Fungal Based Biopolymer Composites for Construction Materials
Abstract
:1. Introduction
2. Materials and Methods
2.1. Microorganisms
2.2. Conditions of Microbial Cultivation
2.3. Spray-Drying of Bacterial Spores
2.4. Preparation of Composites Consisting in Atomized Bacterial Pellet and Polypropylene (PP)
2.5. Viability of Bacterial Spores
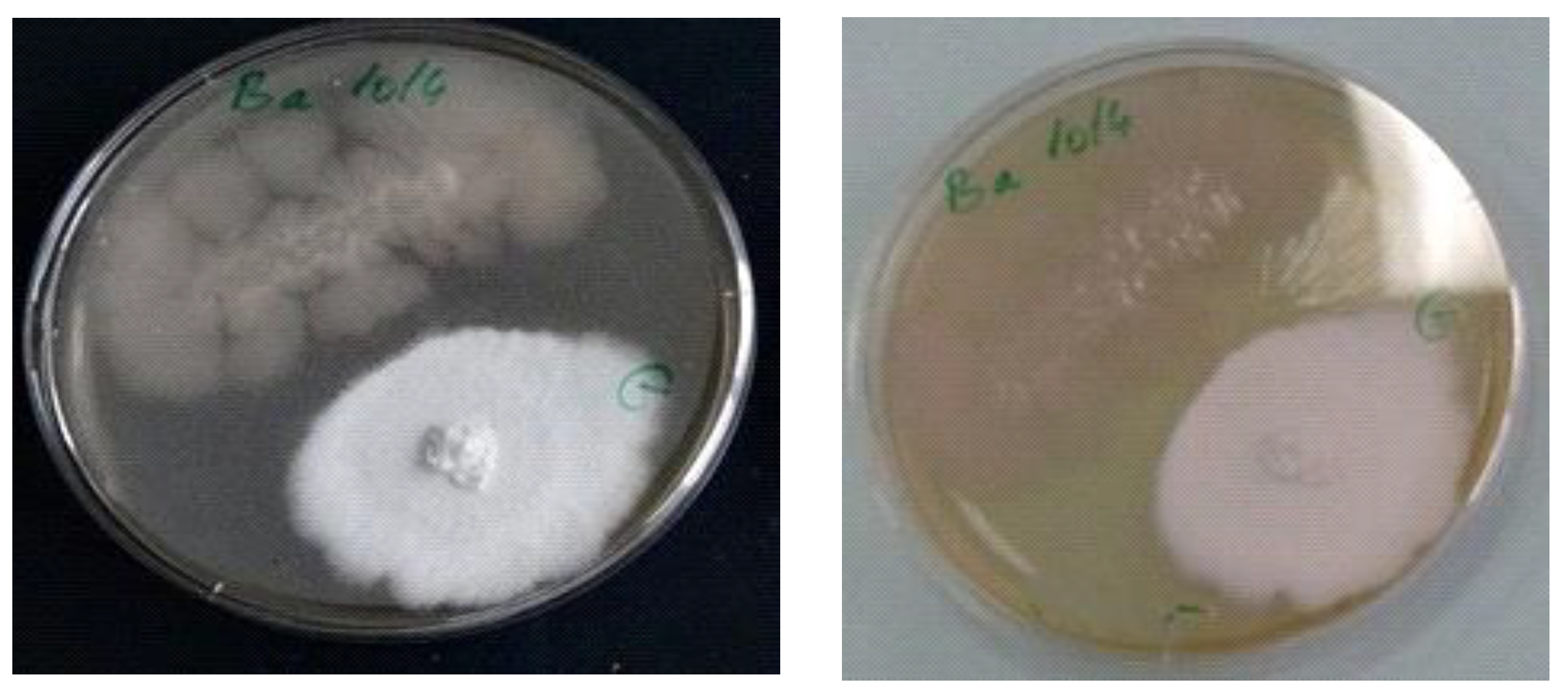
2.6. Antagonism between Ganoderma lucidum and Bacillus amyloliquefaciens 1014
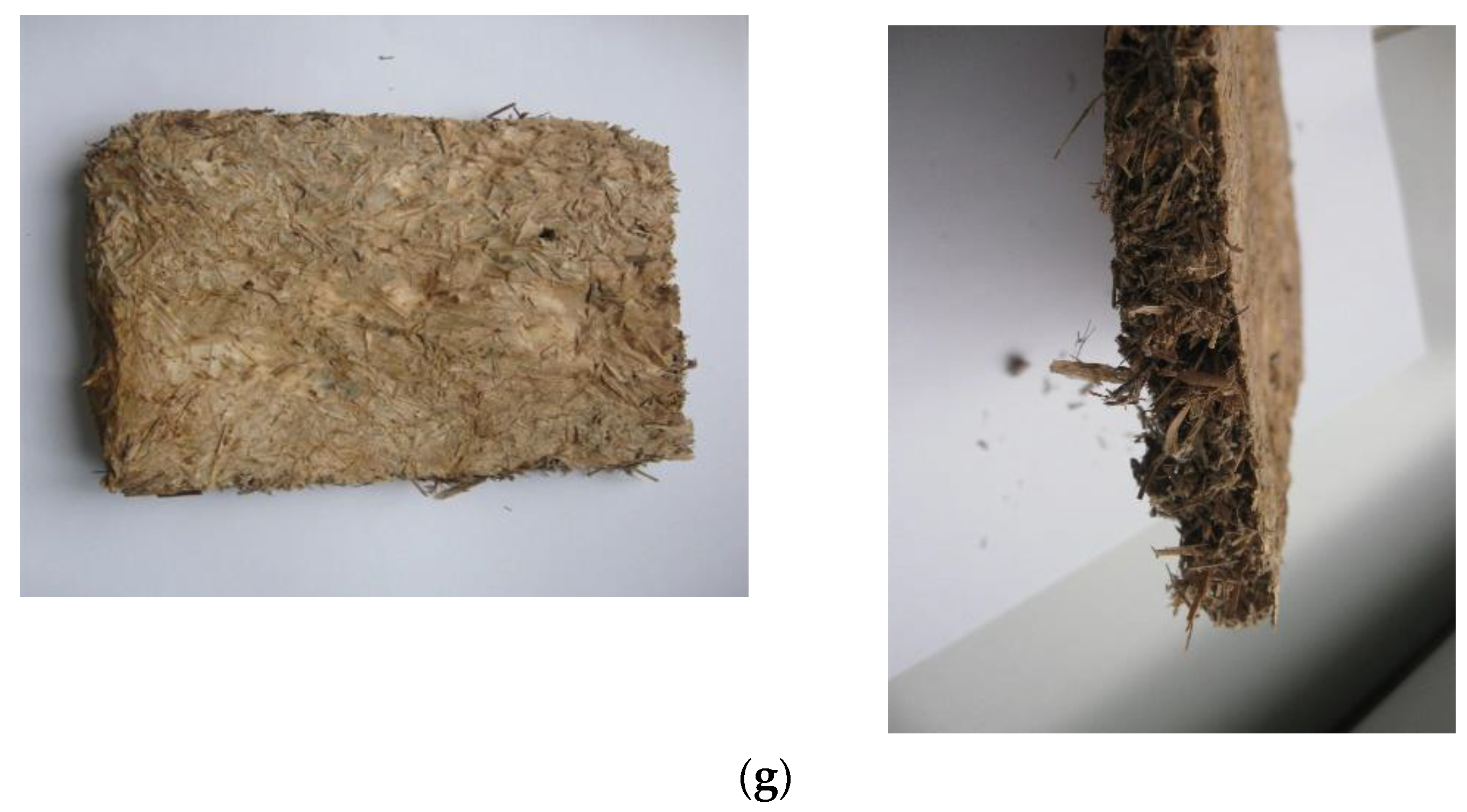
2.7. Preparation of Biomaterials Boards
2.8. FTIR Analysis
2.9. Thermal Conductivity
2.10. Mechanical Testing in Compression
3. Results
3.1. Microbial Cultivation
3.2. Viability of Bacterial Spores Embedded in Polypropylene Matrix
3.3. Antagonism Fungus-Bacteria
3.4. Preparation of Biomaterials Boards
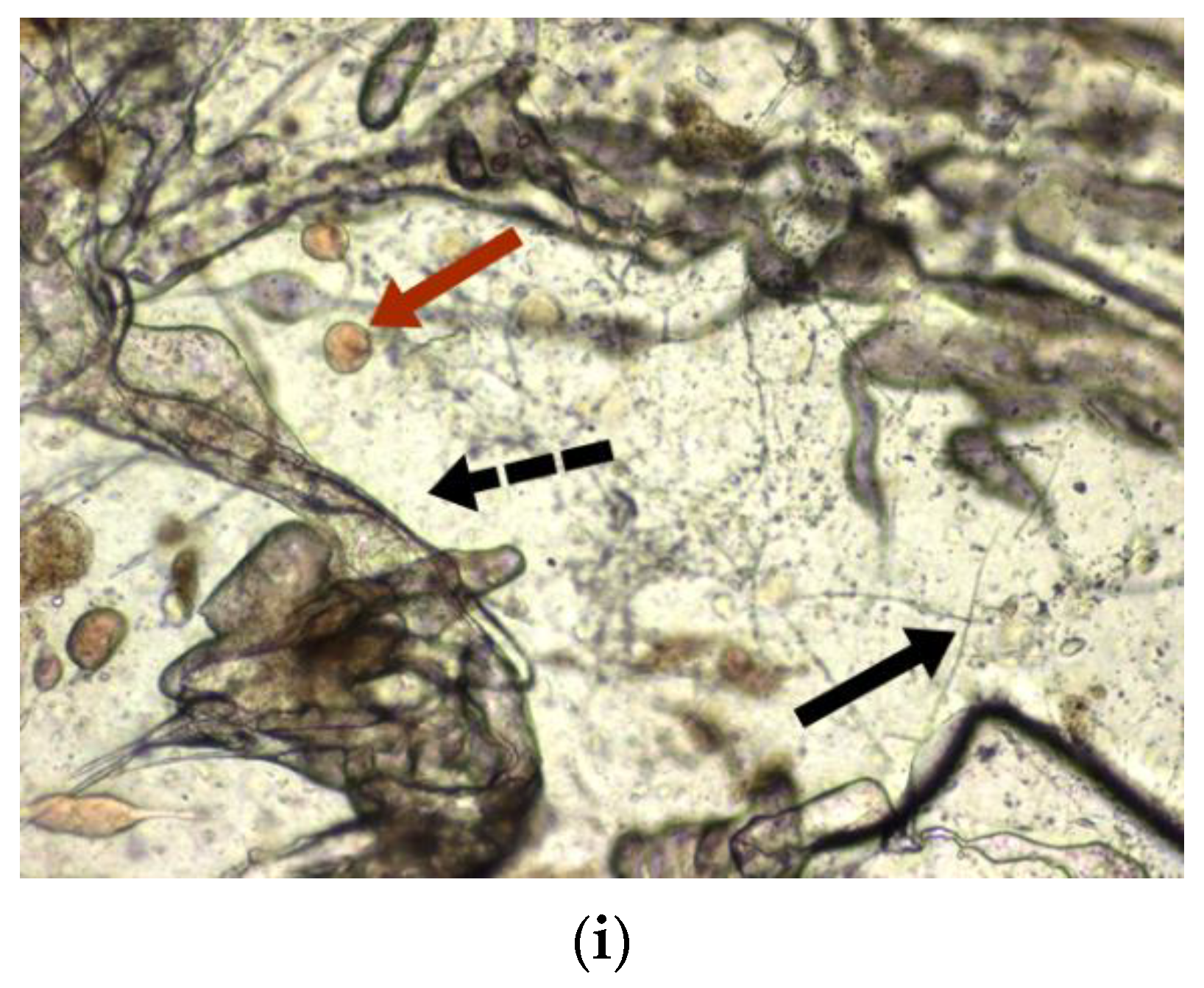
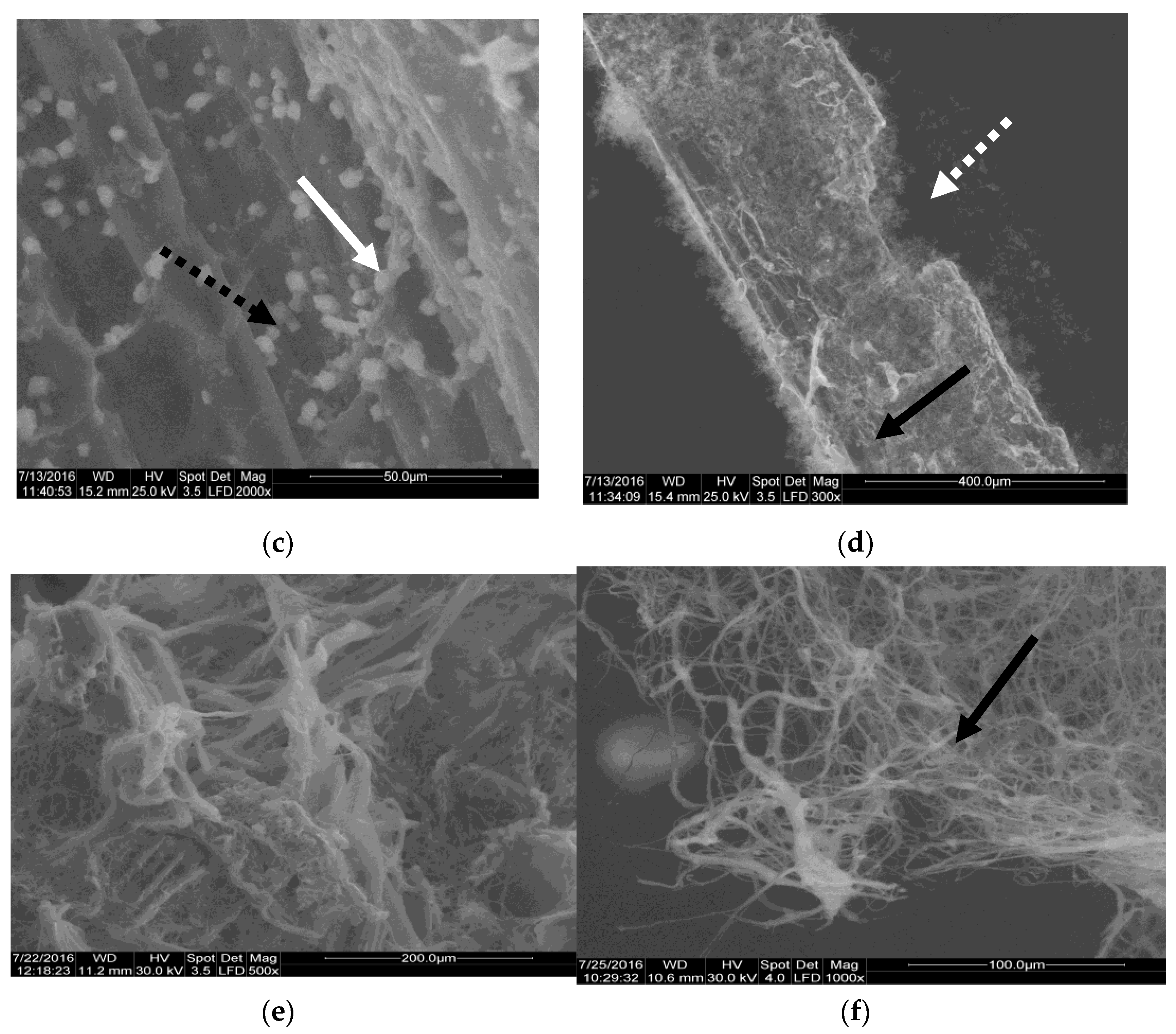
3.5. Morphological Analysis of Fungal Biomaterial
3.6. Chemical Characterization FTIR Spectrum Analysis
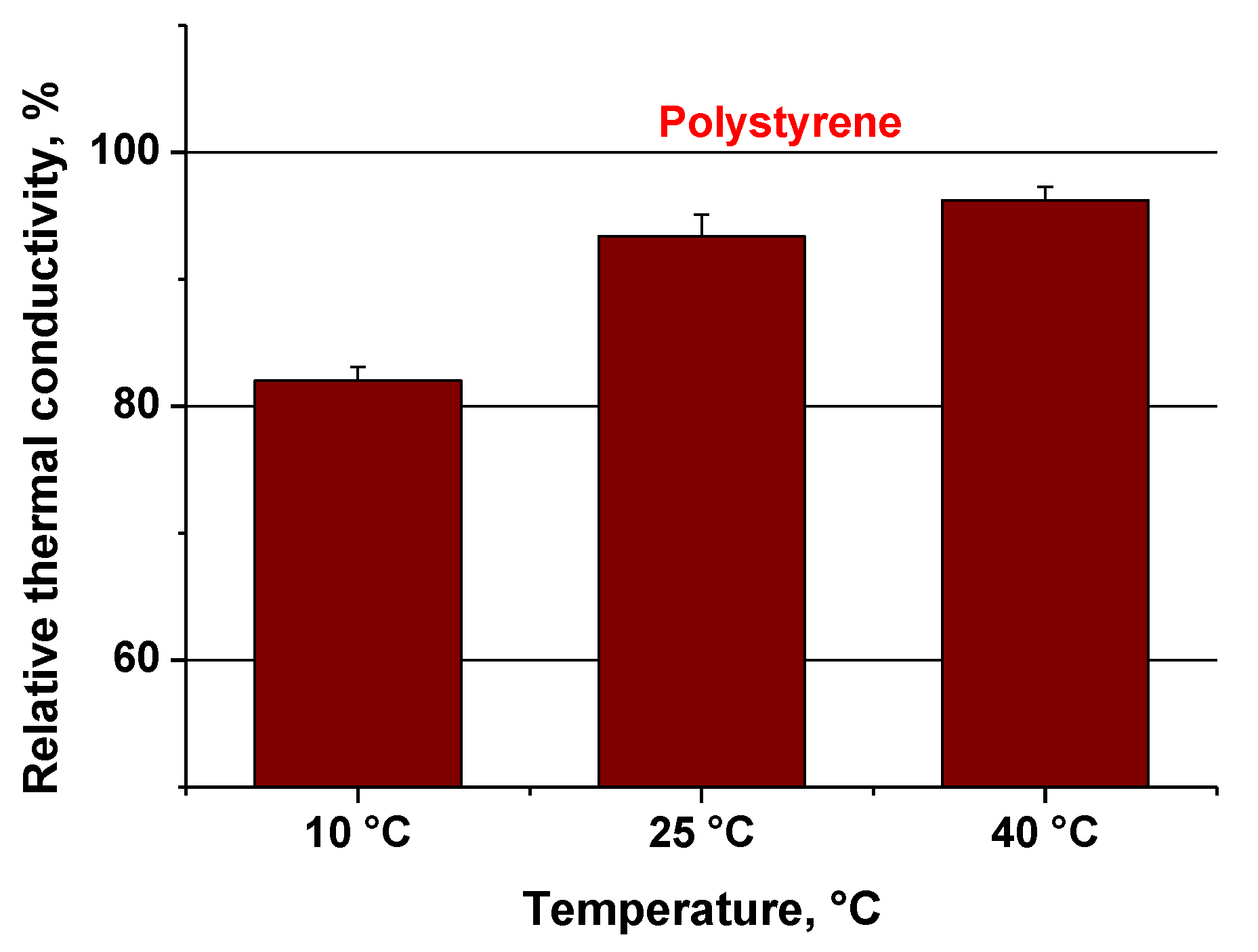
3.7. Thermal Conductivity
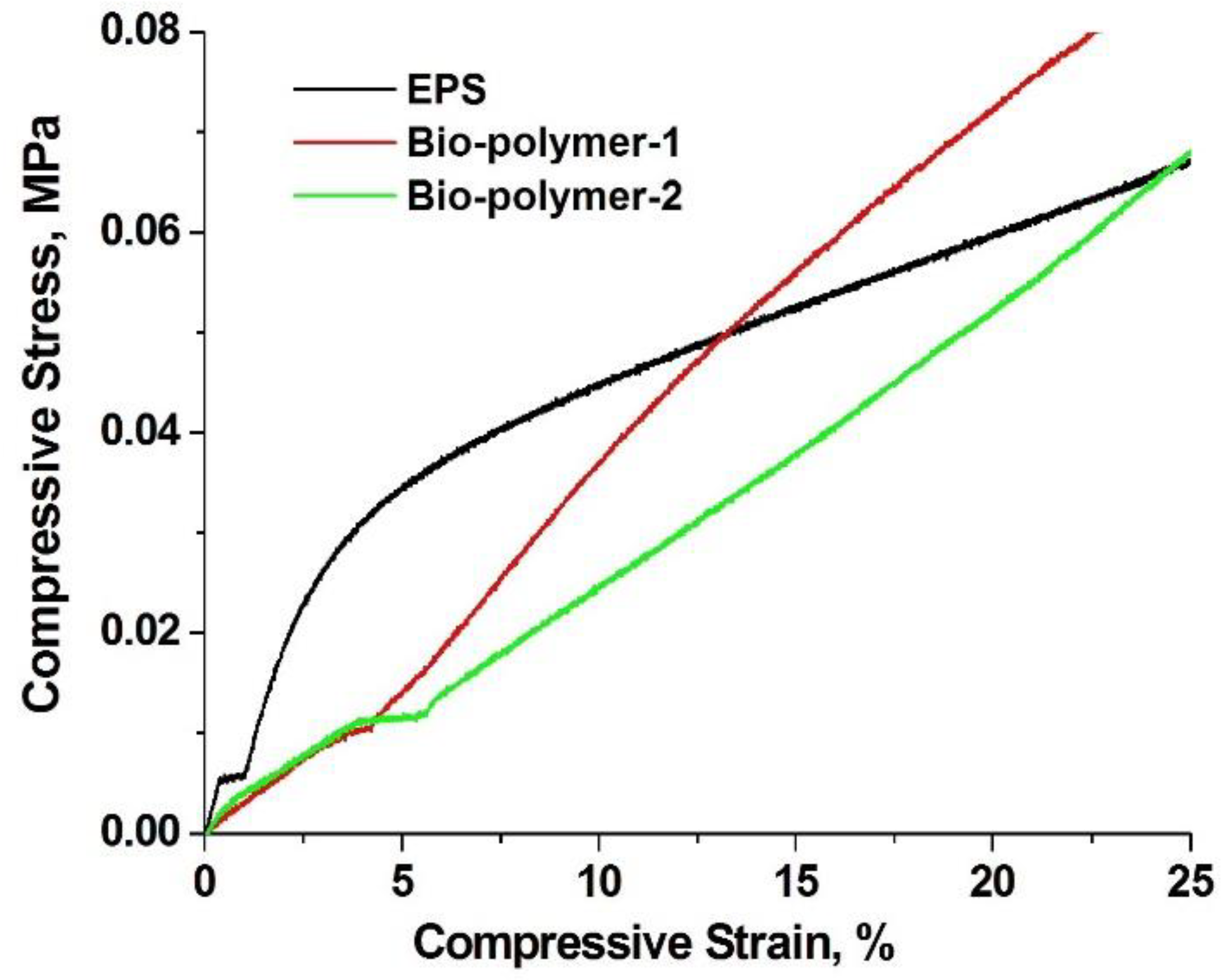
3.8. Mechanical Testing in Compression
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Arkatkar, A.; Arutchelvi, J.; Sudhakar, M.; Bhaduri, S.; Uppara, P.V.; Doble, M. Approaches to enhance the biodegradation of polyolefins. Open Environ. Eng. J. 2009, 2, 68–80. [Google Scholar] [CrossRef]
- Sheikh, S.; Chandrashekar, K.R.; Kumaraswamy, S.; Somashekarappa, H.M. Biodegradation of gamma irradiated low density polyethylene and polypropylene by endophytic fungi. Intern. Biodeter. Biodegrad. 2015, 105, 21–29. [Google Scholar] [CrossRef]
