Highly Effective Adsorption Process of Ni(II) Ions with the Use of Sewage Sludge Fly Ash Generated by Circulating Fluidized Bed Combustion (CFBC) Technology
Abstract
1. Introduction
2. Experimental Procedure
2.1. Materials and Methods
2.1.1. Sewage Sludge Fly Ash Preparation
2.1.2. Sewage Sludge Fly Ash Characterization
2.1.3. Nickel(II) Adsorption Process
3. Results and Discussion
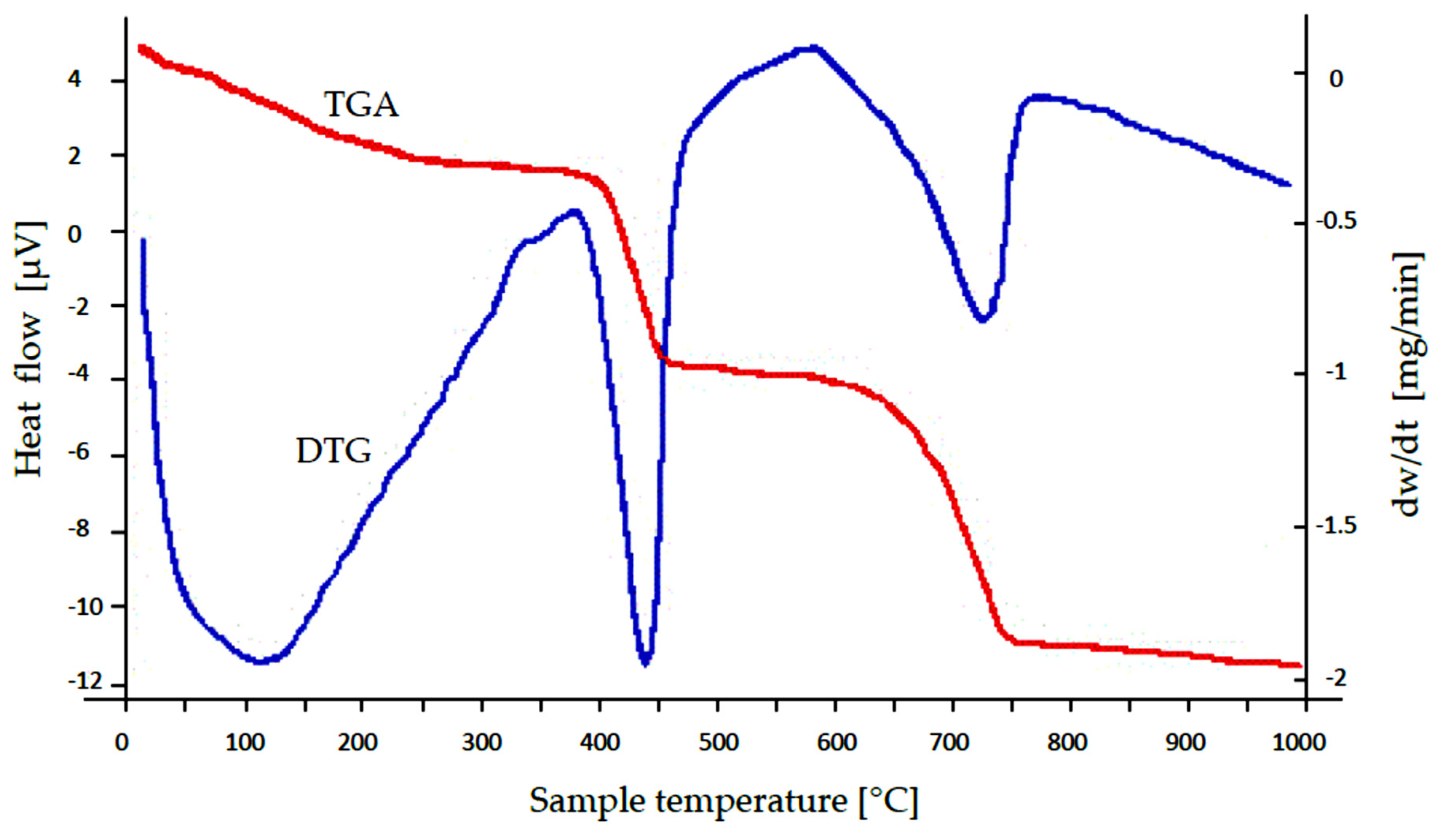
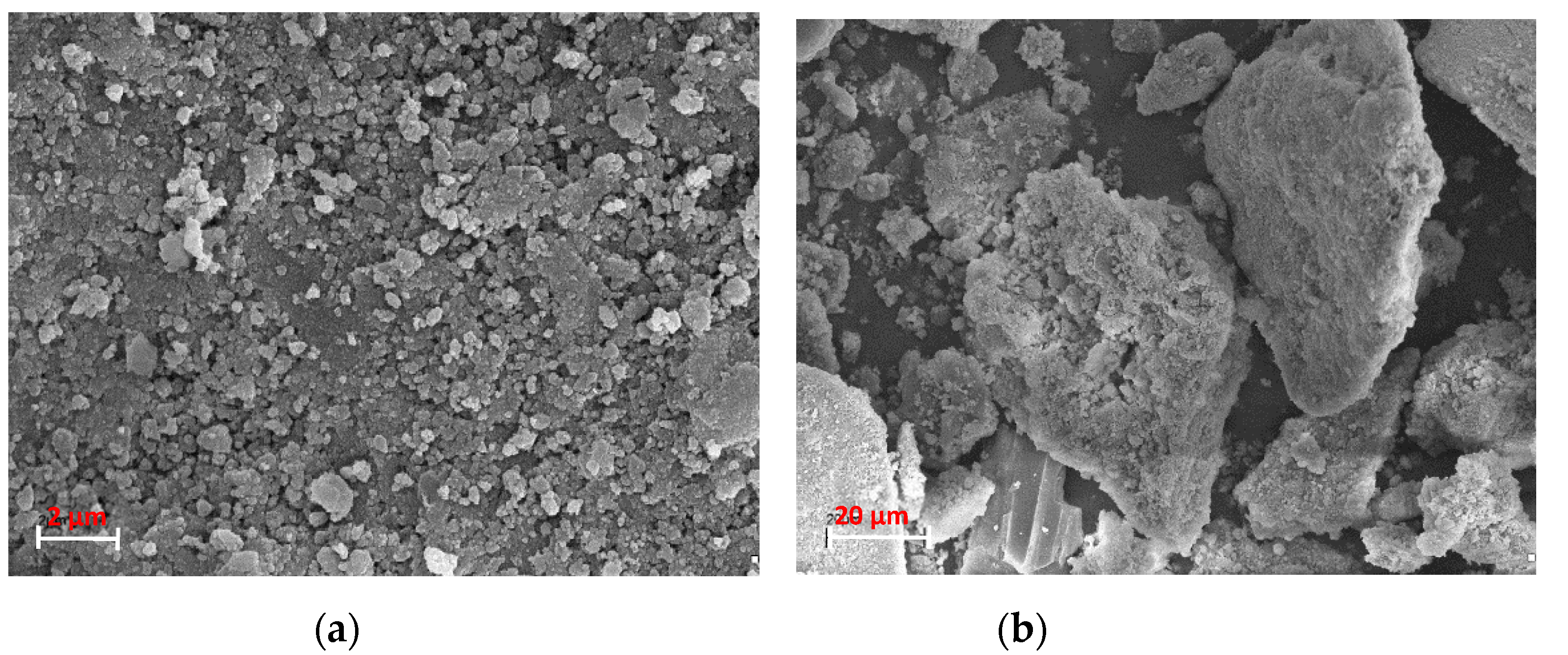
3.1. Characterization of the SS-FA Adsorptive Material
3.2. Adsorption Analysis of Ni(II) Ions
3.2.1. Analysis of pH Profile
3.2.2. Effect of Adsorbent Dosage
3.2.3. Effect of Initial Concentration of Ni(II) Ions
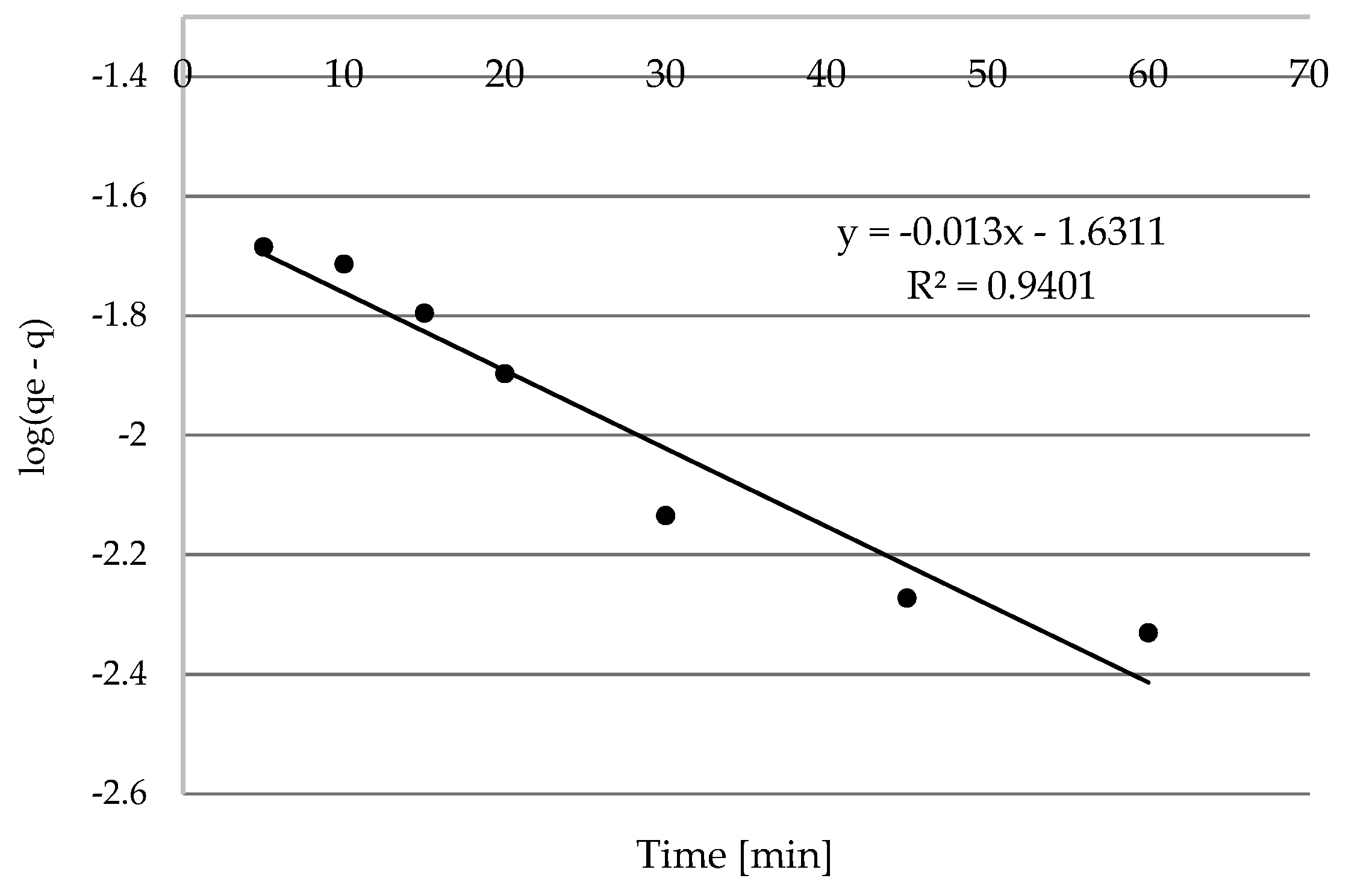
3.2.4. Kinetics Analysis
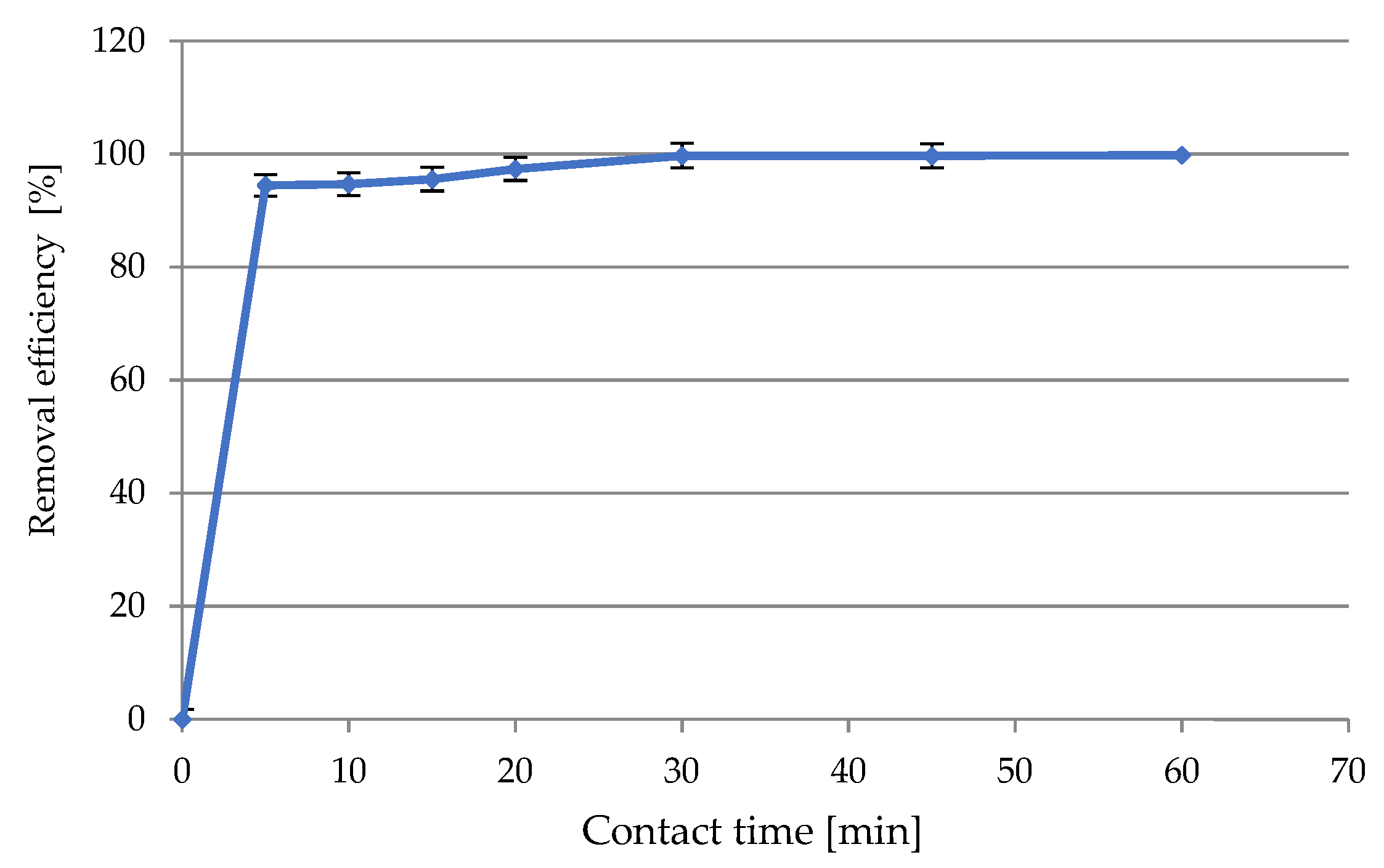
Studies of Contact Time
Pseudo-First-Order and Pseudo-Second-Order Kinetic Models
3.2.5. Isothermal Analysis
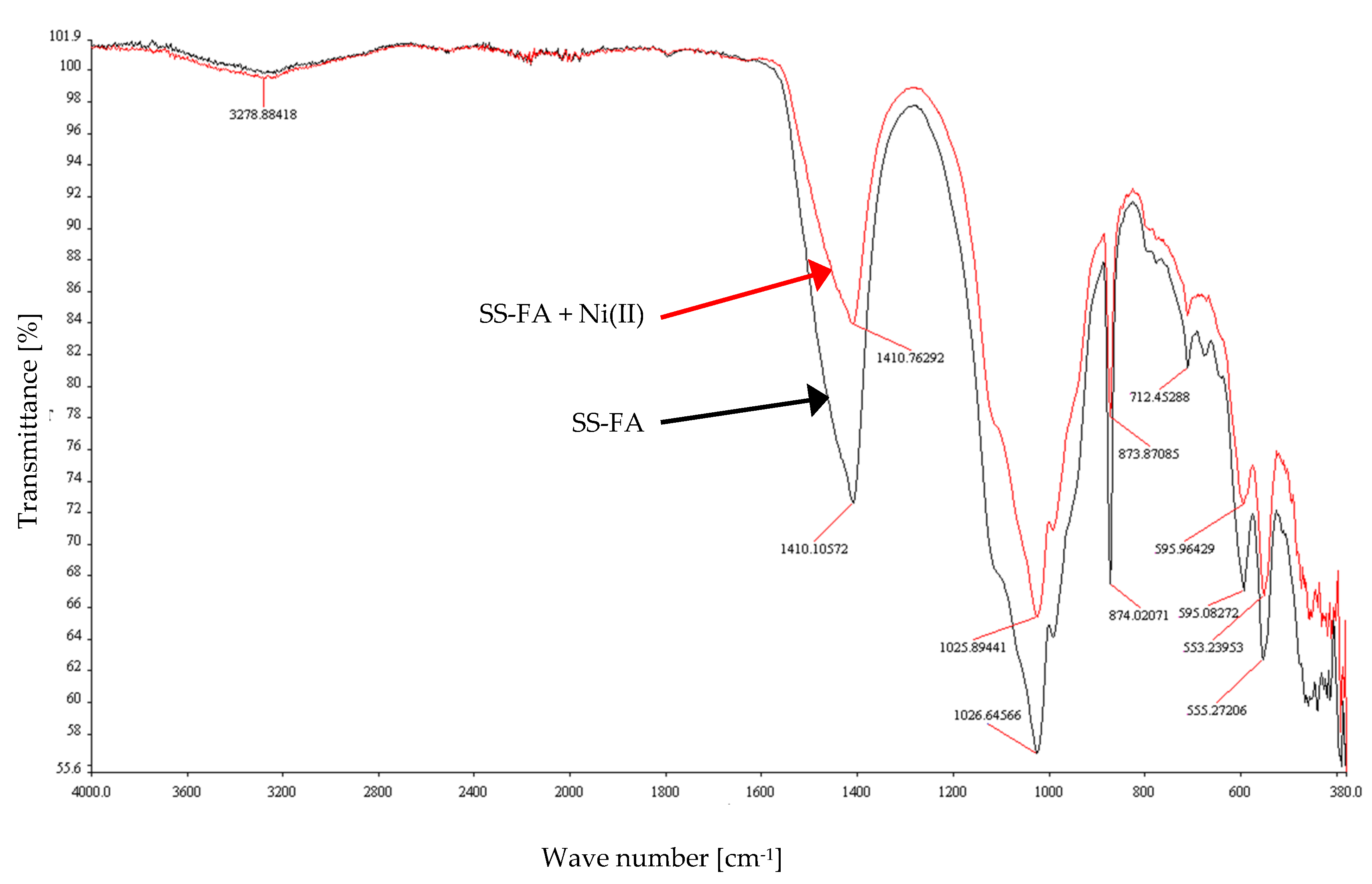
3.3. FT-IR Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kalak, T.; Cierpiszewski, R.; Ulewicz, M. High Efficiency of the Removal Process of Pb(II) and Cu(II) Ions with the Use of Fly Ash from Incineration of Sunflower and Wood Waste Using the CFBC Technology. Energies 2021, 14, 1771. [Google Scholar] [CrossRef]
- Erdoğan, S.; Önal, Y.; Akmil-Başar, C.; Bilmez-Erdemoğlu, S.; Sarıcı-Özdemir, Ç.; Köseoğlu, E.; İçduygu, G. Optimization of nickel adsorption from aqueous solution by using activated carbon prepared from waste apricot by chemical activation. Appl. Surf. Sci. 2005, 252, 1324–1331. [Google Scholar] [CrossRef]
- Demirbaş, E.; Kobya, M.; Öncel, S.; Şencan, S. Removal of Ni(II) from aqueous solution by adsorptiononto hazelnut shell activated carbon: Equilibrium studies. Bioresour. Technol. 2002, 84, 291–293. [Google Scholar] [CrossRef]
- Raji, M.; Ibrahim, Y.; Ehinmidu, J. Physico-chemical characteristics and Heavy metal levels in Drinking Water sources in Sokoto metropolis in North-western Nigeria. J. Appl. Sci. Environ. Manag. 2010, 14, 81–85. [Google Scholar] [CrossRef]
- Gautam, P.K.; Gautam, R.K.; Banerjee, S.; Chattopadhyaya, M.C.; Pandey, J.D. Heavy metals in the environment: Fate, transport, toxicity and remediation technologies. Heavy Met. 2016, 4, 1–29. [Google Scholar]
- Bhalerao, S.A.; Sharma, A.S.; Poojari, A.C. Toxicity of Nickel in Plants. Int. J. Pure App. Biosci. 2015, 3, 345–355. [Google Scholar]
- Tchounwou, P.B.; Yedjou, C.G.; Patlolla, A.K.; Sutton, D.J. Heavy metal toxicity and the environment. Exp. Suppl. 2012, 101, 133–164. [Google Scholar] [PubMed]
- Maarof, H.; Daud, W.; Aroua, M. Recent trends in removal and recovery of heavy metals from wastewater by electrochemical technologies. Rev. Chem. Eng. 2017, 33, 359–386. [Google Scholar] [CrossRef]
- Bazrafshan, E.; Mohammadi, L.; Ansari-Moghaddam, A.; Mahvi, A.H. Heavy metals removal from aqueous environments by electrocoagulation process—A systematic review. J. Environ. Health Sci. Eng. 2015, 13, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-H.; Eom, J.-H.; Lee, S.-L.; Hwang, S.-W.; Kim, S.-H.; Kang, S.-W.; Yun, J.-J.; Cho, J.-S.; Lee, Y.-H.; Seo, D.-C. Exploration of the potential capacity of fly ash and bottom ash derived from wood pellet-based thermal power plant for heavy metal removal. Sci. Total Environ. 2020, 740, 140205. [Google Scholar] [CrossRef]
