Effect of the Alloying Metal on the Corrosion Resistance of Pd-Rich Binary Alloys with Pt, Rh, and Ru in Sulfuric Acid
Abstract
1. Introduction
2. Materials and Methods
2.1. Material Preparation
2.2. Electrochemical Measurements
2.3. Material Characteristics Methods
3. Results and Discussion
3.1. Morphology and Bulk Composition of the Pd-Alloys before and after Corrosion
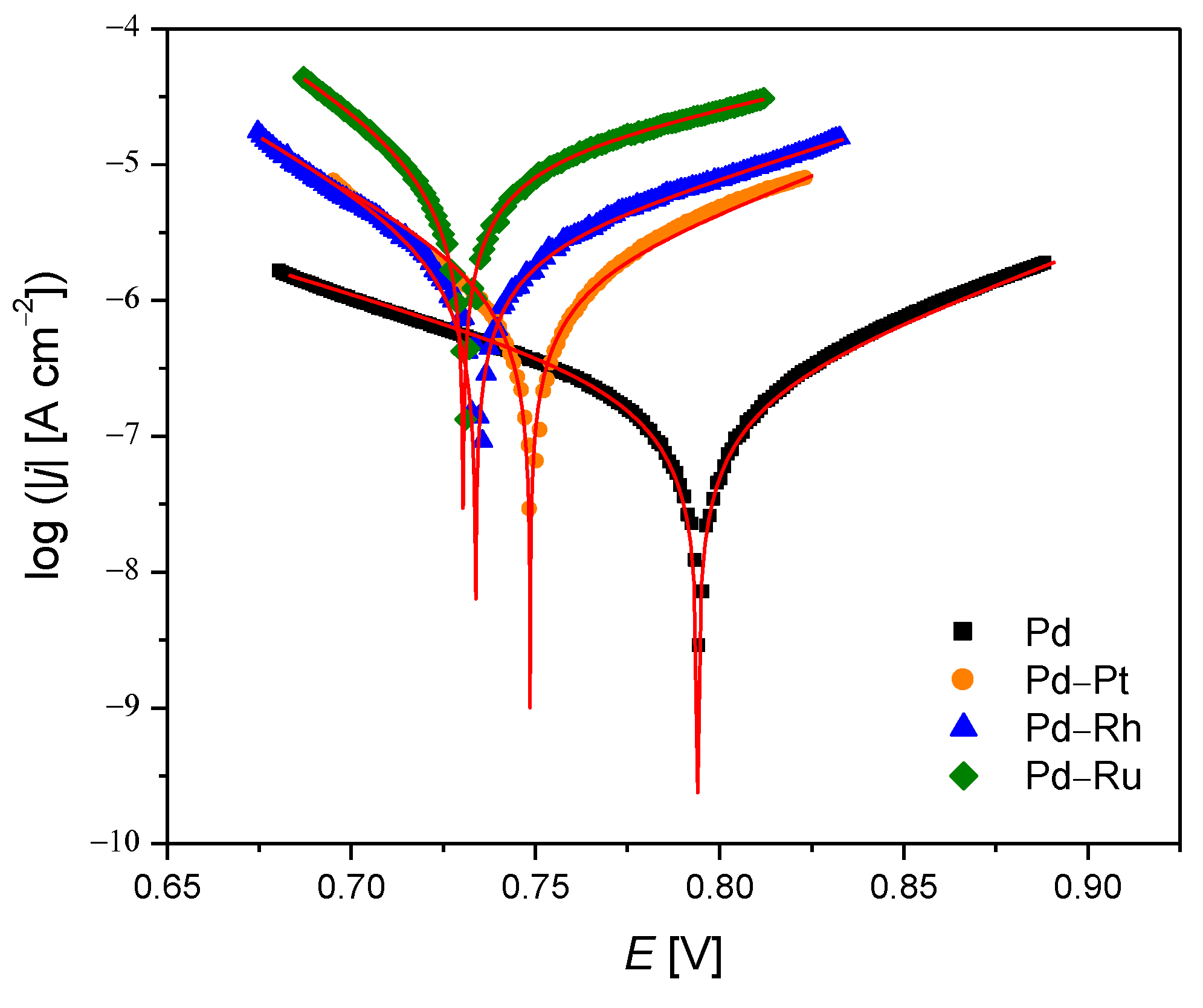
3.2. Potentiodynamic Measurements
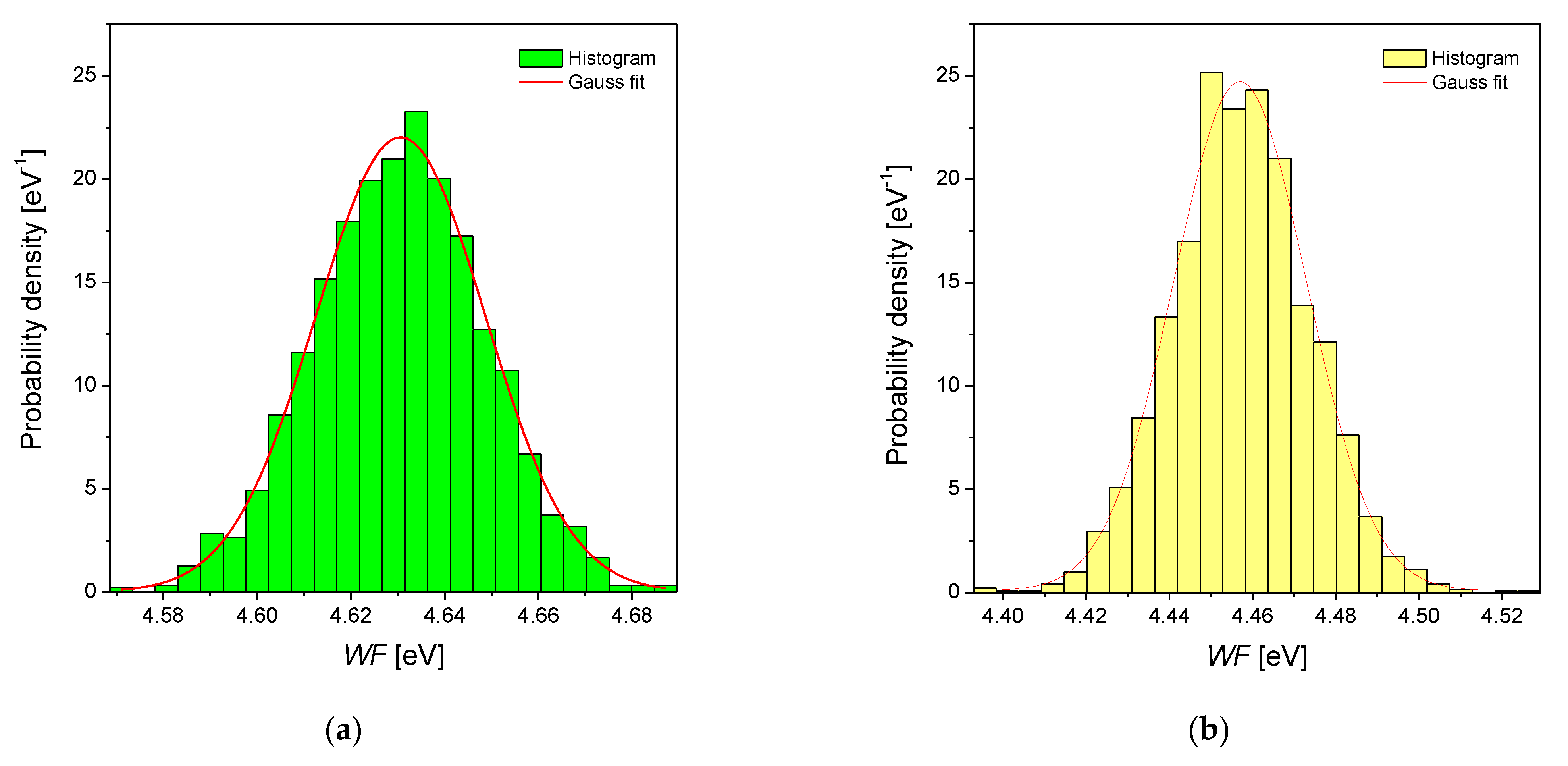
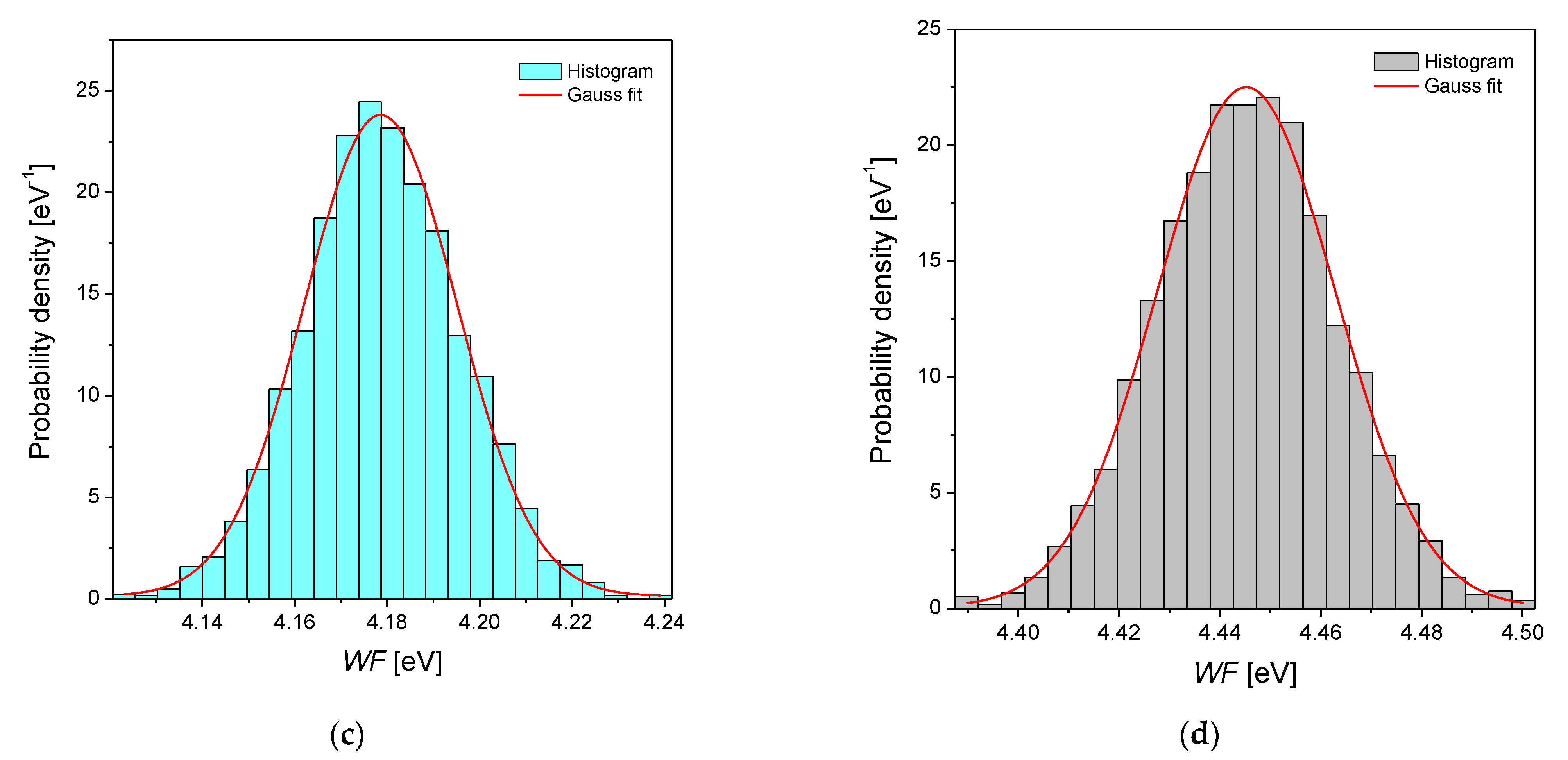
3.3. Scanning Kelvin Probe Measurements
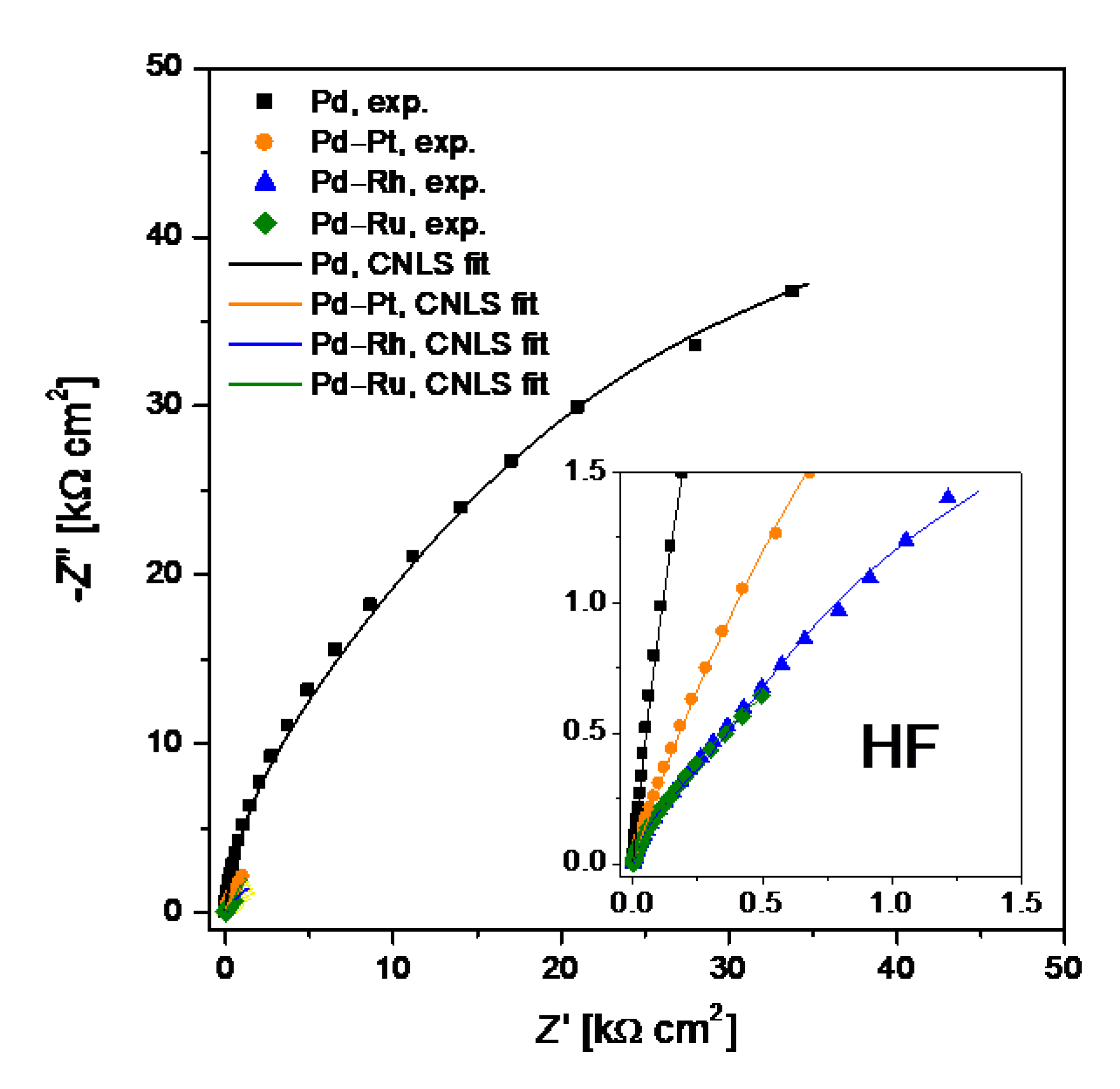
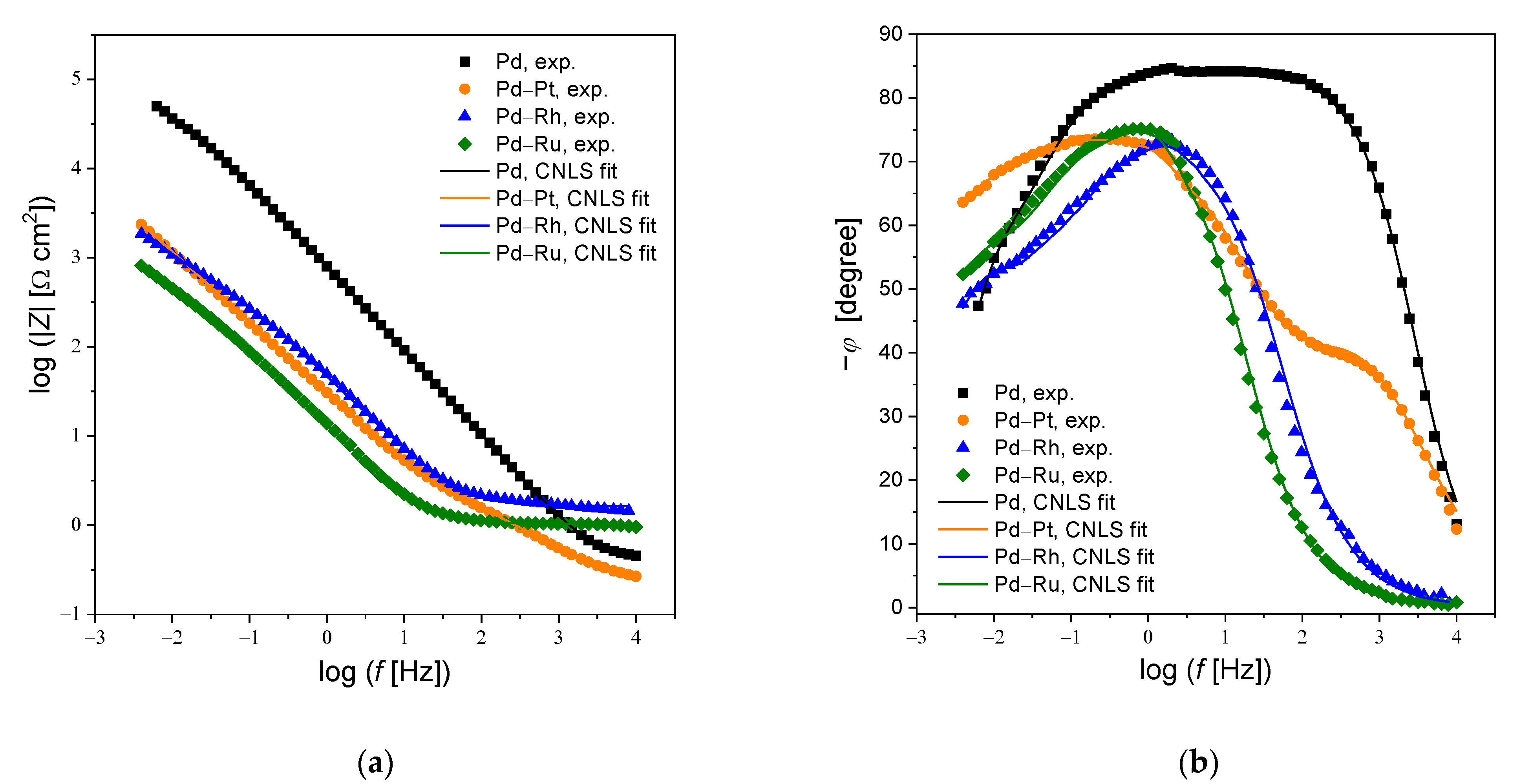
3.4. Electrochemical Impedance Spectroscopy
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sun, D.; Brantley, W.A.; Frankel, G.S.; Heshmati, R.H.; Johnston, W.M. Potentiodynamic polarization study of the corrosion behavior of palladium–silver dental alloys. J. Prosthet. Dent. 2018, 119, 650–656. [Google Scholar] [CrossRef]
- Feng, D.; Taskinen, P. Thermodynamic properties of silver–palladium alloys determined by a solid state electrochemical method. J. Mater. Sci. 2014, 49, 5790–5798. [Google Scholar] [CrossRef]
- Picard, C.; Kleppa, O.J.; Boureau, G. High–temperature thermodynamics of the solutions of hydrogen in palladium–silver alloys. J. Chem. Phys. 1979, 70, 2710–2719. [Google Scholar] [CrossRef]
- Li, S.; Zuo, Y.; Ju, P. Erosion–corrosion resistance of electroplated Co–Pd film on 316L stainless steel in a hot sulfuric acid slurry environment. Appl. Surf. Sci. 2015, 331, 200–209. [Google Scholar] [CrossRef]
