The Influence of Temperature on the Hydration Rate of Cements Based on Calorimetric Measurements
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Test Procedure
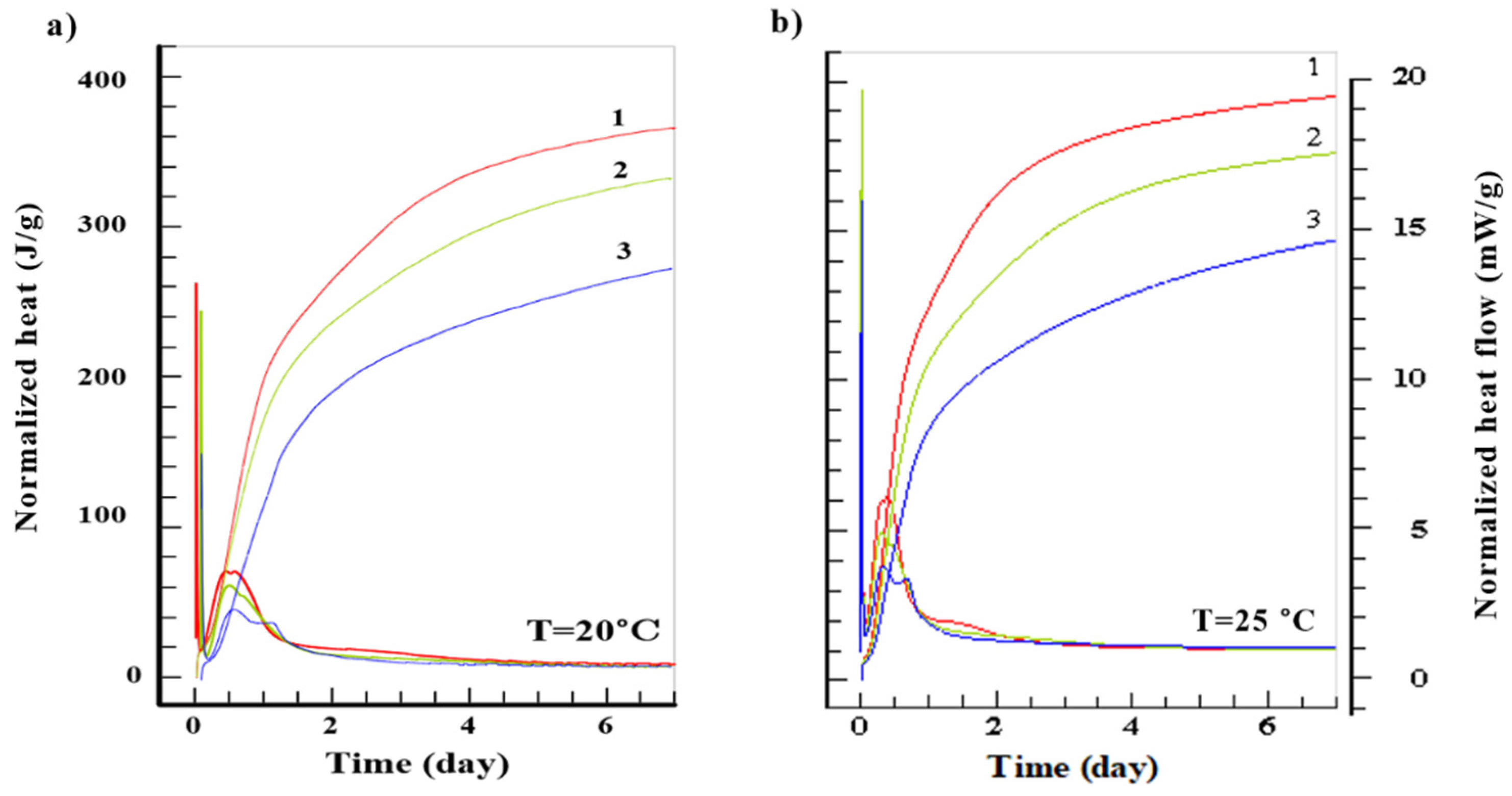
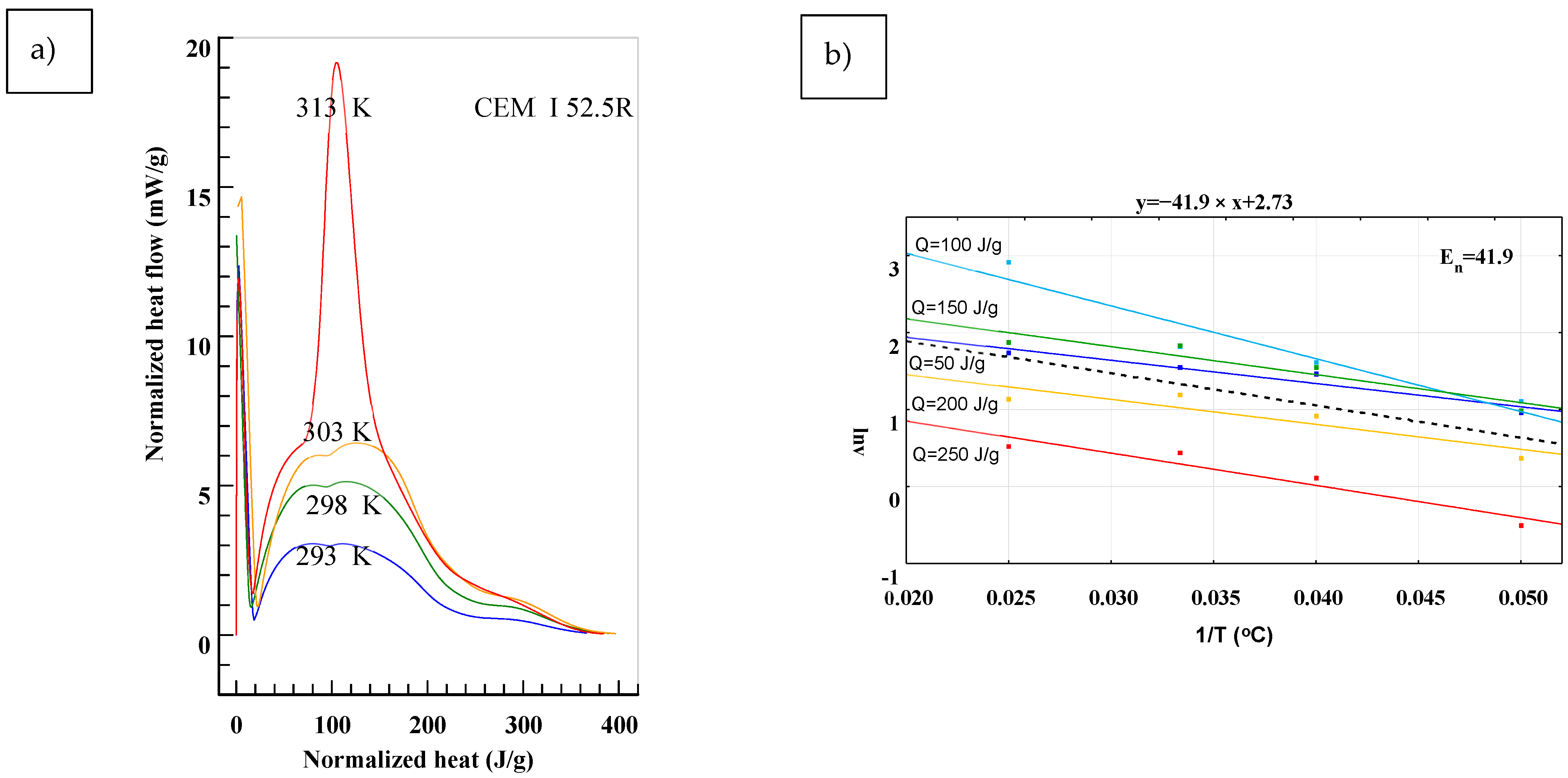
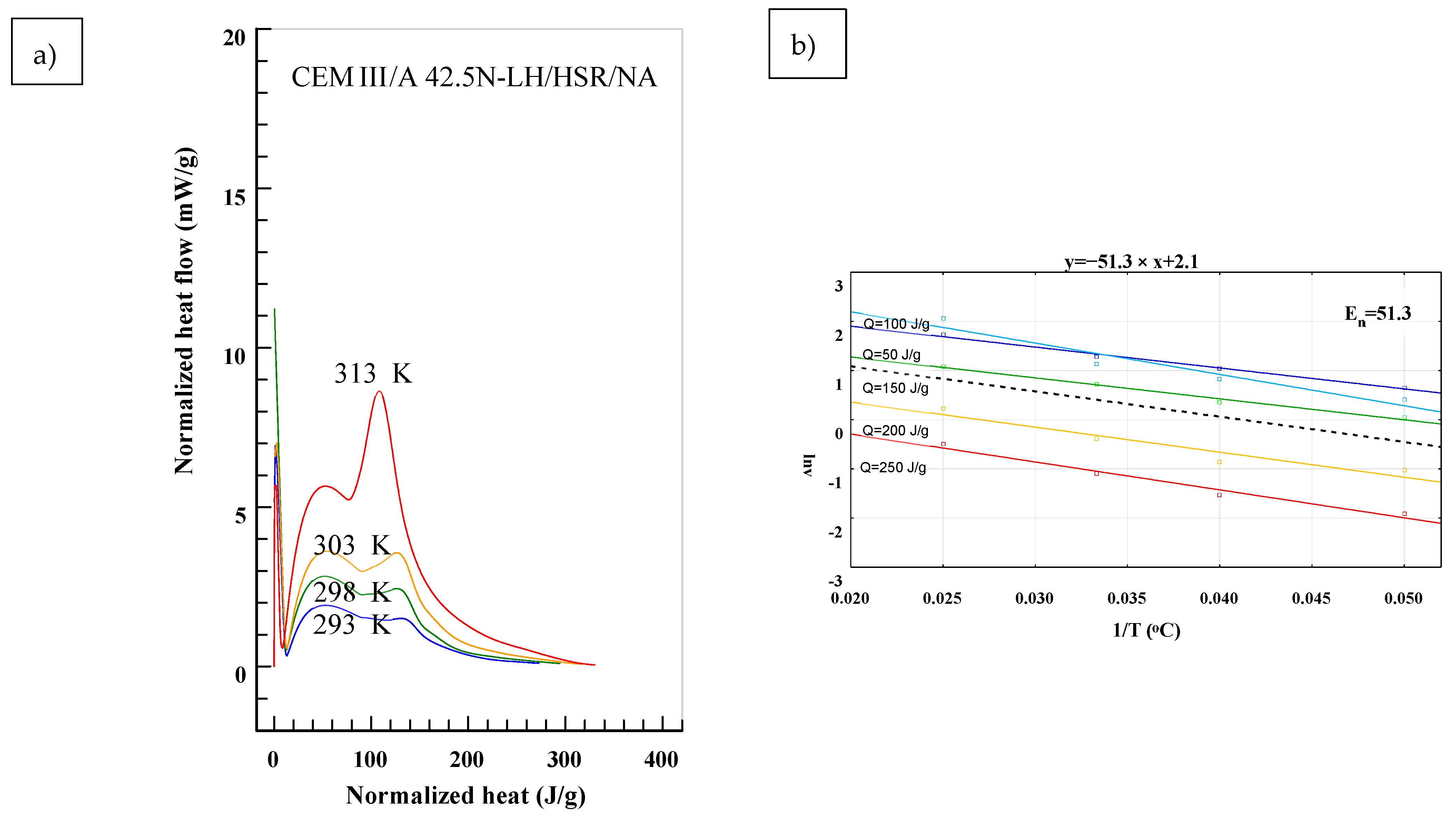
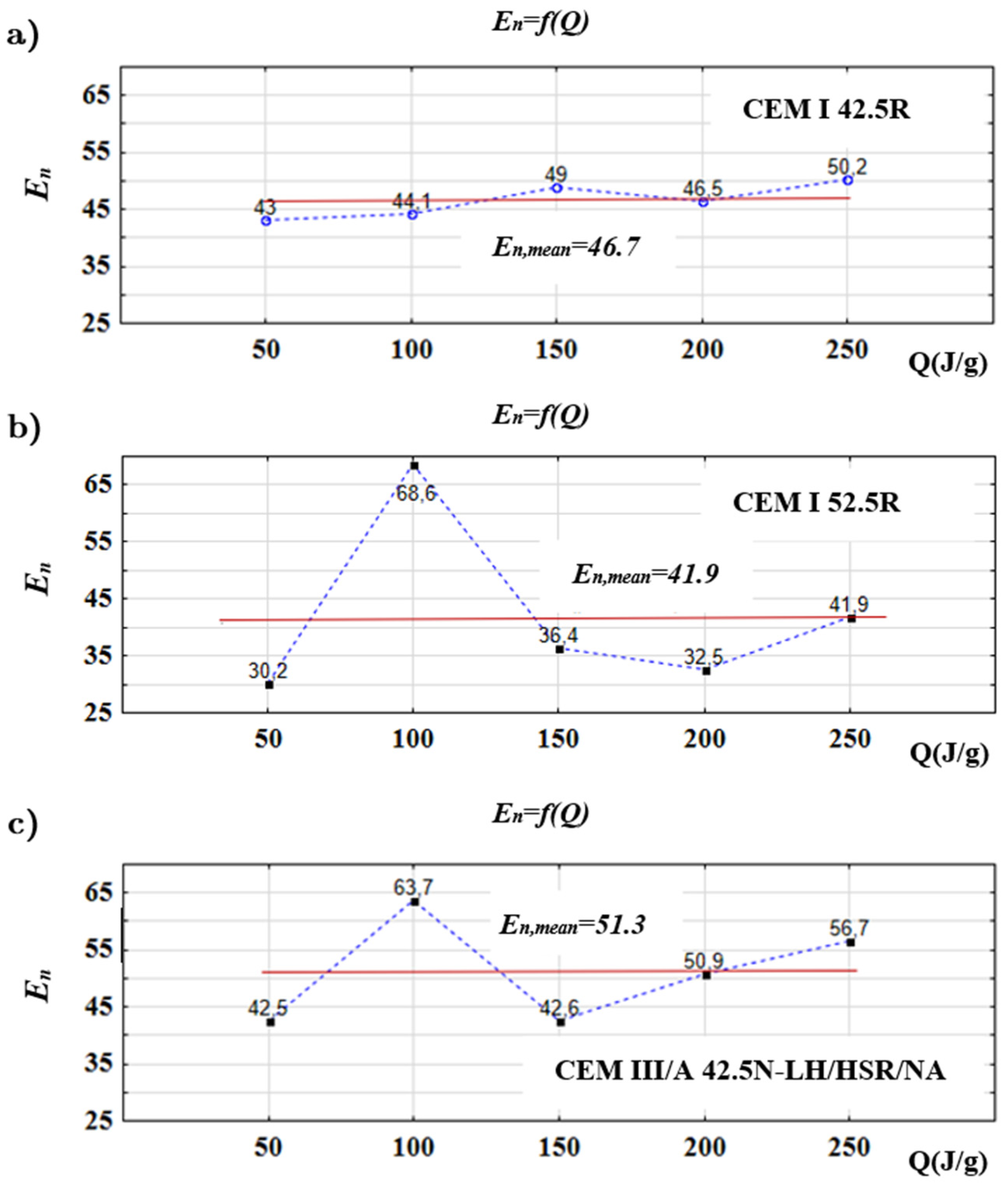
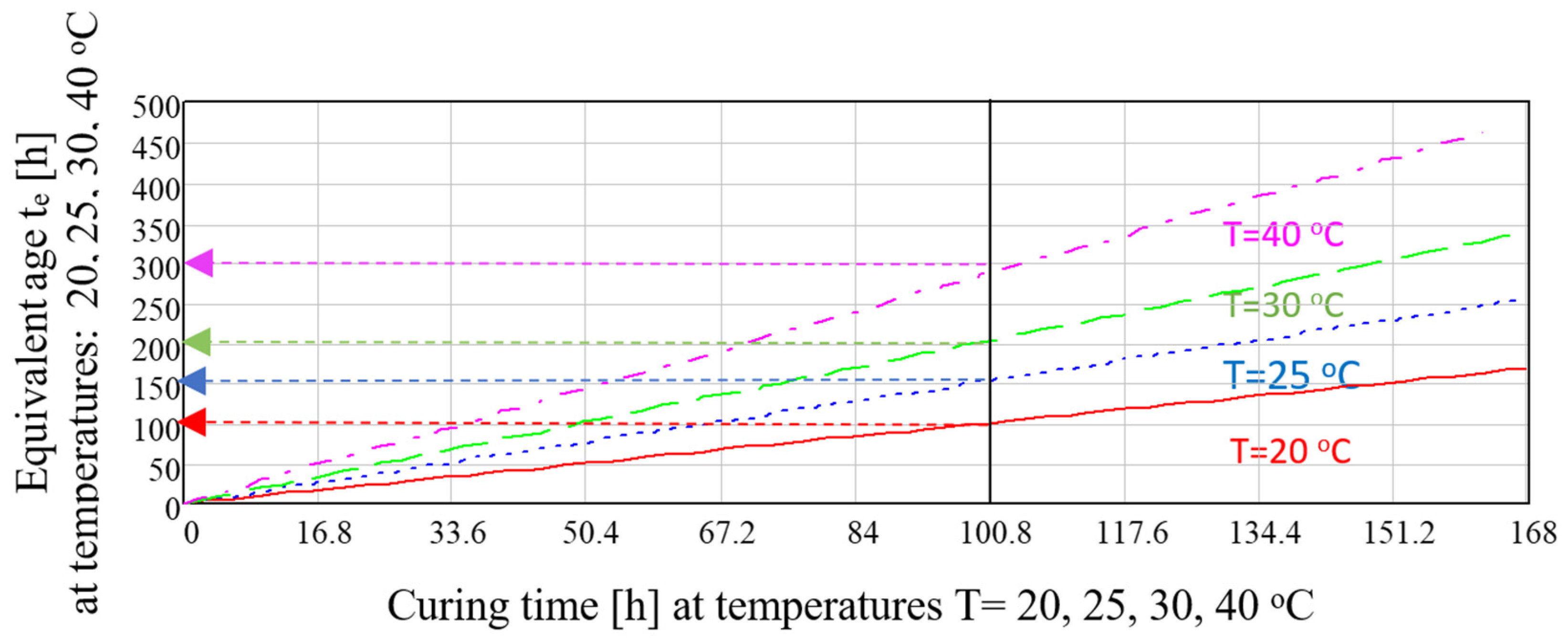
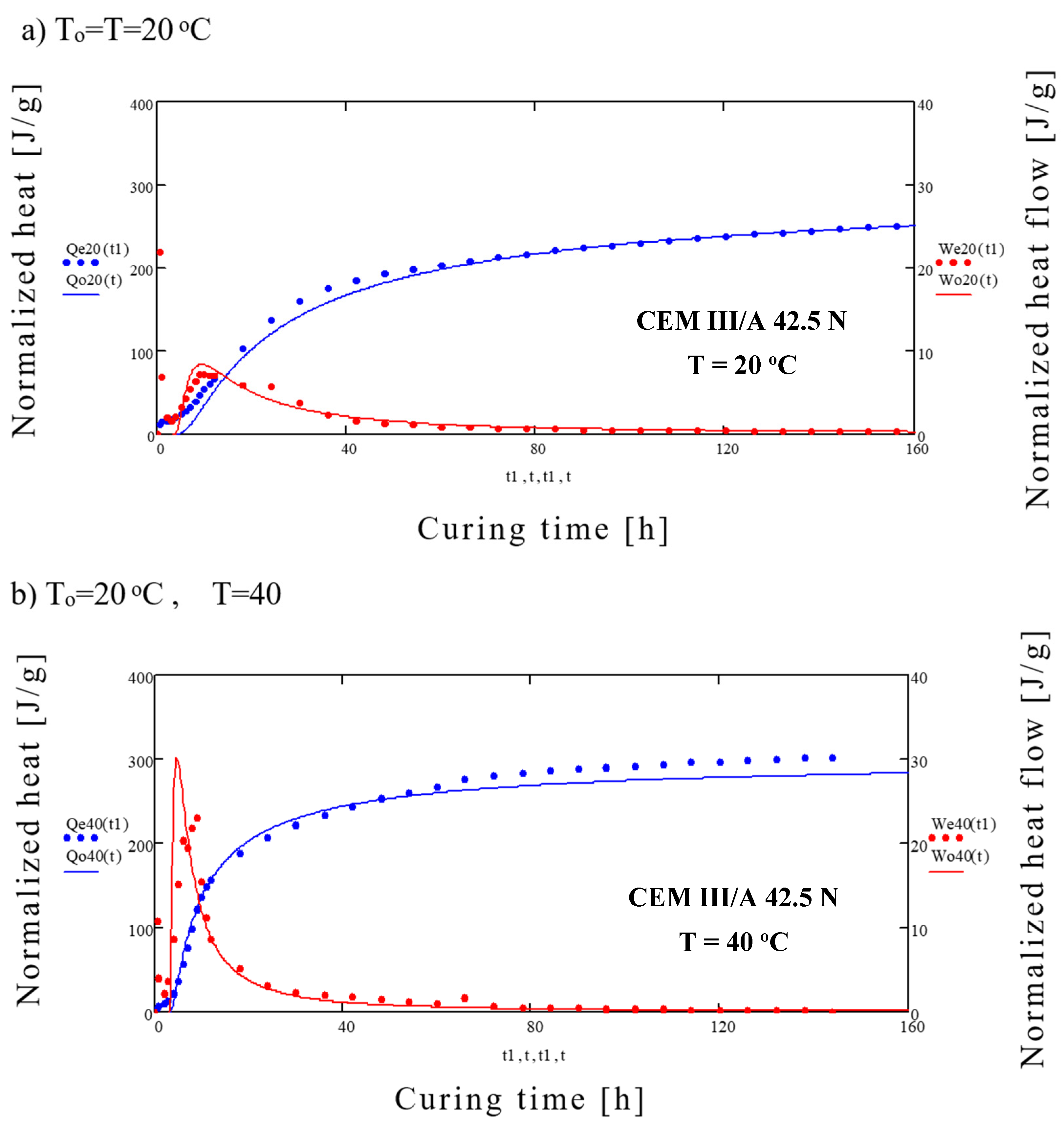
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nasir, M.; Baghabra Al-Amoudi, O.S.; Maslehuddin, M. Effect of placement temperature and curing method on plastic shrinkage of plain and pozzolanic cement concretes under hot weather. Constr. Build. Mater. 2017, 152, 943–953. [Google Scholar] [CrossRef]
- Klemczak, B.; Żmij, A. Insight into Thermal Stress Distribution and Required Reinforcement Reducing Early-Age Cracking in Mass Foundation Slabs. Materials 2021, 14, 477. [Google Scholar] [CrossRef]
