Important Synthesis Parameters Affecting Crystallization of Zeolite T: A Review
Abstract
1. Introduction
1.1. Hydrothermal Conventional Method
1.2. Microwave-Assisted Method
1.3. Sonochemical-Assisted Method
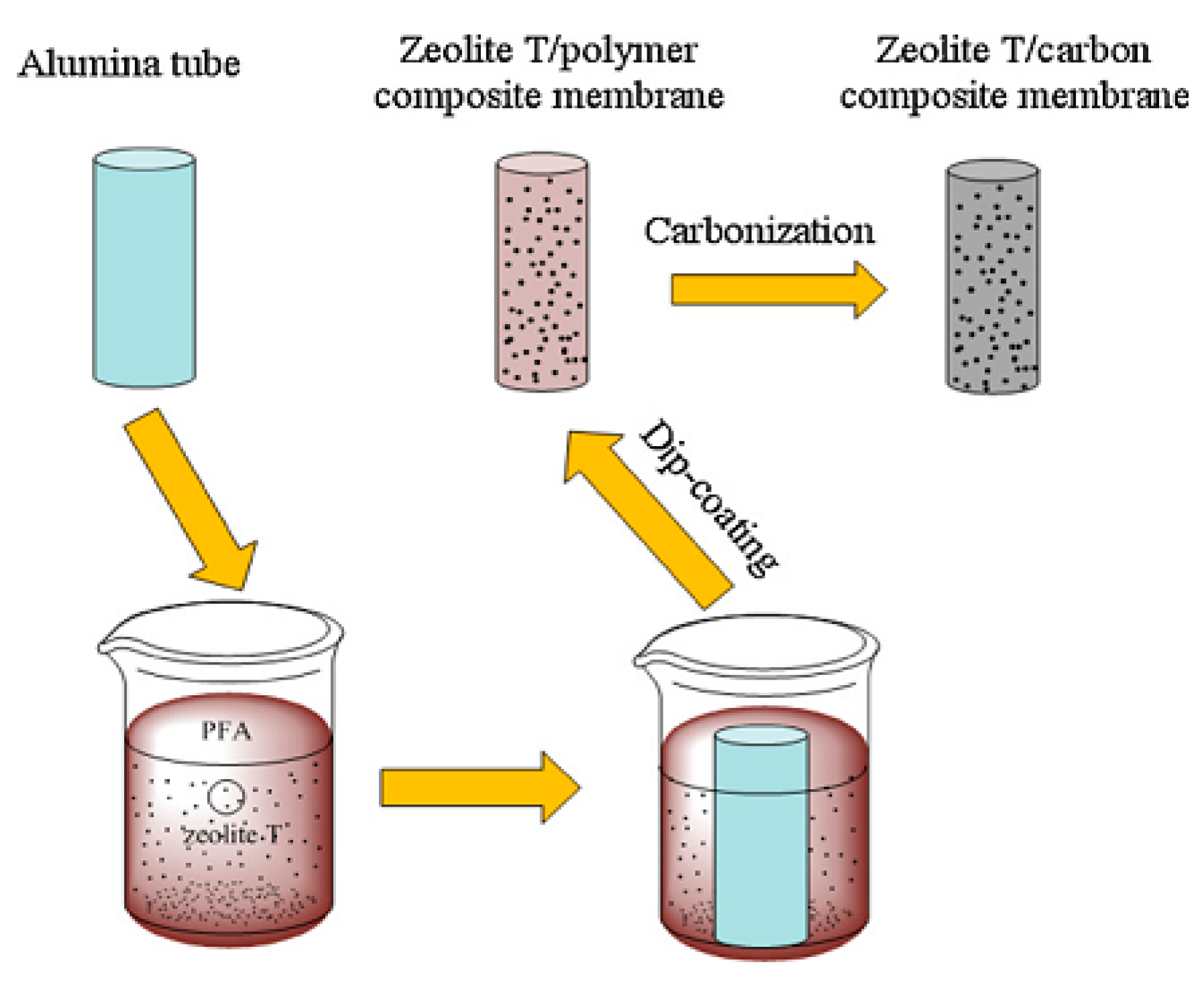
1.4. Secondary Growth/Dip-Coating
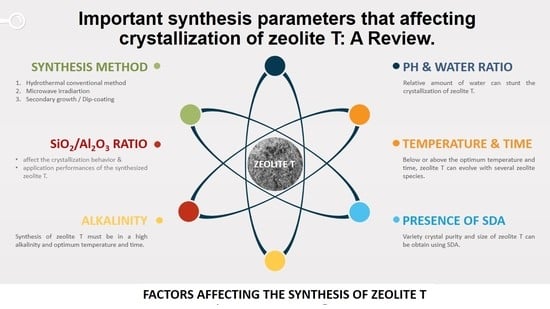
2. Factors Affecting Crystallization of Zeolite T
2.1. Effects of SiO2/Al2O3 Ratio
2.2. Effects of Alkalinity
2.3. Effects of pH and Water Ratio
2.4. Effects of Synthesis Temperature and Crystallization Time
2.5. Effects of the Addition of SDA
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mirfendereski, M.; Mohammadi, T. Investigation of hydrothermal synthesis parameters on characteristics of T type zeolite crystal structure. Powder Technol. 2011, 206, 345–352. [Google Scholar] [CrossRef]
- Rad, M.D.; Fatemi, S.; Mirfendereski, S.M. Development of T type zeolite for separation of CO2 from CH4 in adsorption processes. Chem. Eng. Res. Des. 2012, 90, 1687–1695. [Google Scholar] [CrossRef]
- Yin, X.; Li, Z.; Wang, S.; Chu, N.; Yang, J.; Wang, J. Hydrothermal synthesis of hierarchial zeolite T aggregates using tetramethylammonium hydroxide as single template. Microporous Mesoporous Mater. 2015, 201, 247–257. [Google Scholar] [CrossRef]