- Zhang, H.; Li, Y.; Zuo, Y.; Zhao, X.; Tang, Y. Electrodeposition of multi–layer Pd–Ni coatings on 316L stainless steel and their corrosion resistance in hot sulfuric acid solution. Trans. Nonferr. Met. Soc. China 2017, 27, 1543–1550. [Google Scholar] [CrossRef]
- Tang, J.; Zhang, Z.; Wang, Y.; Ju, P.; Tang, Y.; Zuo, Y. Corrosion resistance mechanism of palladium film–plated stainless steel in boiling H2SO4 solution. Corros. Sci. 2018, 135, 222–232. [Google Scholar] [CrossRef]
- Song, J.; Wang, L.; Zibart, A.; Koch, C. Corrosion protection of electrically conductive surfaces. Metals 2012, 2, 450–477. [Google Scholar] [CrossRef]
- Huang, Y.H.; Yang, S.P.; Lee, P.T.; Kuo, T.T.; Ho, C.E. Significant improvement of the thermal stability and electrochemical corrosion resistance of the Au/Pd surface finish through catalytic modification. Corros. Sci. 2019, 146, 112–120. [Google Scholar] [CrossRef]
- Shao, M.; Liu, P.; Zhang, J.; Adzic, R. Origin of enhanced activity in palladium alloy electrocatalysts for oxygen reduction reaction. J. Phys. Chem. B 2007, 111, 6772–6775. [Google Scholar] [CrossRef] [PubMed]
- Pötzelberger, I.; Mardare, C.C.; Uiberlacker, L.M.; Hild, S.; Mardare, A.I.; Hassel, A.W. Electrocatalysis on copper–palladium alloys for amperometric formaldehyde sensing. RSC Adv. 2017, 7, 6031–6039. [Google Scholar] [CrossRef]