- Chu, I.; Lee, Y.; Amin, M.N.; Jang, B.-S.; Kim, J.-K. Application of a thermal stress device for the prediction of stresses due to hydration heat in mass concrete structure. Constr. Build. Mater. 2013, 45, 192–198. [Google Scholar] [CrossRef]
- Batog, M.; Giergiczny, Z. Influence of mass concrete constituents on its properties. Constr. Build. Mater. 2017, 146, 221–230. [Google Scholar] [CrossRef]
- Su, J.; Zuo, G.W.; Li, W. Temperature Control Technique and Analysis of Mass Concrete in the Pile Cap of Main Pier in Yangtze River Bridge. Dev. Ind. Manuf. 2014, 587–589, 1407–1411. [Google Scholar] [CrossRef]
- Lee, M.H.; Chae, Y.S.; Khil, B.S.; Yun, H.D. Influence of Casting Temperature on the Heat of Hydration in Mass Concrete Foundation with Ternary Cements. Dev. Ind. Manuf. 2014, 525, 478–481. [Google Scholar] [CrossRef]
- Xu, W.; Qiang, S.; Hu, Z.; Ding, B.; Yang, B. Effect of Hydration Heat Inhibitor on Thermal Stress of Hydraulic Structures with Different Thicknesses. Adv. Civ. Eng. 2020, 2020, 5029865. [Google Scholar] [CrossRef]