- Mougenel, J.C.; Kessler, H. Ionic conductivity of offretite, erionite, and zeolite T: Application to the determination of stacking faults. Zeolites 1991, 11, 81–84. [Google Scholar] [CrossRef]
- Zhou, R.; Hu, L.; Zhng, Y.; Hu, N.; Chen, X.; Lin, X.; Kita, H. Synthesis of oriented zeolite T membranes from clear solutions and their pervaporation properties. Microporous Mesoporous Mater. 2013, 174, 81–89. [Google Scholar] [CrossRef]
- Wang, X.; Yang, Z.; Yu, C.; Yin, L.; Zhang, C.; Gu, X. Preparation of T-type zeolite membranes using a dip-coating seeding suspension containing colloidal SiO2. Microporous Mesoporous Mater. 2014, 197, 17–25. [Google Scholar] [CrossRef]
- Yang, S.; Evmiridis, N.P. Synthesis and characterization of an offretite/erionite type zeolite. Microporous Mater. 1996, 6, 19–26. [Google Scholar] [CrossRef]
- Johnson, E.B.; Arshad, S.E. Hydrothermal synthesized zeolite based on kaolinite: A review. Appl. Clay Sci. 2014, 97, 215–221. [Google Scholar] [CrossRef]
- Ji, M.; Gao, X.; Wang, X.; Zhang, Y.; Jiang, J.; Gu, X. An ensemble synthesis strategy for fabrication of hollow fibre T-type zeolite membrane modules. J. Mmbrane Sci. 2018, 563, 460–469. [Google Scholar] [CrossRef]