- Gunji, T.; Ochiai, H.; Ohira, T.; Liu, Y.; Nakajima, Y.; Matsumoto, F. Preparation of various Pd–based alloys for electrocatalytic CO2 reduction reaction—selectivity depending on secondary elements. Chem. Mater. 2020, 32, 6855–6863. [Google Scholar] [CrossRef]
- Vedyagin, A.A.; Shubin, Y.V.; Kenzhin, R.M.; Plyusnin, P.E.; Stoyanovskii, V.O. The Attractiveness of the Ternary Rh-Pd-Pt Alloys for CO Oxidation Process. Processes 2020, 8, 928. [Google Scholar] [CrossRef]
- Vedyagin, A.A.; Plyusnin, P.E.; Rybinskaya, A.A.; Shubin, Y.V.; Mishakov, I.V.; Korenev, S.V. Synthesis and study of Pd-Rh alloy nanoparticles and alumina-supported low-content Pd-Rh catalysts for CO oxidation. Mater. Res. Bull. 2018, 102, 196–202. [Google Scholar] [CrossRef]
- Vedyagin, A.A.; Volodin, A.M.; Stoyanovskii, V.O.; Kenzhin, R.M.; Slavinskaya, E.M.; Mishakov, I.V.; Plyusnin, P.E.; Shubin, Y.V. Stabilization of active sites in alloyed Pd–Rh catalysts on γ-Al2O3 support. Catal. Today 2014, 238, 80–86. [Google Scholar] [CrossRef]
- Fazle Kibria, A.K.M.; Sakamoto, Y. The effect of alloying of palladium with silver and rhodium on the hydrogen solubility, miscibility gap and hysteresis. Int. J. Hydrogen Energy 2000, 25, 853–859. [Google Scholar] [CrossRef]
- Comisso, N.; De Ninno, A.; Del Giudice, E.; Mengoli, G.; Soldan, P. Electrolytic hydriding of Pd79.5Rh20.5 alloy. Electrochim. Acta 2004, 49, 1379–1388. [Google Scholar] [CrossRef]
- Duncan, H.; Lasia, A. Hydrogen adsorption/absorption on Pd/Pt(1 1 1) multilayers. J. Electroanal. Chem. 2008, 621, 62–68. [Google Scholar] [CrossRef]