- Oancea, F.; Vuluga, Z.; Calin, M.; Raut, I.; Doni, M.; Jecu, L.; Paceagiu, J.; Iorga, M.; Dorel, F. Process for Increasing the Biocompatibility of Plastics and Biocomposite Product Resulting from It. RO Patent 132559 B1, 30 March 2020. [Google Scholar]
- Zielinska, D.; Rydzkowskib, T.; Thakurc, V.K.; Borysiak, S. Enzymatic engineering of nanometric cellulose for sustainable polypropylene nanocomposites. Inds. Crops Prod. 2021, 161, 1131. [Google Scholar]
- Jones, M.; Huynh, T.; Dekiwadia, C.; Daver, F.; John, S. Mycelium composites: A review of engineering characteristics and growth kinetics. Bionanoscience 2017, 11, 241–257. [Google Scholar] [CrossRef]
- Haneef, M.; Ceseracciu, L.; Canale, C.; Bayer, I.S.; Heredia-Guerrero, J.A.; Athanassiou, A. Advanced materials from fungal mycelium: Fabrication and tuning of physical properties. Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef]
- López Nava, J.A.; Méndez González, J.; Ruelas Chacón, X.; Nájera Luna, J.A. Assessment of edible fungi and films bio-based material simulating expanded polystyrene. Mater. Manuf. Process. 2016, 31, 1085–1090. [Google Scholar] [CrossRef]
- Bishop, K.S.; Kaob, C.H.J.; Xub, Y.; Glucinac, M.P.; Paterson, R.M.; Ferguson, L.R. From 2000 years of Ganoderma lucidum to recent developments in nutraceuticals. Phytochemistry 2015, 114, 56–65. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Zhang, H.; Zuo, J.; Gong, X.; Yi, F.; Zhu, W.; Li, L. Advances in research on the active constituents and physiological effects of Ganoderma lucidum. Biomed. Dermatol. 2019, 3, 6. [Google Scholar] [CrossRef]