- Zhou, H.; Li, Y.; Zhu, G.; Liu, J.; Yang, W. Preparation of zeolite T membranes by microwave-assisted in situ nucleation and secondary growth. Mater. Lett. 2009, 63, 255–257. [Google Scholar] [CrossRef]
- Zhou, H.; Li, Y.; Zhu, G.; Liu, J.; Yang, W. Microwave-assisted hydrothermal synthesis of a&b-oriented zeolite T membranes and their pervaporation properties. Sep. Purif. Technol. 2009, 65, 164–172. [Google Scholar]
- Jusoh, N.; Yeong, Y.F.; Mohamad, M.; Lau, K.K.; M. Shariff, A. Rapid-synthesis pf Zeolite T via sonochemical-assisted hydrothermal growth method. Ultrason. Sonochemistry 2017, 34, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Zheng, Y.; Hu, L.; Hu, N.; Zhu, M.; Zhou, R.; Chen, X.; Kita, H. Preparation of high-flux zeolite T membranes using reusable macroporous stainless steel supports in fluoride media. J. Membr. Sci. 2014, 456, 107–116. [Google Scholar] [CrossRef]
- Pal, P.; Das, J.K.; Bandyopadhyay, S. Synthesis of NaP zeolite at room temperature and short crystallization time by sonochemical method. Ultrason. Sonochemistry 2013, 20, 314–321. [Google Scholar] [CrossRef]
