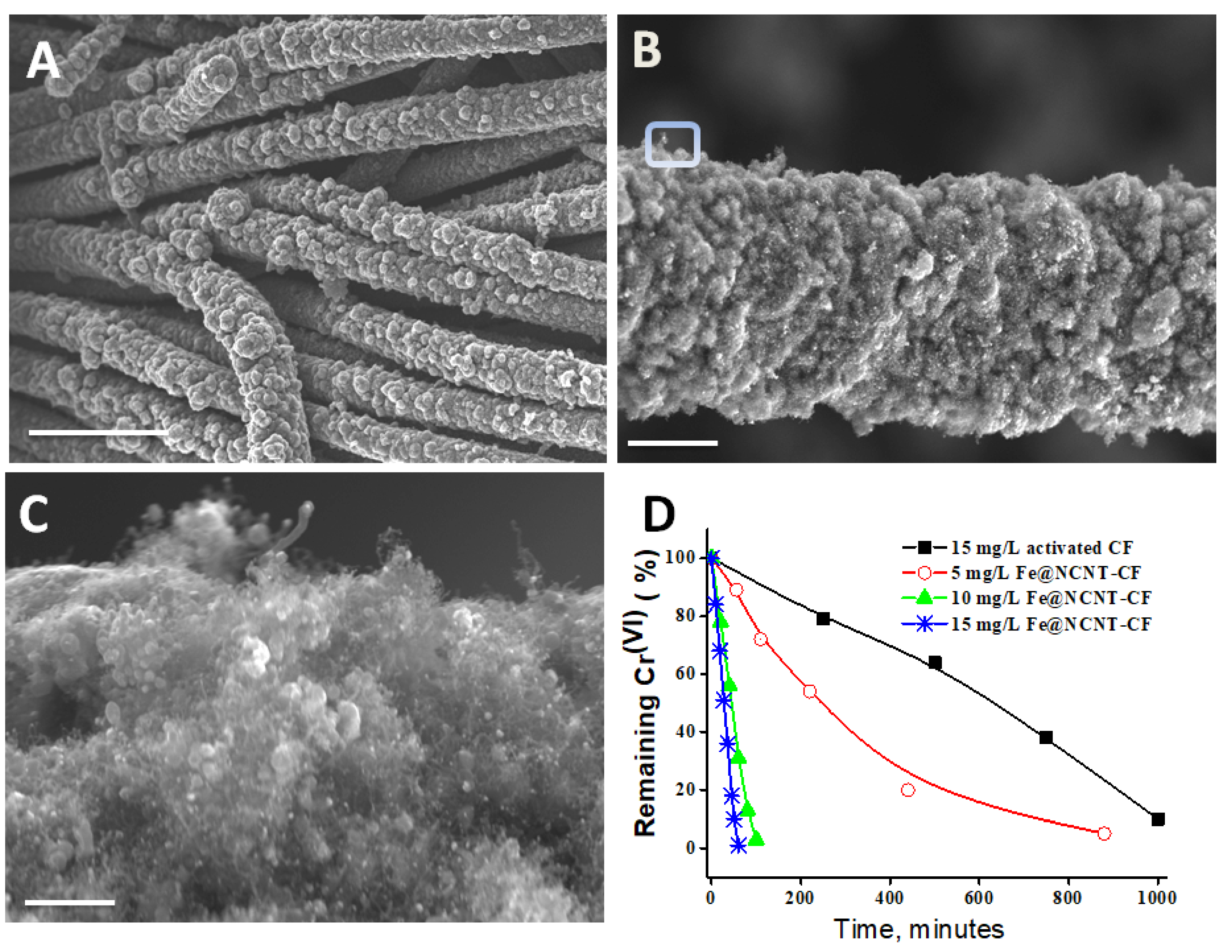
2.4. Chromium (Cr(VI)) Adsorption Studies
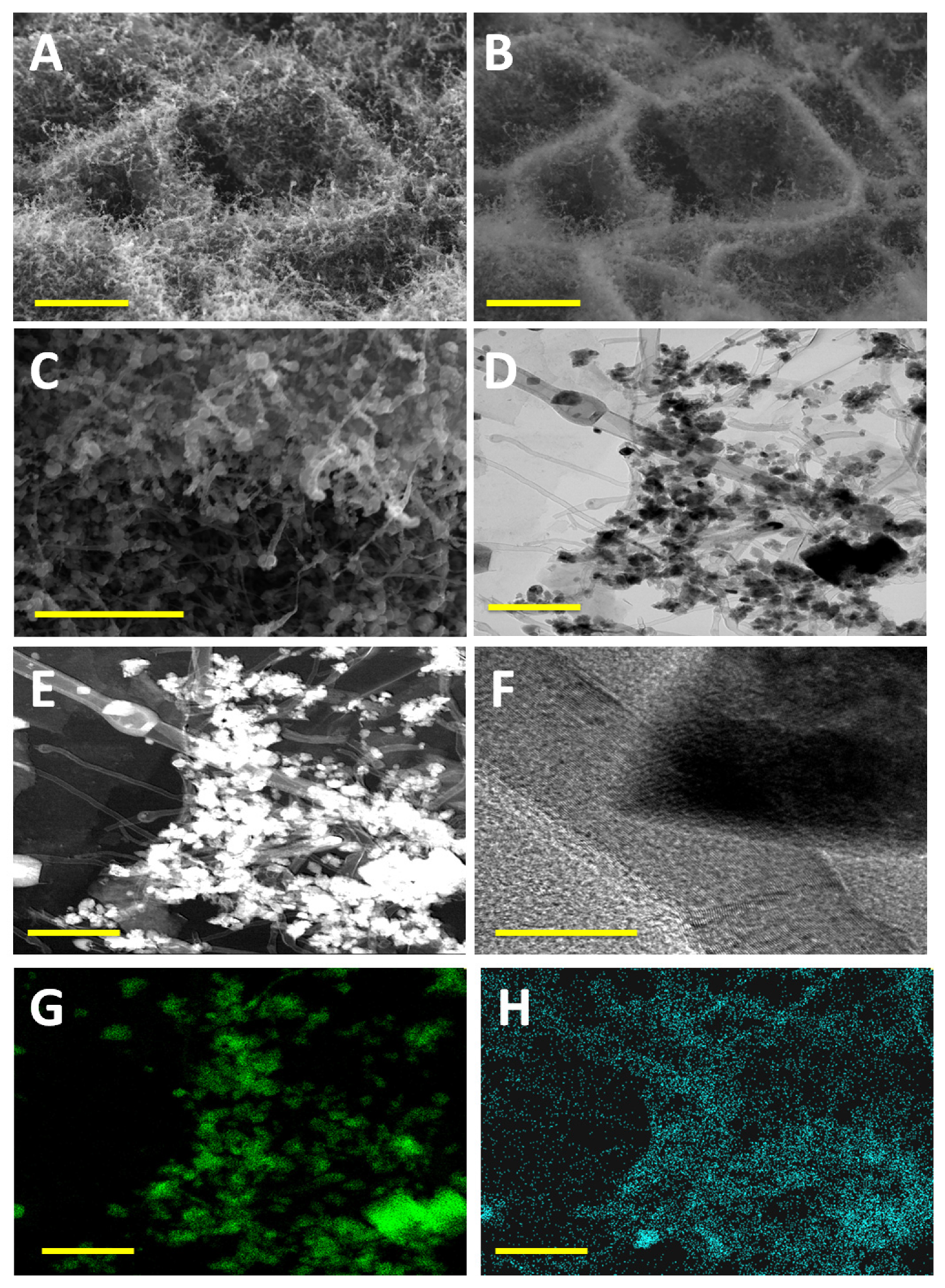
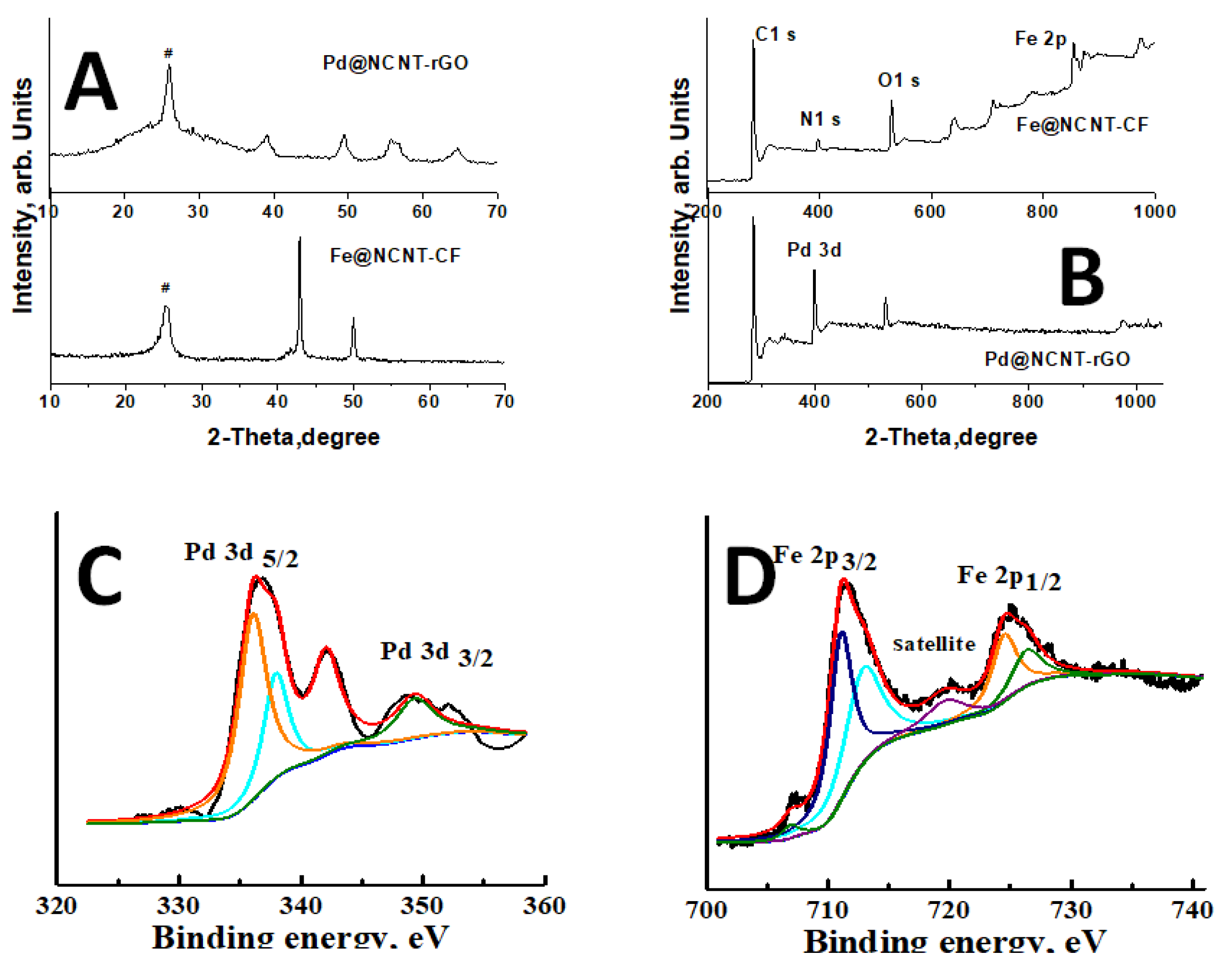
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, J.; Fan, X.; Sun, C.Q.; Zhu, W. DFT Study on Intermetallic Pd–Cu Alloy with Cover Layer Pd as Efficient Catalyst for Oxygen Reduction Reaction. Materials 2018, 11, 33. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Wang, P.; Luo, L.; Liu, L.; Wang, F. Green Synthesis of Fluorescent Palladium Nanoclusters. Materials 2018, 11, 191. [Google Scholar] [CrossRef]
- Mohamed Aslam, N.; Tsujiguchi, T.; Osaka, Y.; Kodama, A. The Origins of the High Performance of Pd Catalysts Supported on Carbon Black-Embedded Carbon Nanofiber for Formic Acid Oxidation. Appl. Sci. 2019, 9, 5542. [Google Scholar] [CrossRef]
- Truong-Phuoc, L.; Pham-Huu, C.; Da Costa, V.; Janowska, I. Few-layered graphene-supported palladium as a highly efficient catalyst in oxygen reduction reaction. Chem. Commun. 2014, 50, 14433–14435. [Google Scholar] [CrossRef] [PubMed]
- Arrigo, R.; Schuster, M.E.; Xie, Z.; Yi, Y.; Wowsnick, G.; Sun, L.L.; Hermann, K.E.; Friedrich, M.; Kast, P.; Hävecker, M.; et al. Nature of the N-Pd Interaction in Nitrogen-Doped Carbon Nanotube Catalysts. ACS Catal. 2015, 5, 2740–2753. [Google Scholar] [CrossRef]
- Mahy, J.G.; Sotrez, V.; Tasseroul, L.; Hermans, S.; Lambert, S.D. Activation Treatments and SiO2/Pd Modification of Sol–Gel TiO2 Photocatalysts for Enhanced Photoactivity under UV Radiation. Catalysts 2020, 10, 1184. [Google Scholar] [CrossRef]
