Al Matrix Composites Reinforced by Ti and C Dedicated to Work at Elevated Temperature
Abstract
1. Introduction
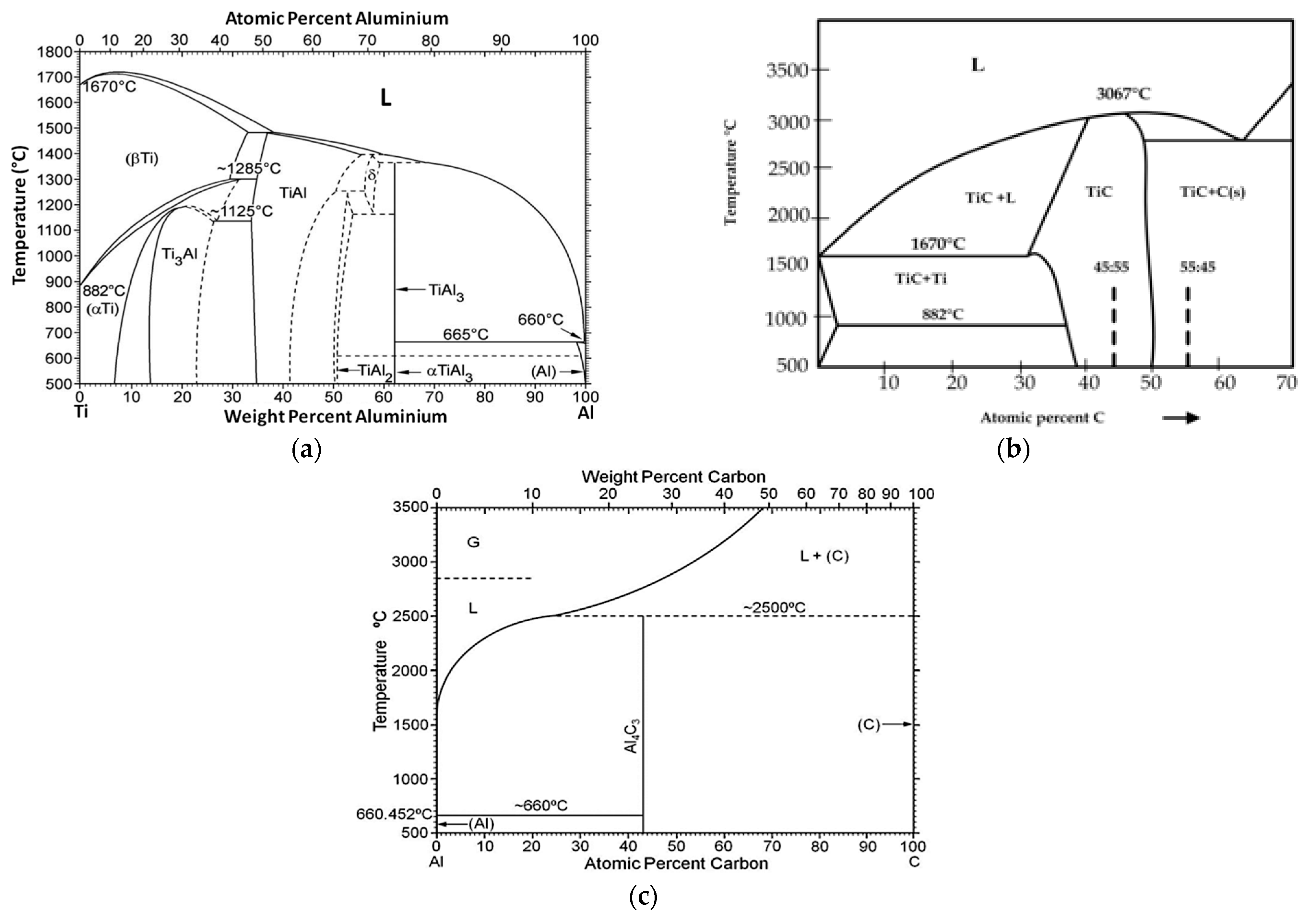
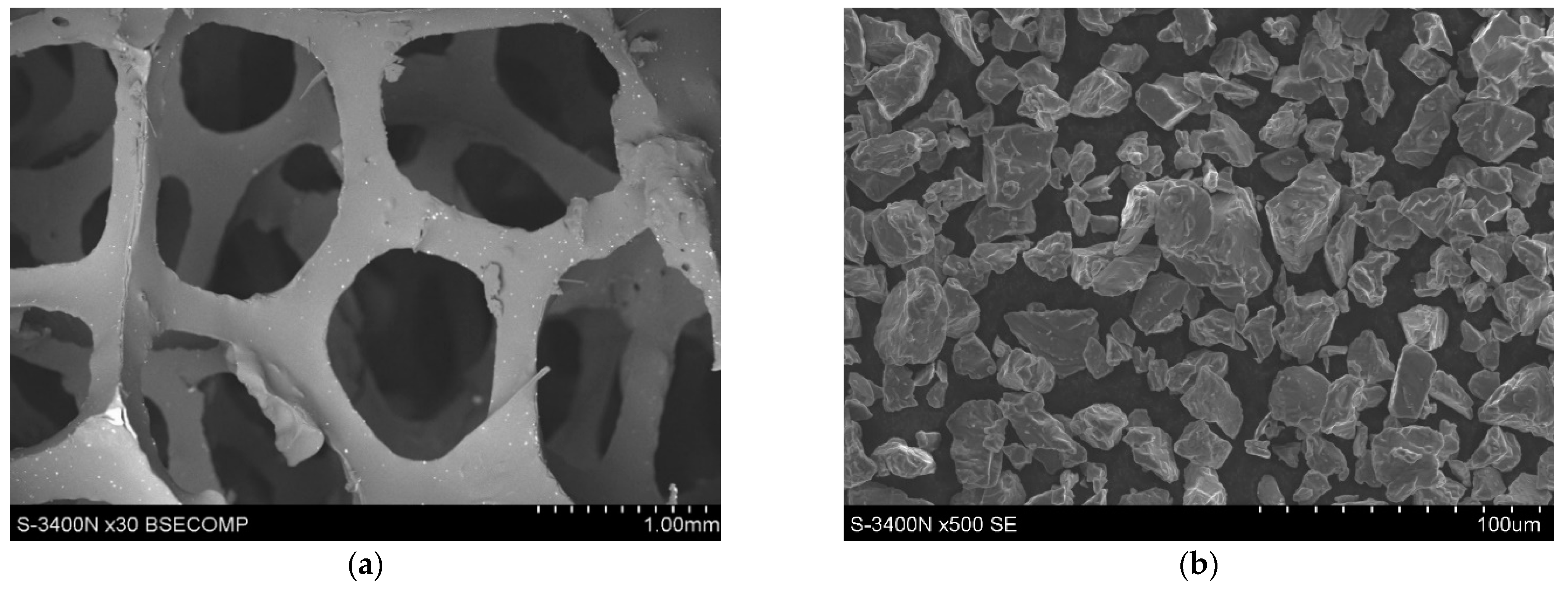