- Martin, M.H.; Lasia, A. Study of the hydrogen absorption in Pd in alkaline solution. Electrochim. Acta 2008, 53, 6317–6322. [Google Scholar] [CrossRef]
- Hubkowska, K.; Łukaszewski, M.; Czerwiński, A. Influence of temperature on hydrogen electrosorption into palladium–noble metal alloys. Part 2–Palladium–platinum alloys. Electrochim. Acta 2011, 56, 2344–2350. [Google Scholar] [CrossRef]
- Hubkowska, K.; Łukaszewski, M.; Czerwiński, A. Pd–Ru electrodeposits with high hydrogen absorption capacity. Electrochem. Commun. 2012, 20, 175–177. [Google Scholar] [CrossRef]
- Koss, U.; Hubkowska, K.; Łukaszewski, M.; Czerwiński, A. Influence of temperature on hydrogen electrosorption into palladium–noble metal alloys. Part 3: Palladium–rhodium alloys. Electrochim. Acta 2013, 107, 269–275. [Google Scholar] [CrossRef]
- Losiewicz, B.; Kubisztal, J.; Osak, P.; Starczewska, O. Effect of hydrogen electrosorption on mechanical and electronic properties of Pd80Rh20 alloy. Materials 2020, 13, 162. [Google Scholar] [CrossRef]
- Losiewicz, B.; Kubisztal, J. Effect of hydrogen electrosorption on corrosion resistance of Pd80Rh20 alloy in sulfuric acid: EIS and LEIS study. Int. J. Hydrogen Energy 2018, 43, 20004–20010. [Google Scholar] [CrossRef]
- Tremblay, J.; Nguyen, N.L.; Rochefort, D. Hydrogen absorption by a palladium electrode from a protic ionic liquid at temperatures exceeding 100 °C. Electrochem. Commun. 2013, 34, 102–104. [Google Scholar] [CrossRef]