- Ju, Y.; Lei, H. Actual Temperature Evolution of Thick Raft Concrete Foundations and Cracking Risk Analysis. Adv. Mater. Sci. Eng. 2019, 2019, 7029671. [Google Scholar] [CrossRef] [Green Version]
- Bentz, D.; Waller, V.; de Larrard, F. Prediction of Adiabatic Temperature Rise in Conventional and High-Performance Concretes Using a 3-D Microstructural Model. Cem. Concr. Res. 1998, 28, 285–297. [Google Scholar] [CrossRef]
- Statens Byggeforskningsinstitut. Winter Concreting–Theory and Practice: Proceedings of the RILEM Symposium, Copenhagen, 1956; SBI forlag: København, Denmark, 1956. [Google Scholar]
- Schindler, A.K. Effect of Temperature on Hydration of Cementitious Materials. MJ 2004, 101, 72–81. [Google Scholar] [CrossRef]
- Malhotra, V.M. Maturity Concept and The Estimation of Concrete Strength: A Review; Department of Mines and Technical Surveys: Ottawa, ON, Canada, 1971. [Google Scholar]
- Mariak, A. Wyznaczanie wytrzymałości betonu na podstawie funkcji dojrzałości wg amerykańskiej normy ASTM C1074-11. Constr. Mater. 2015, 1, 70–73. [Google Scholar] [CrossRef]