- Sudheer, S.; Alzorqi, I.; Manickam, S.; Ali, A. Bioactive compounds of the wonder medicinal mushroom “Ganoderma lucidum”. In Bioactive Molecules in Food; Reference Series in Phytochemistry; Mérillon, J.M., Ramawat, K., Eds.; Springer: Cham, Switzerland, 2019. [Google Scholar] [CrossRef]
- Tudryn, G.J.; Smith, L.C.; Freitag, J.; Bucinell, R.; Schadler, L.S. Processing and morphology impacts on mechanical properties of fungal based biopolymer composites. J. Polym. Environ. 2018, 26, 1473–1483. [Google Scholar] [CrossRef]
- Teixeira, J.L.; Matos, M.P.; Nascimento, B.L.; Griza, S.; Holanda, F.S.R.; Marino, R.H. Production and mechanical evaluation of biodegradable composites by white rot fungi. Cienc. Agrotec. 2018, 42, 676–684. [Google Scholar] [CrossRef]
- Jones, M.; Mautner, A.; Luenco, S.; Bismarck, A.; John, S. Engineered mycelium composite construction materials from fungal biorefineries: A critical review. Mater. Des. 2020, 187, 108397. [Google Scholar] [CrossRef]
- Ghafoor, A.; Hasnain, S. Production dynamics of Bacillus subtilis strain AG-1 and EAG-2, producing moderately alkaline proteases. Afr. J. Microbiol. Res. 2009, 3, 258–263. [Google Scholar]
- Tzeng, Y.M.; Rao, Y.K.; Tsay, K.J.; Wu, W.S. Effect of cultivation conditions on spore production from Bacillus amyloliquefaciens B128 and its antagonism to Botrytis elliptica. J. Appl. Microbiol. 2018, 104, 1275–1282. [Google Scholar] [CrossRef]