- Zingwe, N.; Meyer, E.; Mbese, J. Synthesis, Characterization and Optimization of Hydrothermally Fabricated Binary Palladium Alloys PdNix for Use as Counter Electrode Catalysts in Dye Sensitized Solar Cells. Materials 2019, 12, 3116. [Google Scholar] [CrossRef]
- Zhao, Z.; Wei, J.; Li, F.; Qu, X.; Shi, L.; Zhang, H.; Yu, Q. Synthesis, Characterization and Hexavalent Chromium Adsorption Characteristics of Aluminum- and Sucrose-Incorporated Tobermorite. Materials 2017, 10, 597. [Google Scholar] [CrossRef]
- Bae, S.; Hikaru, F.; Kanematsu, M.; Yoshizawa, C.; Noguchi, T.; Yu, Y.; Ha, J. Removal of Hexavalent Chromium in Portland Cement Using Ground Granulated Blast-Furnace Slag Powder. Materials 2018, 11, 11. [Google Scholar] [CrossRef]
- Peng, H.; Leng, Y.; Cheng, Q.; Shang, Q.; Shu, J.; Guo, J. Efficient Removal of Hexavalent Chromium from Wastewater with Electro-Reduction. Processes 2019, 7, 41. [Google Scholar] [CrossRef]
- Shi, D.; Ouyang, Z.; Zhao, Y.; Xiong, J.; Shi, X. Catalytic Reduction of Hexavalent Chromium Using Iron/Palladium Bimetallic Nanoparticle-Assembled Filter Paper. Nanomaterials 2019, 9, 1183. [Google Scholar] [CrossRef]
- Xing, Y.; Chen, X.; Wang, D. Electrically Regenerated Ion Exchange for Removal and Recovery of Cr(VI) from Wastewater. Environ. Sci. Technol. 2007, 41, 1439–1443. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, Z.; Hursthouse, A.; Ren, B. Gemini Surfactant-Modified Activated Carbon for Remediation of Hexavalent Chromium from Water. Water 2018, 10, 91. [Google Scholar] [CrossRef]