- Pająk, M.; Hubkowska, K.; Czerwiński, A. The study of hydrogen sorption in palladium limited volume electrode from DEMA–TFO ionic liquid. J. Electroanal. Chem. 2018, 825, 73–76. [Google Scholar] [CrossRef]
- Pająk, M.; Hubkowska, K.; Czerwiński, A. Hydrogen sorption capacity as a tunable parameter in aprotic ionic liquids. Electrochem. Commun. 2020, 118, 106805. [Google Scholar] [CrossRef]
- Lewis, F.A. The Palladium/Hydrogen System; Academic Press: London, UK; New York, NY, USA, 1967. [Google Scholar] [CrossRef]
- Burch, R. Theoretical aspects of the absorption of hydrogen by palladium and its alloys. Part 1.–A reassessment and comparison of the various proton models. Trans. Faraday Soc. 1970, 66, 736–748. [Google Scholar] [CrossRef]
- Wicke, E.; Frölich, K. Electronic and elastic effect in the phase diagrams of binary Pd alloy hydrides. Z. Pchys. Chem. 1989, 163, 35–40. [Google Scholar] [CrossRef]
- Moysan, I.; Paul-Boncour, V.; Thiébaut, S.; Sciora, E.; Fournier, J.M.; Cortes, R.; Bourgeois, S.; Percheron-Guégan, A. Pd–Pt alloys: Correlation between electronic structure and hydrogenation properties. J. Alloys Comp. 2001, 322, 14–20. [Google Scholar] [CrossRef]
- Hubkowska, K.; Łukaszewski, M.; Koss, U.; Czerwiński, A. Characterization and electrochemical behavior of Pd-rich Pd-Ru alloys. Electrochim. Acta 2014, 132, 214–222. [Google Scholar] [CrossRef]
- Grdeń, M.; Piaścik, A.; Koczorowski, Z.; Czerwiński, A. Hydrogen electrosorption in Pd–Pt alloys. J. Electroanal. Chem. 2002, 532, 35–42. [Google Scholar] [CrossRef]