- Islam, M.A.; Nain, Z.; Alam, M.K.; Banu, N.A.; Islam, M.R. In vitro study of biocontrol potential of rhizospheric Pseudomonas aeruginosa against Fusarium oxysporum f. sp. Cucumerinum. Egypt. J. Biol. Pest Control 2018, 28, 90. [Google Scholar] [CrossRef]
- Krause, K.; Jung, E.-M.; Lindner, J.; Hardiman, I.; Poetschner, J.; Madhavan, S.; Matthäus, C.; Kai, M.; Menezes, R.C.; Popp, J.; et al. Response of the wood-decay fungus Schizophyllum commune to co-occurring microorganisms. PLoS ONE 2020, 15, e0232145. [Google Scholar] [CrossRef] [Green Version]
- Arun, G.; Eyini, M.; Gunasekaran, P. Characterization and biological activities of extracellular melanin produced by Schizophyllum commune (Fries). Indian J. Exp. Biol. 2015, 53, 380. [Google Scholar] [PubMed]
- Jung, M.R.; Horgen, F.D.; Orski, S.V.; Rodriguez, C.V.; Beers, K.L.; Balazs, G.H.; Jones, T.T.; Work, T.M.; Brignac, K.C.; Royer, S.J.; et al. Validation of ATR FT-IR to identify polymers of plastic marine debris, including those ingested by marine organisms. Mar. Pollut. Bull. 2018, 127, 704–716. [Google Scholar] [CrossRef]
- Ashok, A.; Rejeesh, C.R. Investigating the biodegradability and physical properties of starch derived bioplastic films reinforced with nanosilica. Int. J. Nanosci. 2019, 18, 1850037. [Google Scholar] [CrossRef]
- Jose, J.; Uvais, K.N.; Sreenadh, T.S.; Deepak, A.V.; Rejeesh, C.R. Investigations into the development of a mycelium biocomposite to substitute polystyrene in packaging applications. Arab. J. Sci. Eng. 2021, 46, 2975–2984. [Google Scholar] [CrossRef]