- Qiu, B.; Xu, C.; Sun, D.; Wei, H.; Zhang, X.; Guo, J.; Wang, Q.; Rutman, D.; Guo, Z.; Wei, S. Polyaniline coating on carbon fiber fabrics for improved hexavalent chromium removal. RSC Adv. 2014, 4, 29855–29865. [Google Scholar] [CrossRef]
- Ahmad, W.; Qaiser, S.; Ullah, R.; Mohamed Jan, B.; Karakassides, M.A.; Salmas, C.E.; Kenanakis, G.; Ikram, R. Utilization of Tires Waste-Derived Magnetic-Activated Carbon for the Removal of Hexavalent Chromium from Wastewater. Materials 2021, 14, 34. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Yu, W.; Liu, S.; Xu, C.; Li, J.; Zhang, Y. Adsorption of Hexavalent Chromium Using Banana Pseudostem Biochar and Its Mechanism. Sustainability 2018, 10, 4250. [Google Scholar] [CrossRef]
- Wu, Y.; Luo, H.; Wang, H.; Wang, C.; Zhang, J.; Zhang, Z. Adsorption of hexavalent chromium from aqueous solutions by graphene modified with cetyltrimethylammonium bromide. J. Colloid Interface Sci. 2013, 394, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yu, C.; Wang, C.; Wang, Z.; Qiu, J. Chemically converting graphene oxide to graphene with organic base for Suzuki reaction. Mater. Res. Bull. 2015, 67, 77–82. [Google Scholar] [CrossRef]
- Karousis, N.; Tsotsou, G.-E.; Evangelista, F.; Rudolf, P.; Ragoussis, N.; Tagmatarchis, N. Carbon Nanotubes Decorated with Palladium Nanoparticles: Synthesis, Characterization, and Catalytic Activity. J. Phys. Chem. C 2008, 112, 13463–13469. [Google Scholar] [CrossRef]
- Rahimian-Koloor, S.M.; Hashemianzadeh, S.M.; Shokrieh, M.M. Effect of CNT structural defects on the mechanical properties of CNT/Epoxy nanocomposite. Phys. B Condens. Matter 2018, 540, 16–25. [Google Scholar] [CrossRef]