- Qiu, R.; Cheng, F.; Huang, H. Removal of Cd2+ from aqueous solution using hydrothermally modified circulating fluidized bed fly ash resulting from coal gangue power plant. J. Clean. Prod. 2018, 172, 1918–1927. [Google Scholar] [CrossRef]
- Ahmaruzzaman, M. Industrial wastes as low-cost potential adsorbents for the treatment of wastewater laden with heavy metals. Adv. Colloid Interface Sci. 2011, 166, 36–59. [Google Scholar] [CrossRef]
- Kalak, T.; Kłopotek, A.; Cierpiszewski, R. Effective adsorption of lead ions using fly ash obtained in the novel circulating fluidized bed combustion technology. Microchem. J. 2019, 145, 1011–1025. [Google Scholar]
- Kalak, T.; Cierpiszewski, R. Comparative studies on the adsorption of Pb(II) ions by fly ash and slag obtained from CFBC technology. Pol. J. Chem. Technol. 2019, 21, 72–81. [Google Scholar] [CrossRef]
- Fytili, D.; Zabaniotou, A. Utilization of sewage sludge in EU application of old and new methods—A review. Renew. Sustain. Energy Rev. 2008, 12, 116–140. [Google Scholar] [CrossRef]
- Kalisz, M. Prognozy zmian w gospodarce osadami ściekowymi. Wodociągi Kanalizacja 2007, 3, 30–31. [Google Scholar]
- Ahmaruzzaman, M. A review on the utilization of fly ash. Prog. Energy Combust. Sci. 2010, 36, 327–363. [Google Scholar] [CrossRef]
- Malik, A.; Thapliyal, A. Eco-friendly Fly Ash Utilization: Potential for Land Application. Crit. Rev. Environ. Sci. Technol. 2009, 39, 333–366. [Google Scholar] [CrossRef]
- European Environment Agency. Large Combustion Plants Operating in Europe. Available online: https://www.eea.europa.eu/data-and-maps/indicators/large-combustion-plants-operating-in-europe-3/assessment (accessed on 17 April 2021).
- Wójtowicz, A. Kierunki zagospodarowania komunalnych osadów ściekowych w świetle zmian prawa. In Proceedings of the Conference—Urząd Marszałkowski Województwa Pomorskiego, Gdańsk, Poland, 11 May 2012. [Google Scholar]
- Werle, S.; Dudziak, M. Gasification of sewage sludge. In Industrial and Municipal Sludge; Industrial and Municipal Sludge: Oxford, UK, 2019; pp. 575–593. [Google Scholar]
- Eurostat. Sewage Sludge Production and Disposal. Available online: http://appsso.eurostat.ec.europa.eu/nui/show.do?dataset=env_ww_spd&lang=en (accessed on 17 April 2021).
- Shim, Y.-S.; Kim, Y.-K.; Kong, S.-H.; Rhee, S.-W.; Lee, W.-K. The adsorption characteristics of heavy metals by various particle sizes of MSWI bottom ash. Waste Manag. 2003, 23, 851–857. [Google Scholar] [CrossRef]
- Police, S.; Maity, S.; Chaudhary, D.K.; Dusane, C.K.; Sahu, S.K.; Kumar, A.V. Effect of coal fly ash’s particle size on U adsorption in water samples and thermodynamic study on adsorption. Environ. Chem. Ecotoxicol. 2020, 2, 32–38. [Google Scholar] [CrossRef]
- Itskos, G.; Koukouzas, N.; Vasilatos, C.; Megremi, I.; Moutsatsou, A. Comparative uptake study of toxic elements from aqueous media by the different particle-size-fractions of fly ash. J. Hazard. Mater. 2010, 183, 787–792. [Google Scholar] [CrossRef]