- Manolova, M.; Böck, R.; Scharf, I.; Mehner, T.; Lampke, T. Electrodeposition of Pd alloys from choline chloride/urea deep eutectic solvents. J. Alloys Comp. 2021, 855, 157462. [Google Scholar] [CrossRef]
- Vedyagin, A.A.; Shubin, Y.V.; Kenzhin, R.M.; Plyusnin, P.E.; Stoyanovskii, V.O.; Volodin, A.M. Prospect of Using Nanoalloys of Partly Miscible Rhodium and Palladium in Three-Way Catalysis. Top Catal. 2019, 62, 305–314. [Google Scholar] [CrossRef]
- Kostin, G.A.; Plyusnin, P.E.; Filatov, E.U.; Kuratieva, N.V.; Vedyagin, A.A.; Kal’nyi, D.B. Double complex salts [PdL4][RuNO(NO2)4OH] (L = NH3, Py) synthesis, structure and preparation of bimetallic metastable solid solution Pd0.5Ru0.5. Polyhedron 2019, 159, 217–225. [Google Scholar] [CrossRef]
- Hubkowska, K.; Łukaszewski, M.; Soszko, M.; Koss, U.; Hamankiewicz, B.; Czerwiński, A. Comparative Physicochemical and Electrochemical Characterization of the Structure and Composition of Thin Pd Binary and Ternary Codeposits with Pt, Ru, and Rh. Materials 2018, 11, 798. [Google Scholar] [CrossRef]
- Łukaszewski, M.; Czerwiński, A. Anodic oxidation of Pd alloys with Pt and Rh. J. Alloys Comp. 2009, 473, 220–226. [Google Scholar] [CrossRef]
- Hubkowska, K. The Electrochemical Properties of Pd–Ru Alloys. Ph.D. Thesis, Faculty of Chemistry, University of Warsaw, Warsaw, Poland, 2016. Available online: https://depotuw.ceon.pl/bitstream/handle/item/1429/Hubkowska-Kosi%c5%84ska_rozprawa%20doktorska.pdf?sequence=1 (accessed on 28 May 2021).