- Sridhar, V.; Park, H. Microwave induced transformation of metal organic frameworks into defect rich carbon nanofibers. New J. Chem. 2020, 44, 5666–5672. [Google Scholar] [CrossRef]
- Jourdain, V.; Bichara, C. Current Understanding of the Growth of Carbon Nanotubes in Catalytic Chemical Vapour Deposition. Carbon 2013, 58, 2–39. [Google Scholar] [CrossRef]
- Sridhar, V.; Park, H. Zeolitic imidazolate frameworks as novel precursors for microwave synthesis of carbon nanotubes. J. Alloys Compd. 2019, 781, 166–173. [Google Scholar] [CrossRef]
- Veith, G.M.; Lupini, A.R.; Baggetto, L.; Browning, J.F.; Keum, J.K.; Villa, A.; Prati, L.; Papandrew, A.B.; Goenaga, G.A.; Mullins, D.R.; et al. Evidence for the Formation of Nitrogen-Rich Platinum and Palladium Nitride Nanoparticles. Chem. Mater. 2013, 25, 4936–4945. [Google Scholar] [CrossRef]
- Sato, S.; Hirai, H.; Araki, S.; Wagatsuma, K. Effect of excitation states of nitrogen on the surface nitridation of iron and steel in d.c. glow discharge plasma. Surf. Interface Anal. 2011, 43, 964–970. [Google Scholar] [CrossRef]
- Zhang, H.; Gong, Q.; Ren, S.; Ali Arshid, M.; Chu, W.; Chen, C. Implication of iron nitride species to enhance the catalytic activity and stability of carbon nanotubes supported Fe catalysts for carbon-free hydrogen production via low-temperature ammonia decomposition. Catal. Sci. Technol. 2018, 8, 907–915. [Google Scholar] [CrossRef]