- Lanzerstorfer, C. Fly ash from coal combustion: Dependence of the concentration of various elements on the particle size. Fuel 2018, 228, 263–271. [Google Scholar] [CrossRef]
- Kara, S.; Aydiner, C.; Demirbas, E.; Kobya, M.; Dizge, N. Modeling the effects of adsorbent dose and particle size on the adsorption of reactive textile dyes by fly ash. Desalination 2007, 212, 282–293. [Google Scholar] [CrossRef]
- Feng, S.; Li, Y. Study on coal fly ash classified by bulk density. J. Phys. Conf. Ser. 2021, 1732, 012127. [Google Scholar] [CrossRef]
- Nawaz, I. Disposal and Utilization of Fly Ash to Protectthe Environment. Int. J. Innov. Res. Sci. Eng. Technol. 2013, 2, 5259–5266. [Google Scholar]
- Mahieux, P.-Y.; Aubert, J.-E.; Cyr, M.; Coutand, M.; Husson, B. Quantitative mineralogical composition of complex mineral wastes—Contribution of the Rietveld method. Waste Manag. 2009, 30, 378–388. [Google Scholar] [CrossRef] [PubMed]
- Coutand, M.; Cyr, M.; Clastres, P. Use of sewage sludge ash as mineral admixture in mortars. Proceedings of the Institution of Civil Engineers. Constr. Mater. 2006, 159, 153–162. [Google Scholar] [CrossRef]
- Zhou, Y.-F.; Li, J.-S.; Lu, J.-X.; Cheeseman, C.; Poon, C.S. Sewage sludge ash: A comparative evaluation with fly ash for potential use as lime-pozzolan binders. Constr. Build. Mater. 2020, 242, 118160. [Google Scholar] [CrossRef]
- Li, H.L.; Liu, G.L.; Cao, Y. Content and Distribution of Trace Elements and Polycyclic Aromatic Hydrocarbons in Fly Ash from a Coal-Fired CHP Plant. Aerosol Air Qual. Res. 2014, 14, 1179–1188. [Google Scholar] [CrossRef]
- Xiong, L.; Wan, Z.; Zhang, Y.; Wang, F.; Wang, J.; Kang, Y. Fly Ash Particle Size Effect on Pore Structure and Strength of Fly Ash Foamed Geopolymer. Adv. Polym. Technol. 2019, 1098027, 1–10. [Google Scholar] [CrossRef]
- Dos Santos, R.P.; Martins, J.; Gadelha, C.; Cavada, B.; Albertini, A.V.; Arruda, F.; Vasconcelos, M.; Teixeira, E.; Alves, F.; Lima Filho, J.; et al. Coal fly ash ceramics: Preparation, characterization, and use in the hydrolysis of sucrose. Sci. World J. 2014, 154651, 1–7. [Google Scholar] [CrossRef]
- Paya, J.; Monzo, J.; Borrachero, M.V.; Perris, E.; Amahjour, F. Thermogravimetric methods for determinig carbon content in fly ashes. Cem. Concr. Res. 1998, 28, 675–686. [Google Scholar] [CrossRef]
- Khan, A.A.; de Jong, W.; Jansens, P.J.; Spliethoff, H. Biomass combustion in fluidized bed boilers: Potential problems and remedies. Fuel Process. Technol. 2009, 90, 21–50. [Google Scholar] [CrossRef]
- Yue, Y.; Wang, J.J.; Basheer, P.; Bai, Y. Establishing the Carbonation Profile with Raman Spectroscopy: Effects of Fly Ash and Ground Granulated Blast Furnace Slag. Materials 2021, 14, 1798. [Google Scholar] [CrossRef] [PubMed]
- Du, B.; Li, J.; Fang, W.; Liu, Y.; Yu, S.; Li, Y.; Liu, J. Characterization of naturally aged cement-solidified MSWI fly ash. Waste Manag. 2018, 80, 101–111. [Google Scholar] [CrossRef]
- Villain, G.; Thiery, M.; Platret, G. Measurement methods of carbonation profiles in concrete: Thermogravimetry, chemical analysis and gammadensimetry. Cem. Concr. Res. 2007, 37, 1182–1192. [Google Scholar] [CrossRef]