- Pena, R.; Lang, C.; Naumann, A.; Polle, A. Ectomycorrhizal identification in environmental samples of tree roots by Fourier-transform infrared (FTIR) spectroscopy. Front. Plant Sci. 2014, 5, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Tacer-Caba, Z.; Varis, J.J.; Lankinen, P.; Mikkone, K.S. Comparison of novel fungal mycelia strains and sustainable growth substrates to produce humidity-resistant biocomposites. Mater. Des. 2020, 19, 108728. [Google Scholar] [CrossRef]
- Jiang, L.; Walczyk, D.; McIntyre, G.; Chan, V.W.K. Cost modeling and optimization of a manufacturing system for mycelium-based biocomposite parts. J. Manuf. Syst. 2016, 41, 8–20. [Google Scholar] [CrossRef]
- Hemmati, F.; Garmabi, H. A study on fire retardancy and durability performance of bagasse fiber/polypropylene composite for outdoor applications. J. Thermoplast. Compos. Mater. 2012, 26, 1041–1056. [Google Scholar] [CrossRef]
- Marino, R.H.; Abreu, L.D. Cultivo do cogumelo Shiitake em resíduo de coco suplementado com farelo de trigo e/ou arroz. Braz. J. Agric. Sci. 2009, 4, 11–16. [Google Scholar] [CrossRef] [Green Version]
- Attias, N.; Danai, O.; Tarazi, E.; Pereman, I.; Grobman, Y.J. Implementing bio-design tools to develop mycelium-based products. Des. J. 2019, 22, 1647–1657. [Google Scholar] [CrossRef] [Green Version]
- Elsacker, E.; Vandelook, S.; Brancart, S.; Peeters, E.; De Laet, L. Mechanical, physical and chemical characterisation of mycelium-based composites with different types of lignocellulosic substrates. PLoS ONE 2019, 14, e0213954. [Google Scholar] [CrossRef] [Green Version]