- Zhang, J.; Cusson, D.; Monteiro, P.; Harvey, J. New perspectives on maturity method and approach for high performance concrete applications. Cem. Concr. Res. 2008, 38, 1438–1446. [Google Scholar] [CrossRef] [Green Version]
- Lee, C.; Lee, S.; Nguyen, N. Modeling of Compressive Strength Development of High-Early-Strength-Concrete at Different Curing Temperatures. Int. J. Concr. Struct. Mater. 2016, 10, 205–219. [Google Scholar] [CrossRef] [Green Version]
- Fjellström, P.; Jonasson, J.-E.; Emborg, M.; Hedlund, H. Model for Concrete Strength Development Including Strength Reduction at Elevated Temperatures. Nord. Concr. Res. 2012, 45, 25–44. [Google Scholar]
- CEN. Eurocode 2: Design of Concrete Structures–Part 1-1: General Rules and Rules for Buildings (EN 1992-1-1:2008); British Standards Institution: London, UK, 1992. [Google Scholar]
- Saul, A.G.A. Principles underlying the steam curing of concrete at atmospheric pressure. Mag. Concr. Res. 1951, 2, 127–140. [Google Scholar] [CrossRef]
- Rastrup, E. Heat of hydration in concrete. Mag. Concr. Res. 1954, 6, 79–92. [Google Scholar] [CrossRef]
- Byfors, J. Plain Concrete at Early Ages; Swedish cement and concrete research Institute: Stockholm, Sweden, 1980. [Google Scholar]
- Flaga, K. Temperature function of concrete curing at elevated temperatures. Funkcja temperatury betonu tężejącego w warunkach podwyższonych temperatur. Arch. Civ. Eng. 1969, 15, 1–2. [Google Scholar]
- Bresson, J.E. Prediction of strength of concrete products. In Proceedings of the RILEM International Conference on Concrete at Early Ages, I. Ecole Nationale des Ponts et Chaussees, Paris, France, 6–8 April 1982; pp. 111–115. [Google Scholar]
- Carino, N.J. Maturity functions for concrete. In Proceedings of the RILEM International Conference on Concrete at Early Ages, Paris, France, 6–8 April 1982. [Google Scholar]