- Magdziarz, A.; Werle, S. Analysis of the combustion and pyrolysis of dried sewage sludge by TGA and MS. Waste Manag. 2014, 34, 174–179. [Google Scholar] [CrossRef]
- Liu, X.; Mäki-Arvela, P.; Aho, A.; Vajglova, Z.; Gun’ko, V.M.; Heinmaa, I.; Kumar, N.; Eränen, K.; Salmi, T.; Murzin, D.Y. Zeta Potential of Beta Zeolites: Influence of Structure, Acidity, pH, Temperature and Concentration. Molecules 2018, 23, 946. [Google Scholar] [CrossRef]
- Liberto, T.; Barentin, C.; Colombani, J.; Costa, A.; Gardini, D.; Bellotto, M.; Le Merrer, M. Simple ions control the elasticity of calcite gels via interparticle forces. J. Colloid Inter. Sci. 2019, 553, 280–288. [Google Scholar] [CrossRef]
- Hadadian, Y.; Ramos, A.P.; Pavan, T.Z. Role of zinc substitution in magnetic hyperthermia properties of magnetite nanoparticles: Interplay between intrinsic properties and dipolar interactions. Sci. Rep. 2019, 9, 18048. [Google Scholar] [CrossRef]
- Dedecek, J.; Balgová, V.; Pashkova, V.; Klein, P.; Wichterlová, B. Synthesis of ZSM-5 Zeolites with Defined Distribution of Al Atoms in the Framework and Multinuclear MAS NMR Analysis of the Control of Al Distribution. Chem. Mater. 2012, 24, 3231–3239. [Google Scholar] [CrossRef]
- Hensen, E.J.M.; Poduval, D.G.; Ligthart, D.A.J.M.; van Veen, J.A.R.; Rigutto, M.S. Quantification of Strong Brønsted Acid Sites in Aluminosilicates. J. Phys. Chem. C 2010, 114, 8363–8374. [Google Scholar] [CrossRef]
- Ronson, T.O.; Carney, J.R.; Whitwood, A.C.; Taylor, R.J.K.; Fairlamb, I.J.S. AsCat and FurCat: New Pd catalysts for selective room-temperature Stille cross-couplings of benzyl chlorides with organostannanes. Chem. Commun. 2015, 51, 3466–3469. [Google Scholar] [CrossRef]
- Kong, D.L.Y.; Sanjayan, J.G.; Sagoe-Crentsil, K. Comparative performance of geopolymers made with metakaolin and fly ash after exposure to elevated temperatures. Cem. Concr. Res. 2007, 37, 1583–1589. [Google Scholar] [CrossRef]
- Hardjito, D.; Wallah, S.E.; Sumajouw, D.M.J.; Rangan, B.V. Fly ash-based geopolymer concrete. Aust. J. Struct. Eng. 2005, 6, 77–86. [Google Scholar] [CrossRef]
- Alinnor, I.J. Adsorption of heavy metal ions from aqueous solution by fly ash. Fuel 2007, 86, 853–857. [Google Scholar] [CrossRef]
- Temuujin, J.; Riessen, A.V. Effect of fly ash preliminary calcination on the properties of geopolymer. J. Hazard. Mater. 2009, 164, 634–639. [Google Scholar] [CrossRef]
- Mustafa, A.M.; Kamarudin, H.; Omar Karem, A.K.A.; Ruzaidi, C.M.; Rafiza, A.R.; Norazian, M.N. Optimization of Alkaline Activator/Fly Ash Ratio on The Compressive Strength of Manufacturing Fly Ash-Based Geopolymer. Appl. Mech. Mater. 2011, 110–116, 734–739. [Google Scholar]
- Thokchom, S.; Ghosh, P.; Ghosh, S. Resistance of Fly Ash Based Geopolymer Mortars in Sulfuric Acid. ARPN J. Eng. Appl. Sci. 2009, 4, 65–70. [Google Scholar]
- Bansal, R.C.; Goyal, M. Activated Carbon Adsorption; CRC Press, Taylor and Francis Group, LLC: Boca Raton, FL, USA, 2005. [Google Scholar]