- Insulation Materials and Their Thermal Properties. Available online: https://www.greenspec.co.uk/building-design/insulation-materials-thermal-properties/ (accessed on 11 February 2021).
- Kertesz, M.A.; Thai, M. Compost bacteria and fungi that influence growth and development of Agaricus bisporus and other commercial mushrooms. Appl. Microbiol. Biotechnol. 2018, 102, 1639–1650. [Google Scholar] [CrossRef]
- Carrassco, J.; Preston, G.M. Growing edible mushrooms: A conversation between bacteria and fungi. Environ. Microbiol. 2020, 22, 858–872. [Google Scholar] [CrossRef] [Green Version]
- Setlow, P. Spores of Bacillus subtilis: Their resistance to and killing by radiation, heat and chemicals. J. Appl. Microbiol. 2006, 101, 514–525. [Google Scholar] [CrossRef]
- Cho, W.I.; Chung, M.S. Bacillus spores: A review of their properties and inactivation processing technologies. Food Sci. Biotechnol. 2020, 29, 1447–1461. [Google Scholar] [CrossRef]
- Melly, E.; Genest, P.C.; Gilmore, M.E.; Little, S.; Popham, D.L.; Driks, A.; Setlow, P. Analysis of the properties of spores of Bacillus subtilis prepared at different temperatures. J. Appl. Microbiol. 2002, 92, 1105–1111. [Google Scholar] [CrossRef]
- Margosch, D.; Gänzle, M.G.; Ehrmann, M.A.; Vogel, R. Pressure Inactivation of Bacillus endospores. Appl. Environ. Microbiol. 2004, 70, 7321. [Google Scholar] [CrossRef] [Green Version]