- Caro, J.; Noack, M. Zeolite membranes—Recent development and progress. Microporous Mesoporous Mater. 2008, 115, 215–233. [Google Scholar] [CrossRef]
- Yin, X.; Chu, N.; Yang, J.; Wang, J.; Li, Z. Thin zeolite T/carbon composite membranes supported on the porous alumina tubes for CO2 separation. Int. J. Greenh. Gas Control 2013, 15, 55–64. [Google Scholar] [CrossRef]
- Chen, X.; Wang, J.; Yin, D.; Yang, J.; Lu, J.; Zhang, Y. High-performance zeolite T membrane for dehydration of organics by new varying temperature hot-dip coating method. Am. Inst. Chem. Eng. 2013, 59, 936–947. [Google Scholar] [CrossRef]
- Yeo, Z.Y.; Chai, S.P.; Zhu, P.W.; Mohamed, A.R. Development of hybrid membrane through coupling of high selectivity zeolite T on ZIF-8 intermediate layer on ots performance in carbon dioxide and methane gas separation. Microporous Mesoporous Mater. 2014, 196, 79–88. [Google Scholar] [CrossRef]
- Zhou, R.; Li, Y.; Liu, B.; Hu, N.; Chen, X.; Kita, H. Preparation of chabazite membranes by secondary growth using zeolite-T-directed chabazite seeds. Microporous Mesoporous Mater. 2013, 179, 128–135. [Google Scholar] [CrossRef]