- Magdziarz, A.; Kosowska-Golachowska, M.; Kijo-Kleczkowska, A.; Musiał, T.; Środa, K.; Wolski, K.; Richter, D. Analysis of sewage sludge ashes from air and oxy-fuel combustion in a circulating fluidized-bed. In Proceedings of the 1st International Conference on the Sustainable Energy and Environment Development (SEED 2016), Krakow, Poland, 14–17 November 2017; Volume 10, p. 00054. [Google Scholar]
- Gao, N.; Kamran, K.; Quan, C.; Williams, P.T. Thermochemical conversion of sewage sludge: A critical review. Prog. Energy Combust. Sci. 2020, 79, 100843. [Google Scholar] [CrossRef]
- Gao, J.; Wang, T.; Zhao, J.; Hu, X.; Dong, C. An Experimental Study on the Melting Solidification of Municipal Solid Waste Incineration Fly Ash. Sustainability 2021, 13, 535. [Google Scholar] [CrossRef]
- Chen, C.-H.; Chiou, I.-J.; Wang, K.-S. Sintering effect on cement bonded sewage sludge ash. Cem. Concr. Compos. 2006, 28, 26–32. [Google Scholar] [CrossRef]
- Cui, X.; Fang, S.; Yao, Y.; Li, T.; Ni, Q.; Yang, X.; He, Z. Potential mechanisms of cadmium removal from aqueous solution by Canna indica derived biochar. Sci. Total Environ. 2016, 562, 517–525. [Google Scholar] [CrossRef]
- Erdemoğlu, M.; Sarikaya, M. Effects of heavy metals and oxalate on the zeta potential of magnetite. J. Colloid Interf. Sci. 2006, 300, 795–804. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.-F.; Hutchison, M.J.; Santucci, R.J., Jr.; Scully, J.R.; Rondinelli, J.M. Improved Electrochemical Phase Diagrams from Theory and Experiment: The Ni–Water System and Its Complex Compounds. J. Phys. Chem. C 2017, 121, 9782–9789. [Google Scholar] [CrossRef]
- Mînzatu, V.; Davidescu, C.-M.; Negrea, P.; Ciopec, M.; Muntean, C.; Hulka, I.; Paul, C.; Negrea, A.; Duteanu, N. Synthesis, Characterization and Adsorptive Performances of a Composite Material Based on Carbon and Iron Oxide Particles. Int. J. Mol. Sci. 2019, 20, 1609. [Google Scholar] [CrossRef]
- Langeroodi, N.S.; Farhadravesh, Z.; Khalaji, A.D. Optimization of adsorption parameters for Fe (III) ions removal from aqueous solutions by transition metal oxide nanocomposite. Green Chem. Lett. Rev. 2018, 11, 404–413. [Google Scholar] [CrossRef]
- Onundi, Y.B.; Mamun, A.A.; Khatib, M.F.A.; Al Saadi, M.A.; Suleyman, A.M. Heavy metals removal from synthetic wastewater by a novel nano-size composite adsorbent. Int. J. Environ. Sci. Technol. 2011, 8, 799–806. [Google Scholar] [CrossRef][Green Version]
- Zamani, S.; Salahi, E.; Mobasherpour, I. Removal of Nickel from Aqueous Solution by Nano Hydroxyapatite Originated from Persian Gulf Corals. Can. Chem. Trans. 2013, 1, 173–190. [Google Scholar]
- Ren, Y.; Yan, N.; Wen, Q.; Fan, Z.; Wei, T.; Zhang, M.; Ma, J. Graphene/δ-MnO2 composite as adsorbent for the removal of nickel ions from wastewater. Chem. Eng. J. 2011, 175, 1–7. [Google Scholar] [CrossRef]
- Liew, Y.M.; Heah, C.Y.; Kamarudin, H. Structure and properties of clay-based geopolymer cements: A review. Prog. Mater. Sci. 2016, 83, 595–629. [Google Scholar] [CrossRef]
- Mobasherpour, I.; Salahi, E.; Ebrahimi, M. Removal of divalent nickel cations from aqueous solution by multi-walled carbon nano tubes: Equilibrium and kinetic processes. Res. Chem. Intermed. 2012, 38, 2205–2222. [Google Scholar] [CrossRef]