- Łukaszewski, M.; Czerwiński, A. Dissolution of noble metals and their alloys studied by electrochemical quartz crystal micro–balance. J. Electroanal. Chem. 2006, 589, 38–45. [Google Scholar] [CrossRef]
- Woods, R. Electroanalytical Chemistry; Bard, A.J., Ed.; Marcel Dekker: New York, NY, USA, 1976; Volume 9, pp. 1–162. [Google Scholar]
- Klein, A.; Körber, C.; Wachau, A.; Säuberlich, F.; Gassenbauer, Y.; Harvey, S.P.; Proffit, D.E.; Mason, T.O. Transparent Conducting Oxides for Photovoltaics: Manipulation of Fermi Level, Work Function and Energy Band Alignment. Materials 2010, 3, 4892–4914. [Google Scholar] [CrossRef]
- Hara, M.; Wan, L.; Matsuyama, M.; Watanabe, K. Alloying effect on heat of hydride and deuteride formation for Pd–based binary alloys. J. Alloys Comp. 2007, 428, 252–255. [Google Scholar] [CrossRef]
- Papaconstantopoulos, D.A. Handbook of the Band Structure of Elemental Solids, 2nd ed.; Springer: New York, NY, USA; London, UK, 2015. [Google Scholar]
- Halas, S.; Durakiewicz, T. Work functions of elements expressed in terms of the Fermi energy and the density of free electrons. J. Phys. Condens. Matter 1998, 10, 10815–10826. [Google Scholar] [CrossRef]
- Onuferko, J.H.; Woodruff, D.P.; Holland, B.W. LEED structure analysis of the Ni{100} (2 × 2)C (p4g) structure; A case of adsorbate-induced substrate distortion. Surf. Sci. 1979, 87, 357–374. [Google Scholar] [CrossRef]
- Lasia, A. Electrochemical Impedance Spectroscopy and Its Applications; Springer Science & Business Media: New York, NY, USA, 2014. [Google Scholar]


| Alloy Type | As–Deposited | After Corrosion |
|---|---|---|
| Pd-Rh | 96.9(4)% Pd 3.1(4)% Rh | 97.0(1)% Pd 3.0(1)% Rh |
| Pd-Ru | 96.3(3)% Pd 3.7(3)% Ru | 96.4(3)% Pd 3.6(3)% Ru |
| Pd-Pt | 96.7(7)% Pd 3.3(7)% Pt | 94.7(3)% Pd 5.3(3)% Pt |
| Alloy Type | jcor [µA cm–2] | Ecor [mV] | ba [V dec–1] | bc [V dec–1] | Rp × 104 [Ω cm2] | CR at Ecor [mm yr–1] |
|---|---|---|---|---|---|---|
| Pd | 0.26(1) | 794.5(3) | 0.096(1) | 0.122(1) | 9.0(3) | 3.8(1) × 10−3 |
| Pd-Rh | 2.87(5) | 733.6(3) | 0.116(2) | 0.066(1) | 0.63(1) | 4.08(7) × 10−2 |
| Pd-Ru | 10.1(1) | 730.5(1) | 0.159(2) | 0.062(1) | 0.19(1) | 1.43(1) × 10−1 |
| Pd-Pt | 1.35(4) | 748.5(4) | 0.093(2) | 0.068(2) | 1.26(5) | 1.92(6) × 10−2 |