- Anthierens, T.; Ragaert, P.; Verbrugghe, S.; Ouchchen, A.; De Geest, B.G.; Noseda, B.; Mertens, J.; Beladjal, L.; De Cuyper, D.; Dierickx, W.; et al. Use of endospore-forming bacteria as an active oxygen scavenger in plastic packaging materials. Innov. Food Sci. Emerg. Technol. 2011, 12, 594–599. [Google Scholar] [CrossRef] [Green Version]
- Castiglioni, A.; Castellani, L.; Cuder, G.; Comba, S. Relevant materials parameters in cushioning for EPS foams. Colloids Surf. A Physicochem. Eng. Asp. 2017, 534, 71–77. [Google Scholar] [CrossRef]
- Hwang, J.; Choi, D.; Han, S.; Jung, S.Y.; Choi, J.; Hong, J. Potential toxicity of polystyrene microplastic particles. Sci. Rep. 2020, 10, 7391. [Google Scholar] [CrossRef]
- Abhijith, R.; Ashok, A.; Rejeesh, C.R. Sustainable packaging applications from mycelium to substitute polystyrene: A Review. Mater. Today Proc. 2018, 5, 2139–2145. [Google Scholar] [CrossRef]
- Girometta, C.; Picco, A.M.; Baiguera, R.M.; Dondi, D.; Babbini, S.; Cartabia, M.; Pellegrini, M.; Savino, E. Physico-mechanical and thermodynamic properties of mycelium-based biocomposites: A review. Sustainability 2019, 11, 281. [Google Scholar] [CrossRef] [Green Version]
- Ates, B.; Koytepe, S.; Ulu, A.; Gurses, C.; Thakur, V.K. Chemistry, structures, and advanced applications of nanocomposites from biorenewable resources. Chem. Rev. 2020, 120, 9304–9362. [Google Scholar] [CrossRef]






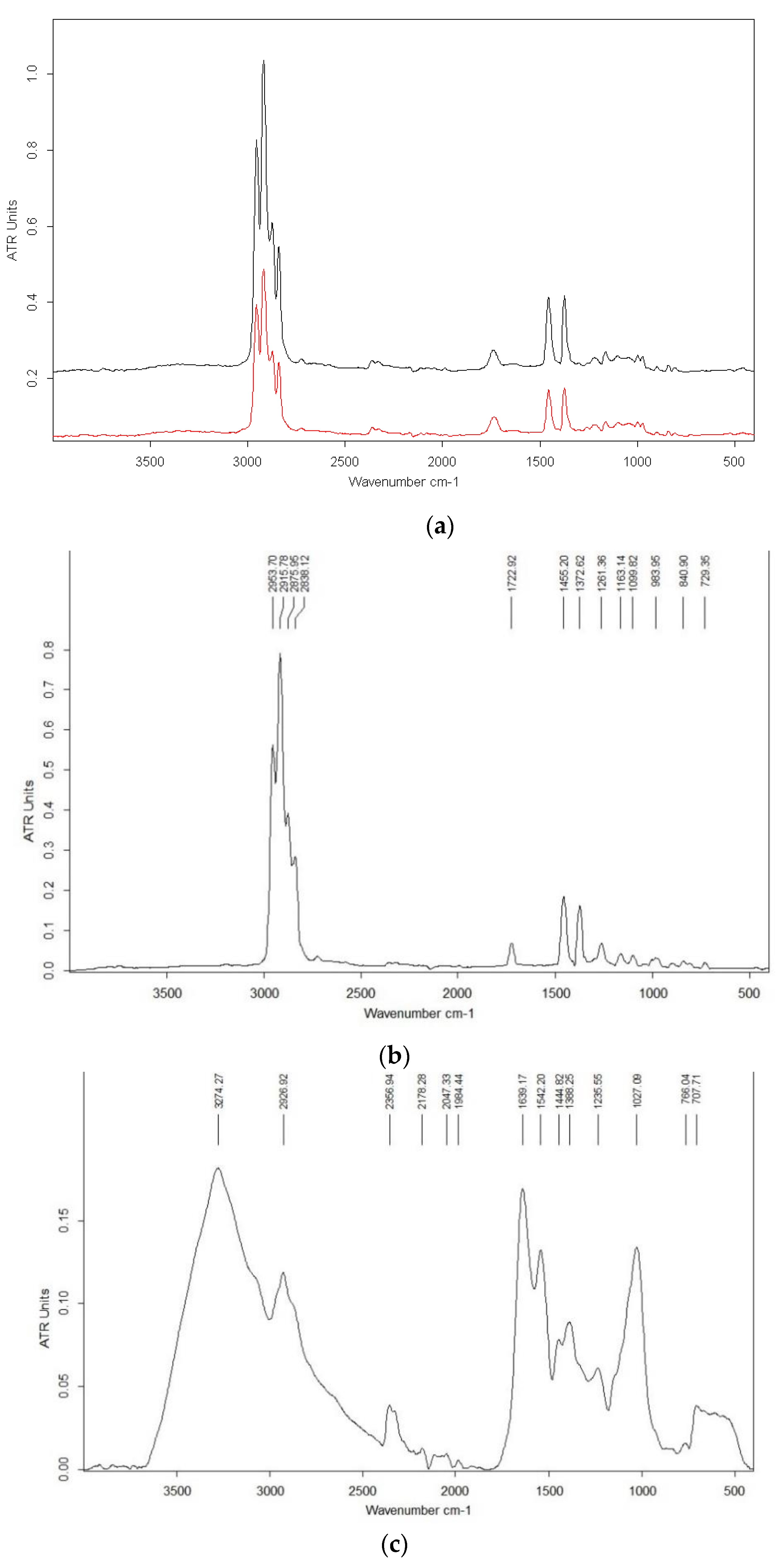
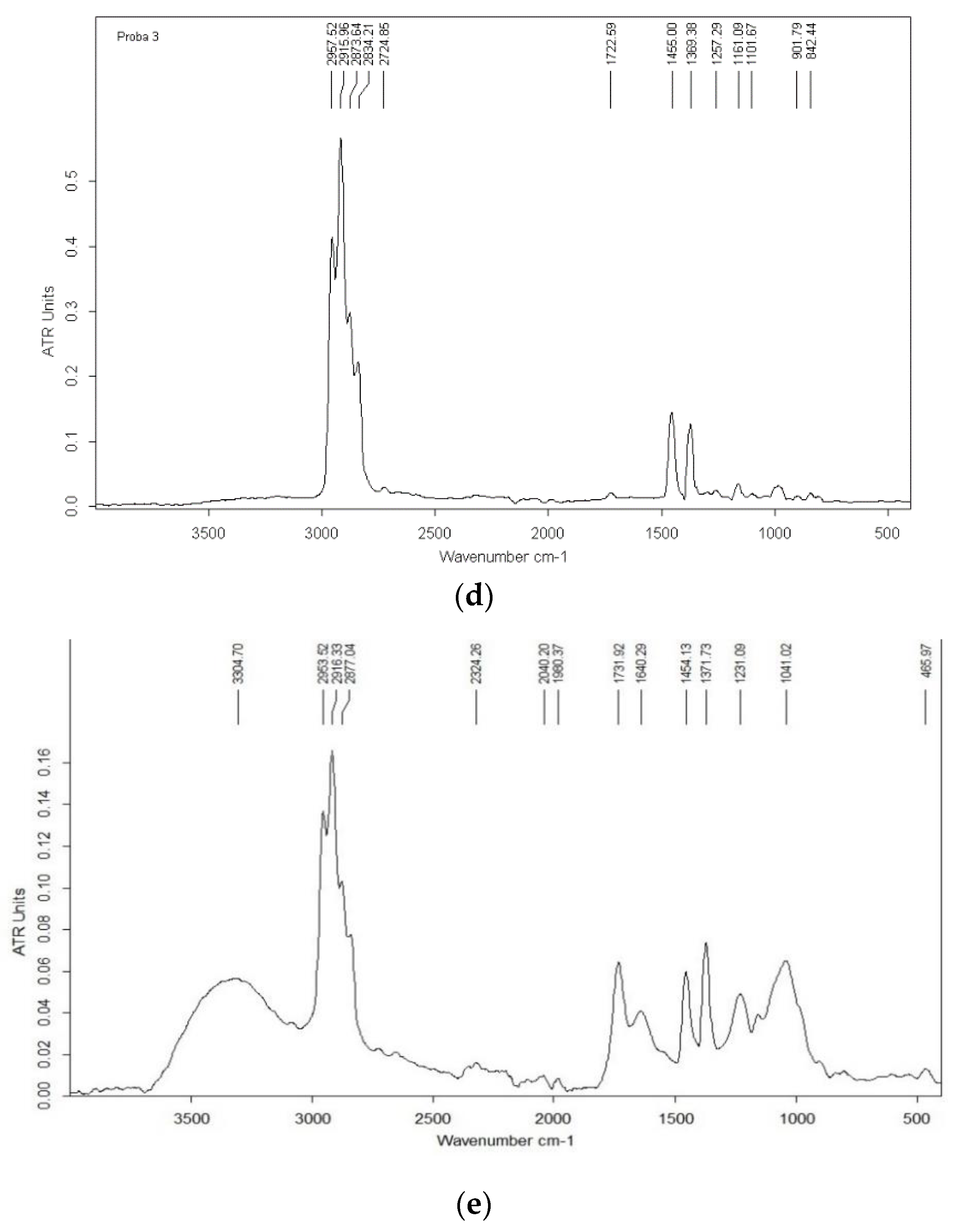
| Variants | Maltodextrin (g) | Bacterial Pellet (g) | Phosphate Buffer (mL) |
|---|---|---|---|
| I | 10 | 10 | 80 |