- Chen, X.; Guo, Y.; Ding, S.; Zhang, H.; Xia, F.; Wang, J.; Zhou, M. Utilization of red mud in geopolymer-based pervious concrete with the function of adsorption of heavy metal ions. J. Clean. Prod. 2019, 207, 789–800. [Google Scholar] [CrossRef]
- Kara, I.; Tunc, D.; Sayin, F.; Akar, S.T. Study on the performance of metakaolin based geopolymer for Mn(II) and Co(II) removal. Appl. Clay Sci. 2018, 161, 184–193. [Google Scholar] [CrossRef]
- Mondal, M.; Dutta, M.; De, S. A novel ultrafiltration grade nickel iron oxide doped hollow fiber mixed matrix membrane: Spinning, characterization and application in heavy metal removal. Sep. Purif. Technol. 2017, 188, 155–166. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Y.; Wang, X.; Sun, Z.; Ma, J.; Wu, T.; Xing, F.; Gao, J. Porous graphene oxide/carboxymethyl cellulose monoliths, with high metal ion adsorption. Carbohydr. Polym. 2014, 101, 392–400. [Google Scholar] [CrossRef] [PubMed]
- Rahmani, A.; Zavvar Mousavi, H.; Fazli, M. Effect of nanostructure alumina on adsorption of heavy metals. Desalination 2010, 253, 94–100. [Google Scholar] [CrossRef]
- Zhou, Z.; Kong, D.; Zhu, H.; Wang, N.; Wang, Z.; Wang, Q.; Liu, W.; Li, Q.; Zhang, W.; Zhongqi Ren, Z. Preparation and adsorption characteristics of an ion-imprinted polymer for fast removal of Ni(II) ions from aqueous solution. J. Hazard. Mater. 2018, 341, 355–364. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Liu, C.; Zhang, L.; Zheng, S.; Zhang, Y. Enhanced boron adsorption onto synthesized MgO nanosheets by ultrasonic method. Ultrason. Sonochem. 2017, 34, 938–946. [Google Scholar] [CrossRef]
- El-Bahy, S.M.; El-Bahy, Z.M. Synthesis and characterization of polyamidoxime chelating resin for adsorption of Cu(II), Mn(II) and Ni(II) by batch and column study. J. Environ. Chem. Eng. 2016, 4, 276–286. [Google Scholar] [CrossRef]
- Shirkhanloo, H.; Khaligh, A.; Mousavi, H.Z.; Rashidi, A. Graphene oxide-packed micro-column solid-phase extraction combined with flame atomic absorption spectrometry for determination of lead (II) and nickel (II) in water samples. Int. J. Environ. Anal. Chem. 2015, 95, 16–32. [Google Scholar] [CrossRef]
- Zare-Dorabei, R.; Ferdowsi, S.M.; Barzin, A.; Tadjarodi, A. Highly efficient simultaneous ultrasonic-assisted adsorption of Pb(II), Cd(II), Ni(II) and Cu (II) ions from aqueous solutions by graphene oxide modified with 2,2′-dipyridylamine: Central composite design optimization. Ultrason. Sonochem. 2016, 32, 265–276. [Google Scholar] [CrossRef]
- Bai, Z.; Zheng, Y.; Zhang, Z. One-pot synthesis of highly efficient MgO for the removal of Congo red in aqueous solution. J. Mater. Chem. A 2017, 5, 6630–6637. [Google Scholar] [CrossRef]
- Mužek, M.N.; Svilović, S.; Zelić, J. Kinetic studies of cobalt ion removal from aqueous solutions using fly ash-based geopolymer and zeolite NaX as sorbents. Sep. Sci. Technol. 2016, 51, 2868–2875. [Google Scholar] [CrossRef]
- Wang, H.; Wang, W.; Zhao, Y.; Xu, Z.; Chen, L.; Zhao, L.; Tian, X.; Sun, W. Superior Adsorption of 3D Nanoporous Architectures for Ni(II) Ions Adsorption Using Polyvinyl Alcohol as Cross-Linking Agent and Adsorption Conveyor. RSC Adv. 2018, 8, 7899–7903. [Google Scholar] [CrossRef]