| Alloy Type | ƒas–deposited | ƒafter corrosion |
|---|---|---|
| Pd | 1.7(1) | 1.3(1) |
| Pd-Rh | 8.9(7) | 15(1) |
| Pd-Ru | 64(3) | 58(3) |
| Pd-Pt | 1.4(1) | 10.4(8) |
| Alloy Type | EF Calculated [eV] 1 | WFav [eV] | σ [eV] | WFlit [eV] | Metal | WFlit [eV] 2 |
|---|---|---|---|---|---|---|
| Pd | 7.06 | 4.63(1) | 18.1(4) × 10−3 | Pd | 5.12 | |
| Pd-Rh | 7.11 | 4.46(1) | 16.0(5) × 10−3 | 4.76 3 | Rh | 4.98 |
| Pd-Ru | 7.19 | 4.18(1) | 16.5(5) × 10−3 | Ru | 4.71 | |
| Pd-Pt | 7.11 | 4.45(1) | 17.1(3) × 10−3 | Pt | 5.65 |
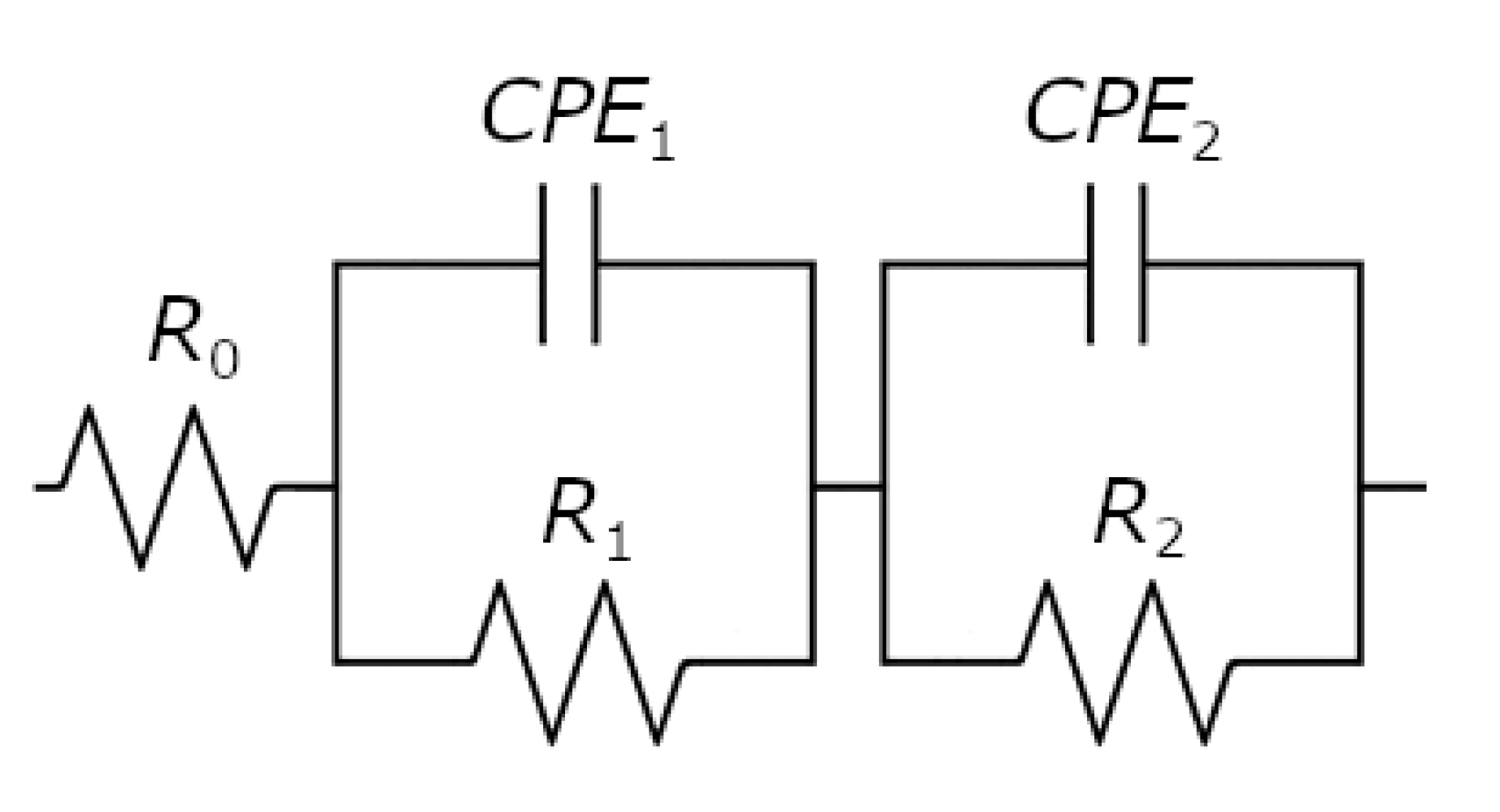
| Alloy Type | R0 | T1 | ϕ1 | R1 | T2 | ϕ2 | R2 |
|---|---|---|---|---|---|---|---|
| [Ω cm2] | [F cm−2 sϕ−1] | [Ω cm2] | [F cm−2 sϕ−1] | [Ω cm2] | |||
| Pd | 0.44(1) | 5.3(3) × 10−4 | 0.94(8) | 8.4(7) × 103 | 3.8(9) × 10−4 | 0.95(7) | 82.9(9) × 103 |
| Pd-Rh | 0.39(1) | 91(9) × 10−4 | 0.88(6) | 225(77) | 90(9) × 10−4 | 0.81(3) | 4.8(7) × 103 |
| Pd-Ru | 0.43(1) | 284(16) × 10−4 | 0.85(5) | 167(12) | 331(19) × 10−4 | 0.90(9) | 2.0(7) × 103 |
| Pd-Pt | 0.38(1) | 76(9) × 10−4 | 0.69(2) | 124(7) | 77.1(4) × 10−4 | 0.80(1) | 13.2(9) × 103 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hubkowska, K.; Kubisztal, J.; Pająk, M.; Łosiewicz, B.; Czerwiński, A. Effect of the Alloying Metal on the Corrosion Resistance of Pd-Rich Binary Alloys with Pt, Rh, and Ru in Sulfuric Acid. Materials 2021, 14, 2923. https://doi.org/10.3390/ma14112923
Hubkowska K, Kubisztal J, Pająk M, Łosiewicz B, Czerwiński A. Effect of the Alloying Metal on the Corrosion Resistance of Pd-Rich Binary Alloys with Pt, Rh, and Ru in Sulfuric Acid. Materials. 2021; 14(11):2923. https://doi.org/10.3390/ma14112923
Chicago/Turabian StyleHubkowska, Katarzyna, Julian Kubisztal, Małgorzata Pająk, Bożena Łosiewicz, and Andrzej Czerwiński. 2021. "Effect of the Alloying Metal on the Corrosion Resistance of Pd-Rich Binary Alloys with Pt, Rh, and Ru in Sulfuric Acid" Materials 14, no. 11: 2923. https://doi.org/10.3390/ma14112923
APA StyleHubkowska, K., Kubisztal, J., Pająk, M., Łosiewicz, B., & Czerwiński, A. (2021). Effect of the Alloying Metal on the Corrosion Resistance of Pd-Rich Binary Alloys with Pt, Rh, and Ru in Sulfuric Acid. Materials, 14(11), 2923. https://doi.org/10.3390/ma14112923