- Sridhar, V.; Kim, H.J.; Jung, J.H.; Lee, C.; Park, S.; Oh, I.K. Defect-Engineered Three-Dimensional Graphene-Nanotube-Palladium Nanostructures with Ultrahigh Capacitance. ACS Nano 2012, 6, 10562–10570. [Google Scholar] [CrossRef]
- Parambhath, V.B.; Nagar, R.; Ramaprabhu, S. Effect of Nitrogen Doping on Hydrogen Storage Capacity of Palladium Decorated Graphene. Langmuir 2012, 28, 7826–7833. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Yao, Y.; Chen, Y. Highly Active and Stable Fe-N-C Oxygen Reduction Electrocatalysts Derived from Electrospinning and In Situ Pyrolysis. Nanoscale Res Lett. 2018, 13, 218. [Google Scholar] [CrossRef] [PubMed]
- Varga, T.; Vásárhelyi, L.; Ballai, G. Noble-Metal-Free Iron Nitride/Nitrogen-Doped Graphene Composite for the Oxygen Reduction Reaction. ACS Omega 2019, 4, 130–139. [Google Scholar] [CrossRef] [PubMed]
- Mu, Y.; Jia, F.; Ai, Z.; Zhang, L. Iron Oxide Shell Mediated Environmental Remediation Properties of Nano Zero-Valent Iron. Environ. Sci. Nano 2017, 4, 27–45. [Google Scholar] [CrossRef]
- Sridhar, V.; Lee, I.; Chun, H.-H.; Park, H. Microwave synthesis of nitrogen-doped carbon nanotubes anchored on graphene substrates. Carbon 2015, 87, 186–192. [Google Scholar] [CrossRef]
- Zhu, C.Z.; Wen, D.; Oschatz, M.; Holzschuh, M.; Liu, W.; Herrmann, A.K.; Simon, F.; Kaskel, S.; Eychmuller, A. Kinetically Controlled Synthesis of PdNi Bimetallic Porous Nanostructures with Enhanced Electrocatalytic Activity. Small 2015, 11, 1430–1434. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Chi, X.; Zou, S.; Zeng, X. Facet Effects of Palladium Nanocrystals for Oxygen Reduction in Ionic Liquids and for Sensing Applications. Nanoscale 2016, 8, 5771–5779. [Google Scholar] [CrossRef] [PubMed]