- Panda, L.; Rath, S.S.; Rao, D.S.; Nayak, B.B.; Das, B.; Misra, P.K. Thorough understanding of the kinetics and mechanism of heavy metal adsorption onto a pyrophyllite mine waste-based geopolymer. J. Mol. Liq. 2018, 263, 428–441. [Google Scholar] [CrossRef]
- Ghani, U.; Hussain, S.; Noor-ul-Amin; Imtiaz, M.; Khan, S. Laterite clay-based geopolymer as a potential adsorbent for the heavy metals removal from aqueous solutions. J. Saudi Chem. Soc. 2020, 24, 874–884. [Google Scholar] [CrossRef]
- Kalembkiewicz, J.; Galas, D.; Sitarz-Palczak, E. The Physicochemical Properties and Composition of Biomass Ash and Evaluating Directions of its Applications. Pol. J. Environ. Stud. 2018, 27, 2593–2603. [Google Scholar] [CrossRef]


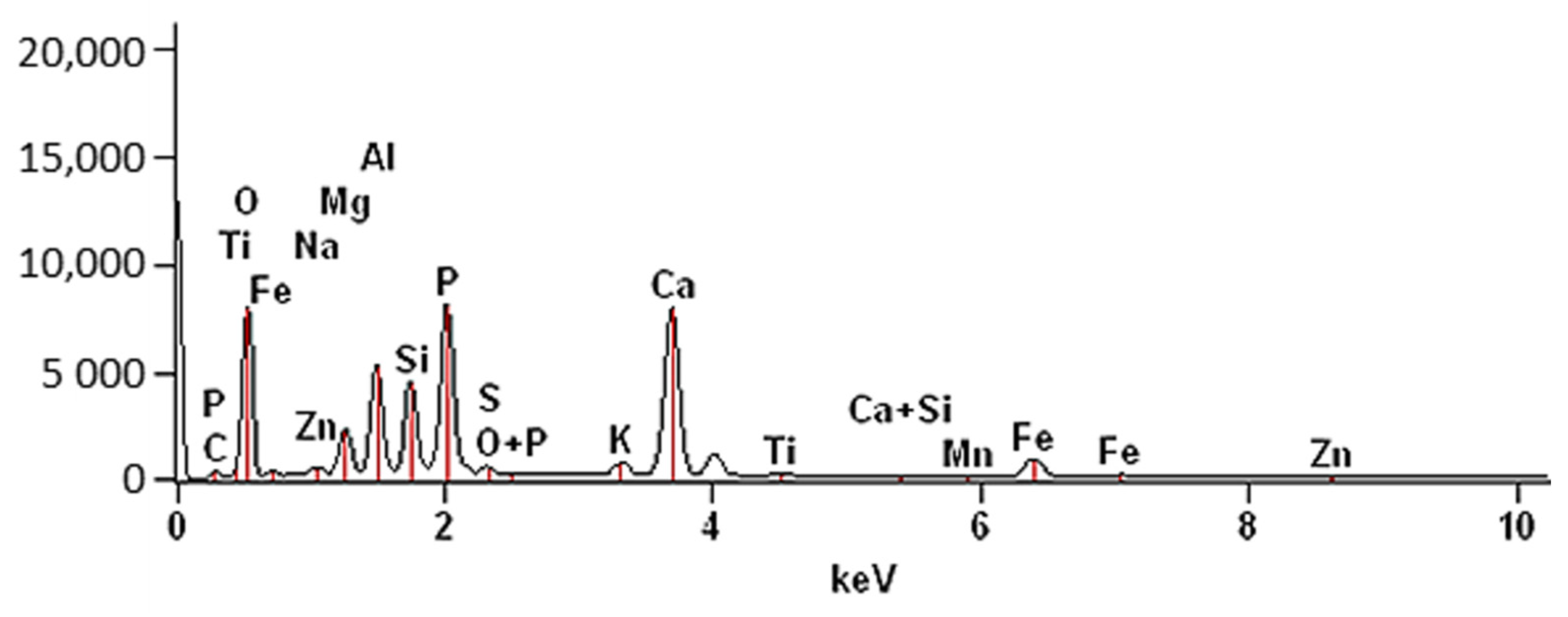





| Elements | C | O | Na | Mg | Al | Si | P | S | K | Ca | Ti | Mn | Fe | Zn |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Weight (%) | 1.85 ± 0.06 | 44.79 ± 0.3 | 0.54 ± 0.03 | 3.28 ± 0.1 | 6.41 ± 0.3 | 5.51 ± 0.2 | 11.35 ± 0.4 | 0.41 ± 0.09 | 1.11 ± 0.1 | 17.68 ± 0.2 | 0.54 ± 0.03 | 0.23 ± 0.02 | 5.43 ± 0.3 | 0.87 ± 0.09 |
| Atomic (%) | 3.41 ± 0.2 | 61.93 ± 0.6 | 0.52 ± 0.03 | 2.99 ± 0.09 | 5.25 ± 0.2 | 4.34 ± 0.3 | 8.11 ± 0.3 | 0.28 ± 0.03 | 0.63 ± 0.05 | 9.76 ± 0.5 | 0.25 ± 0.02 | 0.09 ± 0.01 | 2.15 ± 0.2 | 0.29 ± 0.02 |
| Content of oxides (wt. %) | ||||||||||||||
| References | CO2 | Na2O | MgO | Al2O3 | SiO2 | P2O5 | SO3 | K2O | CaO | TiO2 | MnO | Fe2O3 | ZnO | |
| This research | 6.97 ± 0.3 | 0.73 ± 0.04 | 5.44 ± 0.2 | 12.11 ± 0.6 | 11.78 ± 0.6 | 26.02 ± 0.8 | 1.02 ± 0.1 | 1.33 ± 0.1 | 24.74 ± 0.8 | 0.89 ± 0.03 | 0.29 ± 0.01 | 7.77 ± 0.3 | 1.08 ± 0.08 | |
| [48] | ― | 0.37 | 1.4 | 27.0 | 48.8 | 1.2 | 0.22 | 0.85 | 6.2 | 1.3 | 0.15 | 10.2 | ― | |
| [49] | ― | 0.37 | 0.77 | 26.49 | 53.36 | 1.43 | 0.20 | 0.80 | 1.34 | 1.47 | ― | 10.86 | ― | |
| [50] | ― | ― | 0.97 | 22.03 | 57.25 | ― | 0.76 | 0.52 | 2.97 | 0.68 | ― | 8.36 | ― | |
| [51] | ― | 0.38 | 1.2 | 23.63 | ― | 1.31 | 0.28 | 0.84 | 1.74 | 1.32 | 0.13 | 15.3 | ― | |
| [52] | ― | 0.42 | 0.78 | 23.59 | 52.11 | 1.31 | 0.49 | 0.80 | 2.61 | 0.88 | 0.03 | 7.39 | ― | |
| [53] | ― | 0.61 | 0.3 | 29.8 | 56.01 | 0.44 | ― | 0.73 | 2.36 | 1.75 | ― | 3.58 | ― | |
| Parameters | Values |
|---|---|
| BET adsorption cumulative surface area (SBET) (m2/g) | 9.105 |
| BET desorption cumulative surface area (SBET) (m2/g) | 12.368 |
| BJH adsorption cumulative volume of pores (Vpa) (cm3/g) | 0.032417 |
| BJH desorption cumulative volume of pores (Vpd) (cm3/g) | 0.03302 |
| BJH adsorption average pore diameter (Apda) (nm) | 14.2408 |
| BJH desorption average pore diameter (Apdd) (nm) | 10.6791 |
| Metal Ion | Adsorbent Dosage [g/L] | Pseudo-First-Order Kinetic Model | Pseudo-Second-Order Kinetic Model | ||||
|---|---|---|---|---|---|---|---|
| kad [min−1] | qe [mg/g] | R2 | k [g/mg min] | qe [mg/g] | R2 | ||
| Ni(II) | 30 | 0.030 | 0.023 | 0.940 | 2441.91 | 0.044 | 0.989 |
| Metal Ion | Adsorbent Dosage (g/L) | Langmuir Isotherm | Freundlich Isotherm | ||||
|---|---|---|---|---|---|---|---|
| Calculated qm (mg/g) | KL (L/mg) | R2 | Kf (mg/g) (L/mg)(1/n) | n | R2 | ||
| Ni(II) | 2.5 | 94.72 | 0.0297 | 0.951 | 2.489 | 0.827 | 0.974 |