| II | 10 | 30 | 60 |
| III | 30 | 10 | 60 |
| Sample | Thermal Conductivity (W/mK) | ||
|---|---|---|---|
| 10 °C | 25 °C | 40 °C | |
| Bio-polymer composite (vheat straws, PP with bacterial spores and Ganoderma mycelium) | 0.029 ± 1.04 | 0.034 ± 1.68 | 0.035 ± 1.05 |
| Compressive Strength, at 25% Deformation | Value, kPa | |
| Bio-Polymer | EPS | |
| 70 ± 0.01 | 70 ± 0.01 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Răut, I.; Călin, M.; Vuluga, Z.; Oancea, F.; Paceagiu, J.; Radu, N.; Doni, M.; Alexandrescu, E.; Purcar, V.; Gurban, A.-M.; et al. Fungal Based Biopolymer Composites for Construction Materials. Materials 2021, 14, 2906. https://doi.org/10.3390/ma14112906
Răut I, Călin M, Vuluga Z, Oancea F, Paceagiu J, Radu N, Doni M, Alexandrescu E, Purcar V, Gurban A-M, et al. Fungal Based Biopolymer Composites for Construction Materials. Materials. 2021; 14(11):2906. https://doi.org/10.3390/ma14112906
Chicago/Turabian StyleRăut, Iuliana, Mariana Călin, Zina Vuluga, Florin Oancea, Jenica Paceagiu, Nicoleta Radu, Mihaela Doni, Elvira Alexandrescu, Violeta Purcar, Ana-Maria Gurban, and et al. 2021. "Fungal Based Biopolymer Composites for Construction Materials" Materials 14, no. 11: 2906. https://doi.org/10.3390/ma14112906
APA StyleRăut, I., Călin, M., Vuluga, Z., Oancea, F., Paceagiu, J., Radu, N., Doni, M., Alexandrescu, E., Purcar, V., Gurban, A. -M., Petre, I., & Jecu, L. (2021). Fungal Based Biopolymer Composites for Construction Materials. Materials, 14(11), 2906. https://doi.org/10.3390/ma14112906