- Carino, N.J.; Lew, H.S. The Maturity Method: From Theory to Application. In Structures 2001, Proceedings of the Structures Congress 2001, Washington, DC, USA, 21–23 May 2001; Chang, P.C., Ed.; American Society of Civil Engineers: Reston, VA, USA, 2012; pp. 1–19. ISBN 978-0-7844-0558-1. [Google Scholar]
- Zych, M. Case study of concrete mechanical properties development based on heat temperature measurements. Cem. Wapno Beton 2015, 6, 383–392. [Google Scholar]
- C09 Committee. Practice for Measuring Hydration Kinetics of Hydraulic Cementitious Mixtures Using Isothermal Calorimetry; ASTM International: West Conshohocken, PA, USA, 2008. [Google Scholar]
- C09 Committee. Practice for Estimating Concrete Strength by the Maturity Method; ASTM International: West Conshohocken, PA, USA, 2004. [Google Scholar]
- Kurdowski, W.; Pichniarczyk, P. Kłopoty z równaniem Arrheniusa przy ocenie dojrzałości betonu. Cem. Wapno Beton 2016, 21, 149–156. [Google Scholar]
- Roy, D.M.; Cady, P.D.; Sabol, S.A.; Licastro, P.H. Concrete Microstruccture: Recommended Revisions to Test Methods; National Research Council: Washington, DC, USA, 1993. [Google Scholar]
- Kada-Benameur, H.; Wirquin, E.; Duthoit, B. Determination of apparent activation energy of concrete by isothermal calorimetry. Cem. Concr. Res. 2000, 30, 301–305. [Google Scholar] [CrossRef]
- Kiernożycki, W.; Błyszko, J. Calorimetric measurements and numerical modelling of the effects of selected chemical admixtures on the development of cement hydration at different temperatures. Cem. Wapno Beton 2019, 24, 432–447. [Google Scholar] [CrossRef]
- Wirquin, E.; Broda, M.; Duthoit, B. Determination of the apparent activation energy of one concrete by calorimetric and mechanical means. Cem. Concr. Res. 2002, 32, 1207–1213. [Google Scholar] [CrossRef]
- Nielsen, C.V.; Kaasgaard, M. Activation Energy for the Concrete Maturity Model—Part 1: Compressive Strength Tests at Different Curing Temperatures. Nord. Concr. Res. 2020, 62, 87–106. [Google Scholar] [CrossRef]
- Methods of Testing Cement. Heat of Hydration. Isothermal Conduction Calorimetry Method; BS EN 196-11:2018; BSI British Standards: London, UK, 2018.