| 5 | 96.8 | 0.0208 | 0.946 | 1.954 | 0.745 | 0.958 | |
| 10 | 81.81 | 0.034 | 0.977 | 3.152 | 0.869 | 0.980 | |
| 25 | 126.32 | 0.1166 | 0.992 | 21.169 | 0.902 | 0.995 | |
| 50 | 130.03 | 0.0435 | 0.983 | 11.242 | 0.820 | 0.984 | |
| Adsorbents | Adsorption Capacity (mg/g) | Ref. |
|---|---|---|
| Carbon nanotubes–granular activated carbon (CNT–GAC) | 0.07 | [64] |
| Nano crystalline hydroxyapatite (nano HAp) | 2.28 | [65] |
| Graphene | 10.8 | [66] |
| LD slag | 15 | [67] |
| Multi-walled carbon nano tubes (MWCNT) | 17.86 | [68] |
| Graphene/MnO2 | 46.55 | [66] |
| Dolochar ash geopolymer | 48 | [69] |
| Pyrophyllite-based geopolymer | 49 | [70] |
| Hollow fibers | 62.51 | [71] |
| Graphene oxide/carboxy methyl cellulose | 72.04 | [72] |
| Nanostructured Al2O3 | 83.33 | [73] |
| Ion-imprinted polymer | 86.3 | [74] |
| MgO nanosheets (ultrasonic method) | 87 | [75] |
| Polyamidoxime chelating resin (PAO-AN) three dimensional | 130 | [76] |
| Sewage sludge fly ash (SS-FA) | 130.03 | This study |
| Graphene oxide (GO) | 178 | [77] |
| Graphene oxide modified with 2,20-dipyridylamine (GO-DPA) | 180.89 | [78] |
| MgO nanosheets (precursor calcination) | 185.5 | [79] |
| Activated carbon derived from Xanthoceras | 188 | [80] |
| Polyvinyl alcohol/CNTs nanoporous architectures (3DPCA) | 225.6 | [81] |
| Slag based geopolymer | 414 | [82] |
| Sieved geopolymer sample (SGS) | 520 | [83] |
| FT-IR Band (cm−1) | Assignment (Vibrations, Species) |
|---|---|
| 3278.88 | stretching vibrations O–H |
| 1410.76, 1410.1 | valence vibration of carbonate ions |
| 1025.89, 1026.64 | asymmetric stretching vibrations of silica Si–O–Si |
| 873.87, 874.02 | symmetric stretching of Al–O–M, vibration of carbonates (calcite) |
| 712.45 | symmetric stretching of Si–O–Si and Al–O–Si |
| 595.96, 595.08 | stretching vibrations Al–O, Si–O–M |
| 553.24, 555.27 | O–P–O, O=P–O bending vibration (probably P2O5) |
| 380–440 | bond bending vibrations Si–O–Si |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kalak, T.; Marciszewicz, K.; Piepiórka-Stepuk, J. Highly Effective Adsorption Process of Ni(II) Ions with the Use of Sewage Sludge Fly Ash Generated by Circulating Fluidized Bed Combustion (CFBC) Technology. Materials 2021, 14, 3106. https://doi.org/10.3390/ma14113106
Kalak T, Marciszewicz K, Piepiórka-Stepuk J. Highly Effective Adsorption Process of Ni(II) Ions with the Use of Sewage Sludge Fly Ash Generated by Circulating Fluidized Bed Combustion (CFBC) Technology. Materials. 2021; 14(11):3106. https://doi.org/10.3390/ma14113106
Chicago/Turabian StyleKalak, Tomasz, Kinga Marciszewicz, and Joanna Piepiórka-Stepuk. 2021. "Highly Effective Adsorption Process of Ni(II) Ions with the Use of Sewage Sludge Fly Ash Generated by Circulating Fluidized Bed Combustion (CFBC) Technology" Materials 14, no. 11: 3106. https://doi.org/10.3390/ma14113106
APA StyleKalak, T., Marciszewicz, K., & Piepiórka-Stepuk, J. (2021). Highly Effective Adsorption Process of Ni(II) Ions with the Use of Sewage Sludge Fly Ash Generated by Circulating Fluidized Bed Combustion (CFBC) Technology. Materials, 14(11), 3106. https://doi.org/10.3390/ma14113106