- Sridhar, V.; Jung, K.H.; Park, H. Vitamin Derived Nitrogen Doped Carbon Nanotubes for Efficient Oxygen Reduction Reaction and Arsenic Removal from Contaminated Water. Materials 2020, 13, 1686. [Google Scholar] [CrossRef] [PubMed]
- Sridhar, V.; Park, H. Transforming Waste Poly(Ethylene Terephthalate) into Nitrogen Doped Carbon Nanotubes and Its Utility in Oxygen Reduction Reaction and Bisphenol-A Removal from Contaminated Water. Materials 2020, 13, 4144. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sridhar, V.; Park, H. DABCO Derived Nitrogen-Doped Carbon Nanotubes for Oxygen Reduction Reaction (ORR) and Removal of Hexavalent Chromium from Contaminated Water. Materials 2021, 14, 2871. https://doi.org/10.3390/ma14112871
Sridhar V, Park H. DABCO Derived Nitrogen-Doped Carbon Nanotubes for Oxygen Reduction Reaction (ORR) and Removal of Hexavalent Chromium from Contaminated Water. Materials. 2021; 14(11):2871. https://doi.org/10.3390/ma14112871
Chicago/Turabian StyleSridhar, Vadahanambi, and Hyun Park. 2021. "DABCO Derived Nitrogen-Doped Carbon Nanotubes for Oxygen Reduction Reaction (ORR) and Removal of Hexavalent Chromium from Contaminated Water" Materials 14, no. 11: 2871. https://doi.org/10.3390/ma14112871
APA StyleSridhar, V., & Park, H. (2021). DABCO Derived Nitrogen-Doped Carbon Nanotubes for Oxygen Reduction Reaction (ORR) and Removal of Hexavalent Chromium from Contaminated Water. Materials, 14(11), 2871. https://doi.org/10.3390/ma14112871