- Arshad, S.E.; Yusslee, E.F.; Rahman, M.L.; Sarkar, S.M.; Patuwan, S.Z. Hydrothermal Synthesis of Free-Template Zeolite T from Kaolin. In AIP Conference Proceedings; AIP Publishing LLC: Langkawi, Malaysia, 2017; p. 030004. [Google Scholar]
- Arshad, S.E.; Rahman, M.L.; Sarkar, S.M.; Yusslee, F.F.; Patuwan, S.Z. Hydrothermal synthesis of zeolite T from kaolin using two-different structure directing agents. Mater. Res. Express 2018, 5, 1. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, J.; Zhang, H.; Zhang, F.; Zhu, M.; Hu, N.; Chen, X.; Kita, H. Synthesis of hierarchical zeolite T nanocrystals with the assistance of zeolite. J. Solid Chem. 2020, 285, 121228. [Google Scholar] [CrossRef]
- Zhou, R.; Zhong, S.; Lin, X.; Xu, N. Synthesis of zeolite T by microwave and conventional heating. Microporous Mesoporous Mater. 2009, 124, 117–122. [Google Scholar] [CrossRef]
- Luo, Y.; Raza, W.; Yang, J.; Li, L.; Lu, Y. Recent advances in acid-resistant zeolite T membranes for dehydration of organics. Chin. J. Chem. Eng. 2019, 27, 1449–1457. [Google Scholar] [CrossRef]
- Flanigen, E.M. Molecular sieve zeolite technology—The first twenty-five years. Pure Appl. Chem. 1980, 52, 2191–2211. [Google Scholar] [CrossRef]