- Neville, A.M. Properties of Concrete, 5th ed.; Pearson: London, UK, 2012; ISBN 978-0273755807. [Google Scholar]
- Regourd, M.; Mortureux, C. Caractérisation et activation thermique des ciments an laitier. In Proceedings of the 7th Congrès International de la Chimie des Ciments, Paris, France, 20 June–4 July 1980; Volume IV. [Google Scholar]
- Kurdowski, W. Chemia Cementu; Wydawnictwo Naukowe PWN: Warszawa, Poland, 1991; ISBN 9788301103842. [Google Scholar]
- Wyrzykowski, M.; Gawin, D. Modelling and experimental study of hydration for ordinary Portland cement. Archit. Civ. Eng. Environ. 2010, 3, 43–54. [Google Scholar]
- Kiernożycki, W. Betonowe Konstrukcje Masywne: Teoria, Wymiarowanie, Realizacja; Polski Cement: Kraków, Poland, 2003; ISBN 9788389478009. [Google Scholar]
- Wesche, K. Baustoffkennwerte zur Berechnung von Temperaturfeldern in Betonbauteilen. In Liber Amicorum opgedragen aan F.G. Riessauw ter gelegenheid van zijn zeventigste verjaardag; RUG Magnel Laboratory for reinforced concrete: Gent, Belgum, 1982. [Google Scholar]




| Characteristic | CEM I 52.5 R | CEM I 42.5 R | CEM III 42.5 N |
|---|---|---|---|
| Composition [%] | |||
| Portland clinker | 95 100 | 95 100 | 35 64 |
| Ground granulated blast furnace slag | - | - | 36 65 |
| Secondary components | 0 5 | 0 5 | 0 5 |
| Compressive strength [MPa] | |||
| 2 days | 36.2 | 29.0 | 14.8 |
| 28 days | 63.6 | 56.9 | 58.3 |
| Setting time (initial) [min] | 170 | 184 | 201 |
| Surface area (Blaine) [cm2/g] | 4411 | 3717 | 4636 |
| Chemical composition [%] | |||
| SO3 | 2.93 | 2.93 | 2.70 |
| Cl− | 0.067 | 0.066 | 0.080 |
| Loss on ignition | 3.73 | 3.40 | 1.08 |
| Insoluble residue | 0.72 | 0.70 | 0.48 |
| Heat of hydration (7 days) [J/g] | 325 375 | 325 375 | <270 |
| Cement | Temperature (°C) | Q71 (J/g) | (mW/g) | (h, min) |
|---|---|---|---|---|
| CEM I 52.5 R | 20 | 366 | 3.06 | 12 h 5 min |
| 25 | 390 | 5.10 | 8 h 50 min | |
| 30 | 396 | 6.38 | 7 h 45 min | |
| 40 | 384 | 19.17 | 5 h 50 min | |
| CEM I 42.5 R | 20 | 332 | 2.66 | 10 h 45 min |
| 25 | 352 | 3.92 | 7 h 50 min | |
| 30 | 353 | 4.91 | 6 h 10 min | |
| 40 | 360 | 7.90 | 4 h 10 min | |
| CEM III 42.5 N | 20 | 272 | 1.93 | 11 h 25 min |
| 25 | 294 | 2.77 | 8 h 55 min | |
| 30 | 316 | 3.59 | 7 h 30 min | |
| 40 | 330 | 8.64 | 8 h 15 min |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kiernożycki, W.; Błyszko, J. The Influence of Temperature on the Hydration Rate of Cements Based on Calorimetric Measurements. Materials 2021, 14, 3025. https://doi.org/10.3390/ma14113025
Kiernożycki W, Błyszko J. The Influence of Temperature on the Hydration Rate of Cements Based on Calorimetric Measurements. Materials. 2021; 14(11):3025. https://doi.org/10.3390/ma14113025
Chicago/Turabian StyleKiernożycki, Włodzimierz, and Jarosław Błyszko. 2021. "The Influence of Temperature on the Hydration Rate of Cements Based on Calorimetric Measurements" Materials 14, no. 11: 3025. https://doi.org/10.3390/ma14113025