- Jihong, Y. Chapter 3Synthesis of Zeolites. In Studies in Surface Science and Catalysts; Jiří Cejka, H.V.B.A.C., Ferdi, S., Eds.; Elsevier: New York, NY, USA, 2007; Volume 168, pp. 39–103. [Google Scholar]
- Chen, Z.; Javier, J.C.; Brennaman, M.K.; Kang, P.; Norris, M.R.; Hoertz, P.G.; Meyer, T.J. Splitting CO2 into CO and O2 by a single catalyst. Proc. Natl. Acad. Sci. USA 2012, 109, 15606–15611. [Google Scholar] [CrossRef]
- Martin, A.; Wol, U.; Berndt, H.; Lucke, B. Nature, strength, and concentration of acid sites of erionite-rich zeolite T determined by ammonia-i.r. spectroscopy. Catal. Lett. 1993, 13, 309–314. [Google Scholar] [CrossRef]
- Jiang, Q.; Rentschler, J.; Sethia, G.; Weinman, S.; Perrone, R.; Liu, K. Synthesis of T-type zeolite nanoparticles for the separation of CO2/N2 and CO2/CH4 by adsorption process. Chem. Eng. J. 2013, 230, 380–388. [Google Scholar] [CrossRef]
- Tosheva, L.; Valtchev, V. Nanozeolites: Synthesis, crystallization mechanism, and application. Chem. Mater. 2005, 17, 2494–2513. [Google Scholar] [CrossRef]

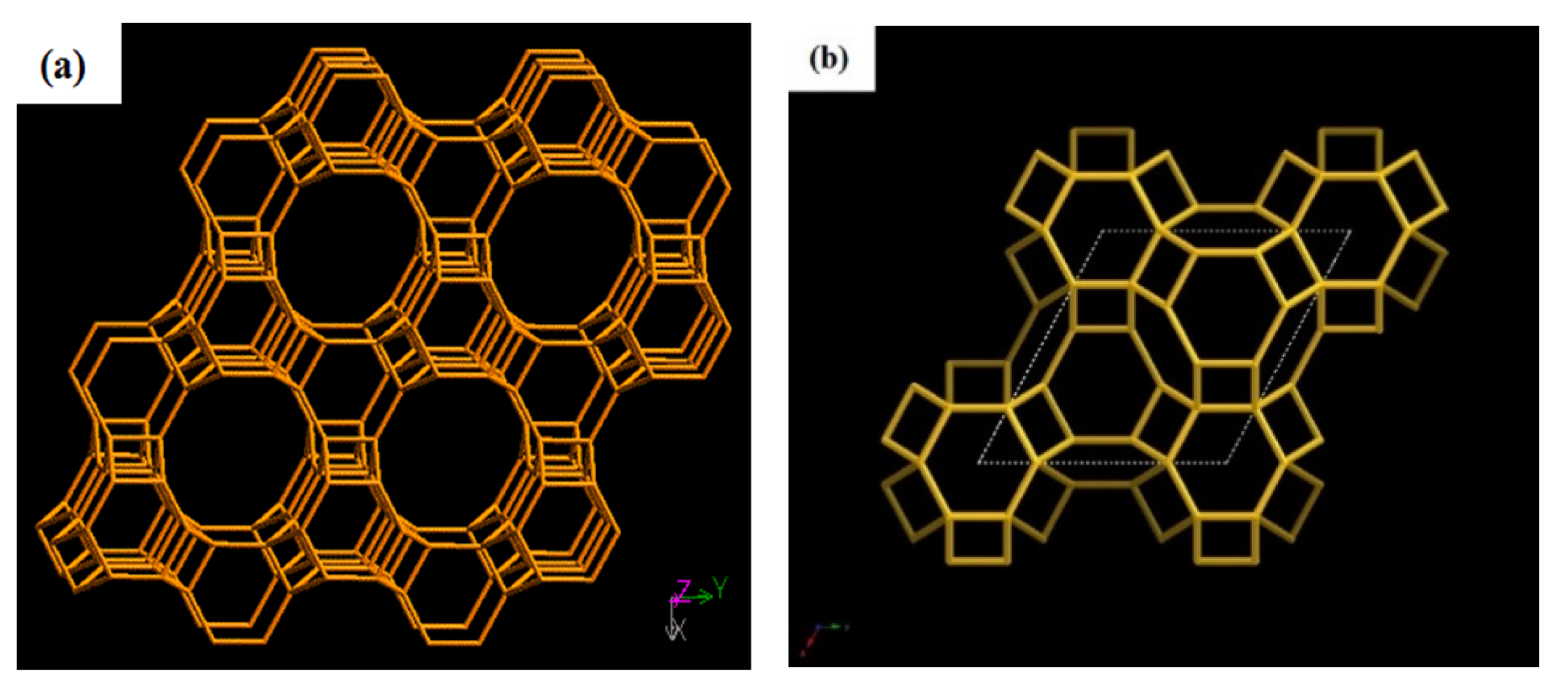

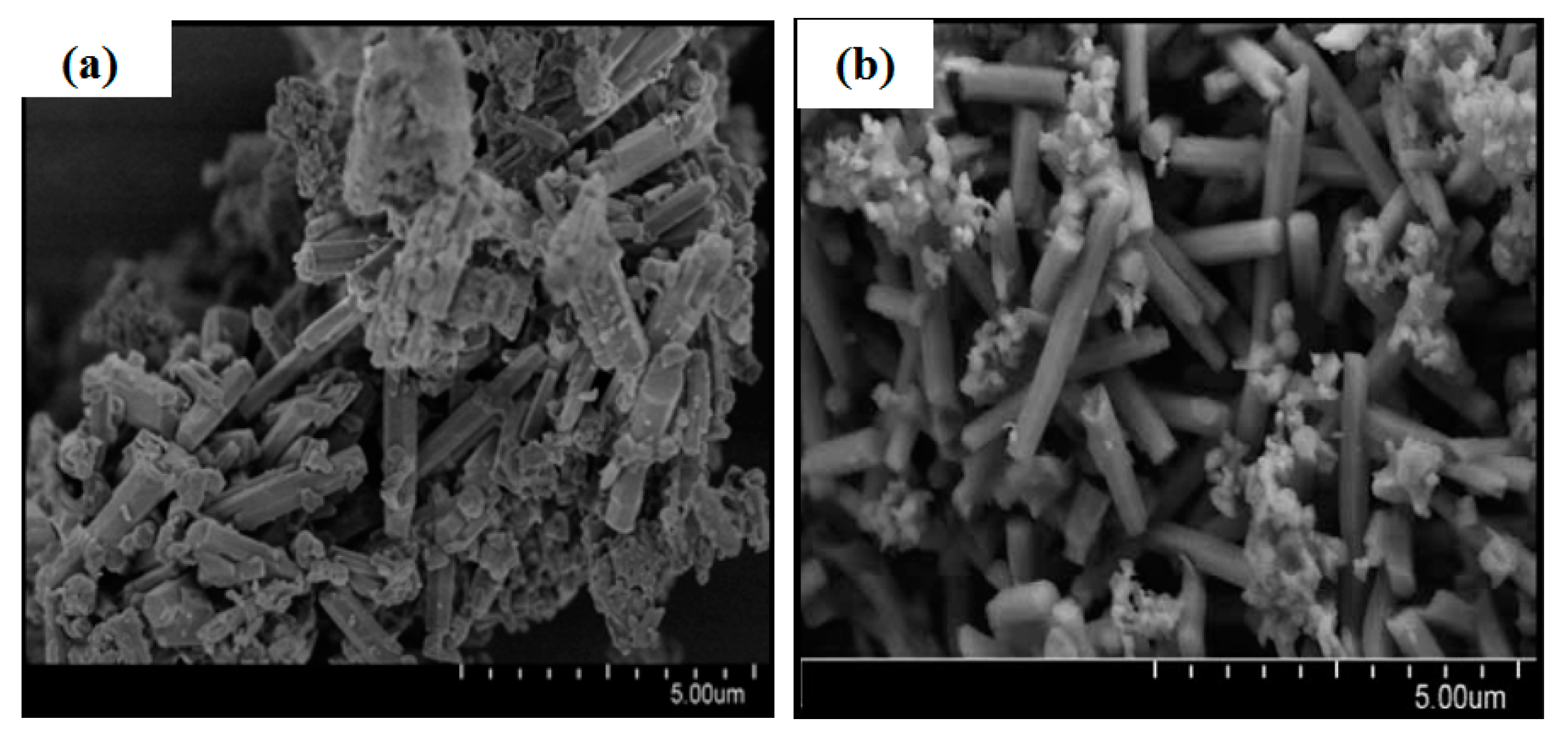
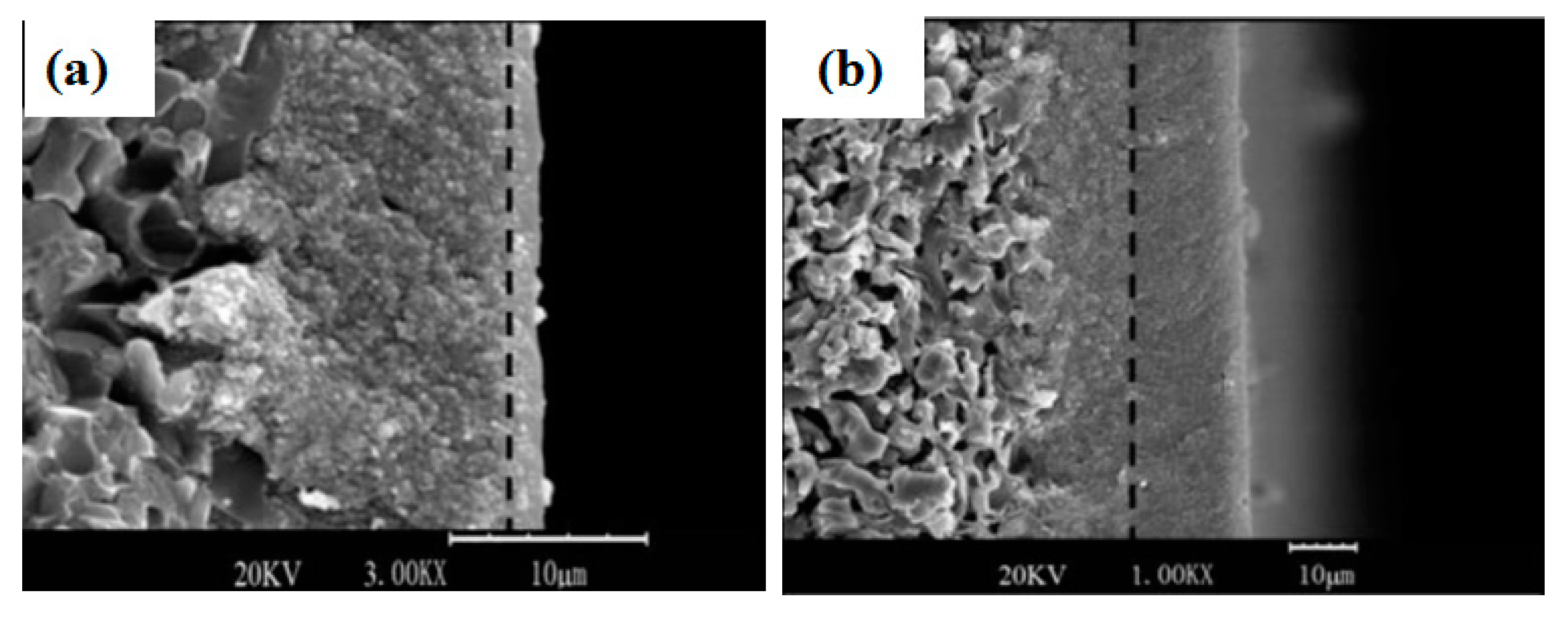
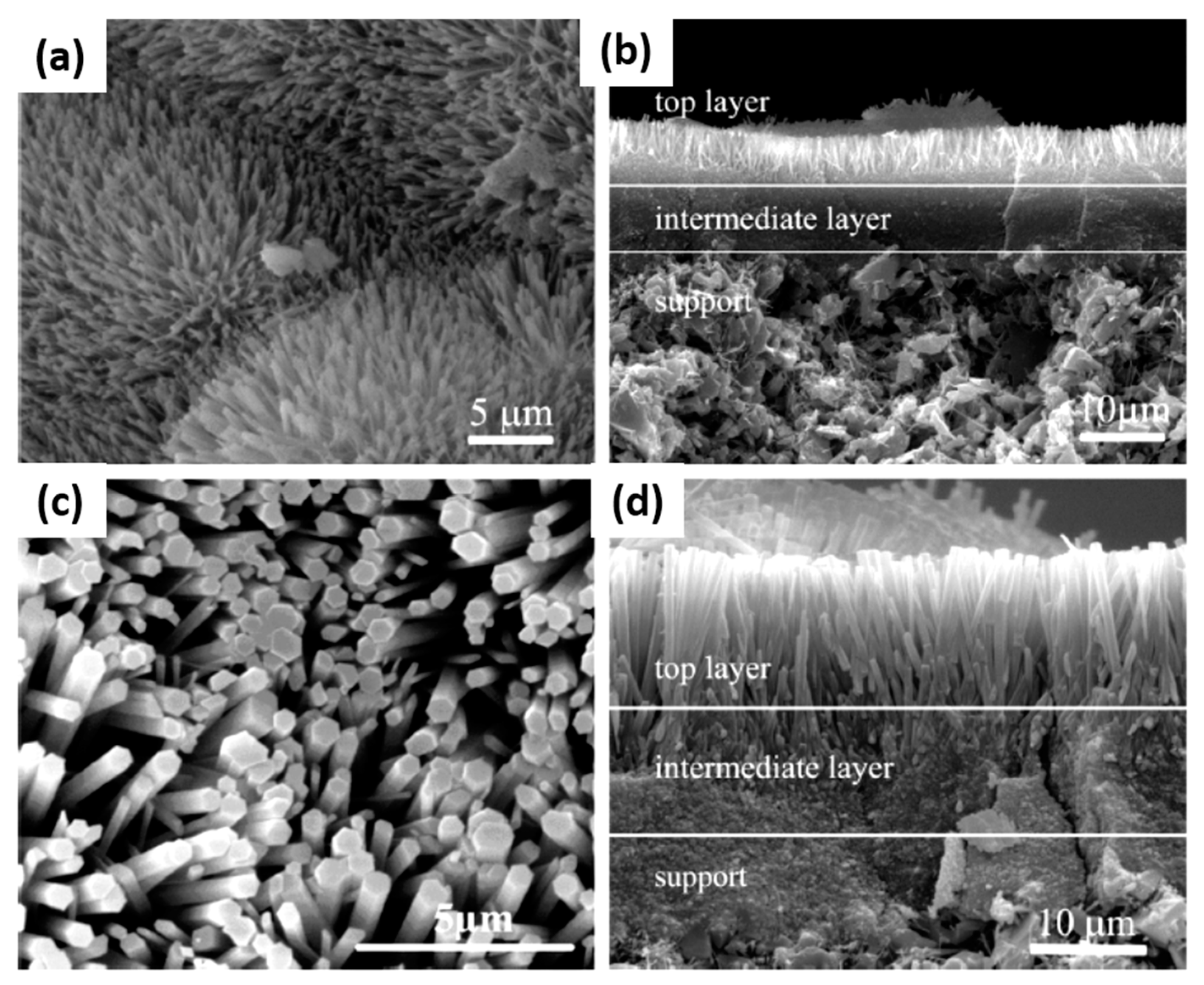

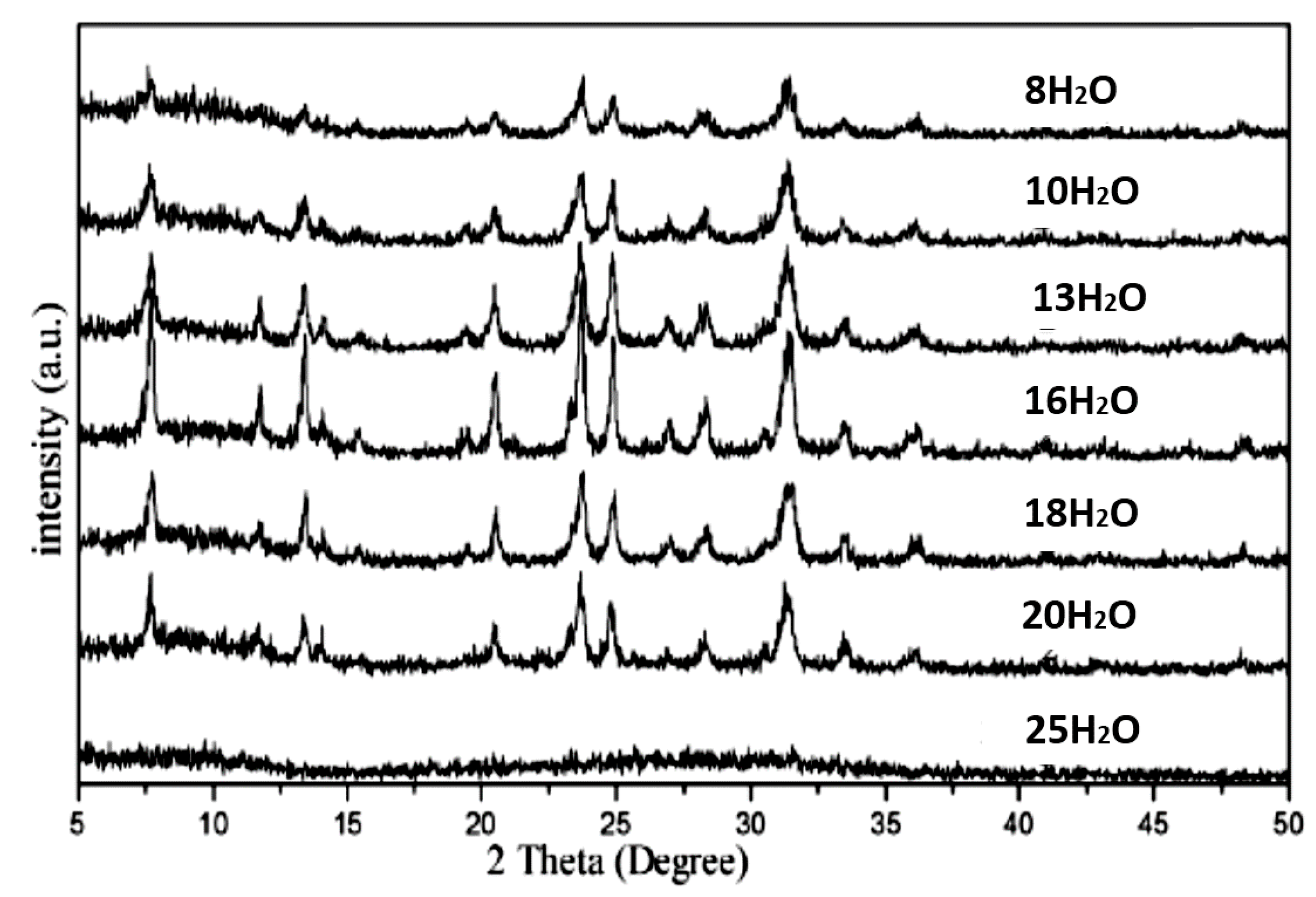


| Sample | ηRM | α | Silica Sources | Relative Crystallinity | Product Phase |
|---|---|---|---|---|---|
| S1 | 20 | 0.82 | Silica acid | 0 | L + W |
| S2 | 20 | 0.71 | Silica acid | 69 | T + L |
| S3 | 25 | 0.71 | Colloidal silica | 80 | T + L |
| S4 | 25 | 0.71 | Colloidal silica | 100 | T |
| S5 | 25 | 0.82 | Colloidal silica | 54 | T + L |
| S6 | 20 | 0.82 | Colloidal silica | 26 | T + L |
| S7 | 20 | 0.71 | Colloidal silica | 82 | T |
| S8 | 25 | 0.82 | Silica acid | 35 | T + L |
| Sample | Crystallization Parameters | Obtained Product(s), Zeolite Type | |
|---|---|---|---|
| Temperature (°C) | Time (h) | ||
| 1 | 100 | 120 | Majority: W |
| 2 | 100 | 168 | Majority: T |
| 3 | 100 | 216 | Majority: T, minor: W |
| 4 | 120 | 120 | Majority: T |
| 5 | 120 | 168 | Majority: T |
| 6 | 120 | 216 | Majority: W, minor: L |
| 7 | 140 | 120 | Majority: W, minor: L |
| 8 | 140 | 168 | Majority: L, minor: T, W |
| 9 | 140 | 216 | Majority: L |
| Experiment | Technique | Time (h) | Crystallization Behavior (XRD) |
|---|---|---|---|
| 1 | CR, T = 100 °C, P = 1 atm | 48 | Amorphous |
| 2 | 72 | Amorphous | |
| 3 | 96 | T + amorphous | |
| 4 | 120 | T | |
| 5 | 168 | T | |
| 6 | MR, T = 100 °C, P = 1 atm | 5 | Amorphous |
| 7 | 10 | Amorphous | |
| 8 | 20 | Amorphous + T | |
| 9 | 30 | T | |
| 10 | 48 | T | |
| 11 | CH, T = 100 °C, P = 1 atm | 48 | Amorphous |
| 12 | 72 | Amorphous + T | |
| 13 | 96 | T | |
| 14 | 120 | T | |
| 15 | 168 | T | |
| 16 | MH, T = 100 °C, P = 1 atm | 5 | Amorphous |
| 17 | 10 | Amorphous | |
| 18 | 15 | Amorphous + PHI | |
| 19 | 20 | PHI | |
| 20 | 30 | PHI |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patuwan, S.Z.; Arshad, S.E. Important Synthesis Parameters Affecting Crystallization of Zeolite T: A Review. Materials 2021, 14, 2890. https://doi.org/10.3390/ma14112890
Patuwan SZ, Arshad SE. Important Synthesis Parameters Affecting Crystallization of Zeolite T: A Review. Materials. 2021; 14(11):2890. https://doi.org/10.3390/ma14112890
Chicago/Turabian StylePatuwan, Siti Z., and Sazmal E. Arshad. 2021. "Important Synthesis Parameters Affecting Crystallization of Zeolite T: A Review" Materials 14, no. 11: 2890. https://doi.org/10.3390/ma14112890
APA StylePatuwan, S. Z., & Arshad, S. E. (2021). Important Synthesis Parameters Affecting Crystallization of Zeolite T: A Review. Materials, 14(11), 2890. https://doi.org/10.3390/ma14112890