2. Materials and Methods
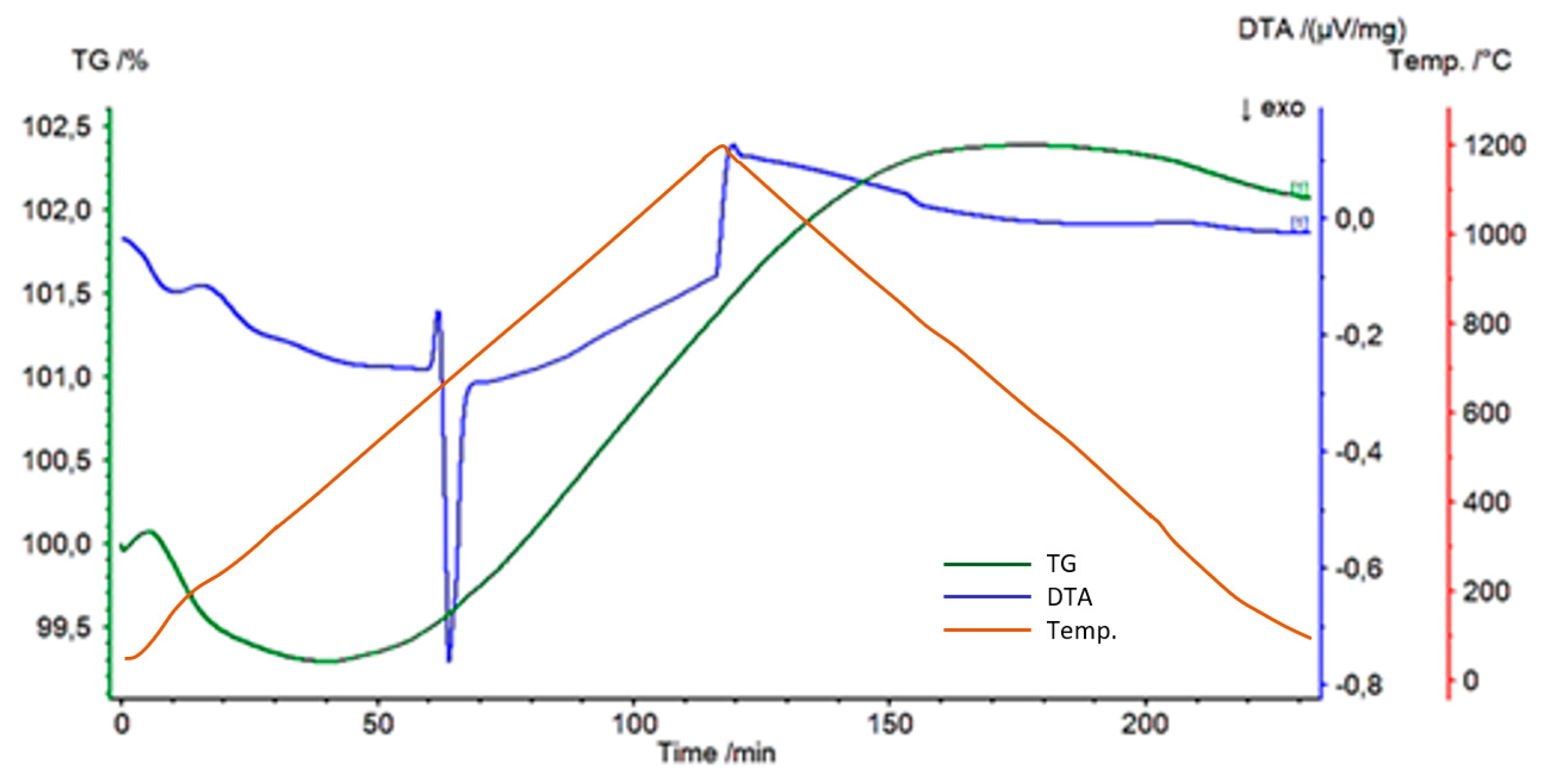
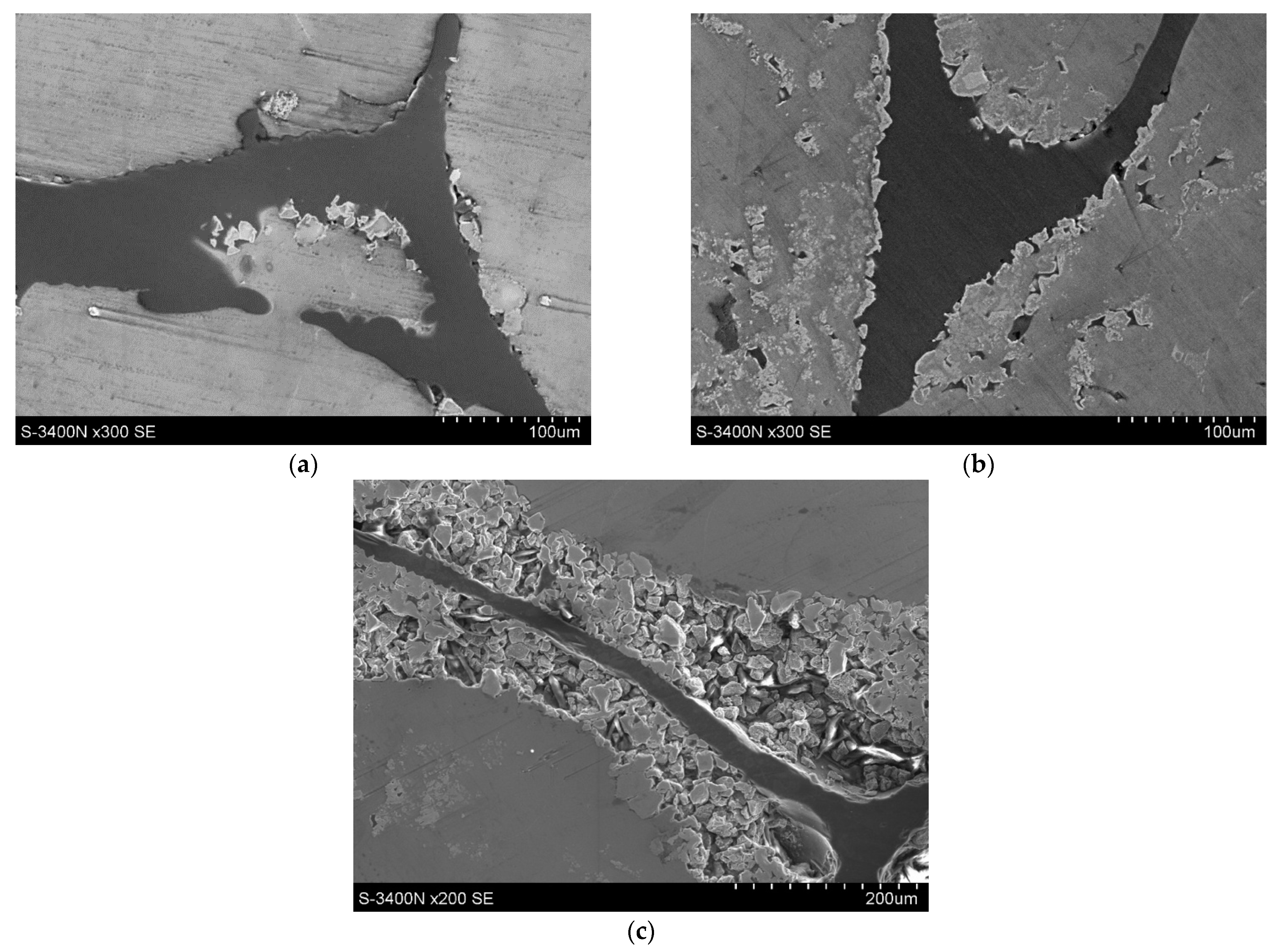
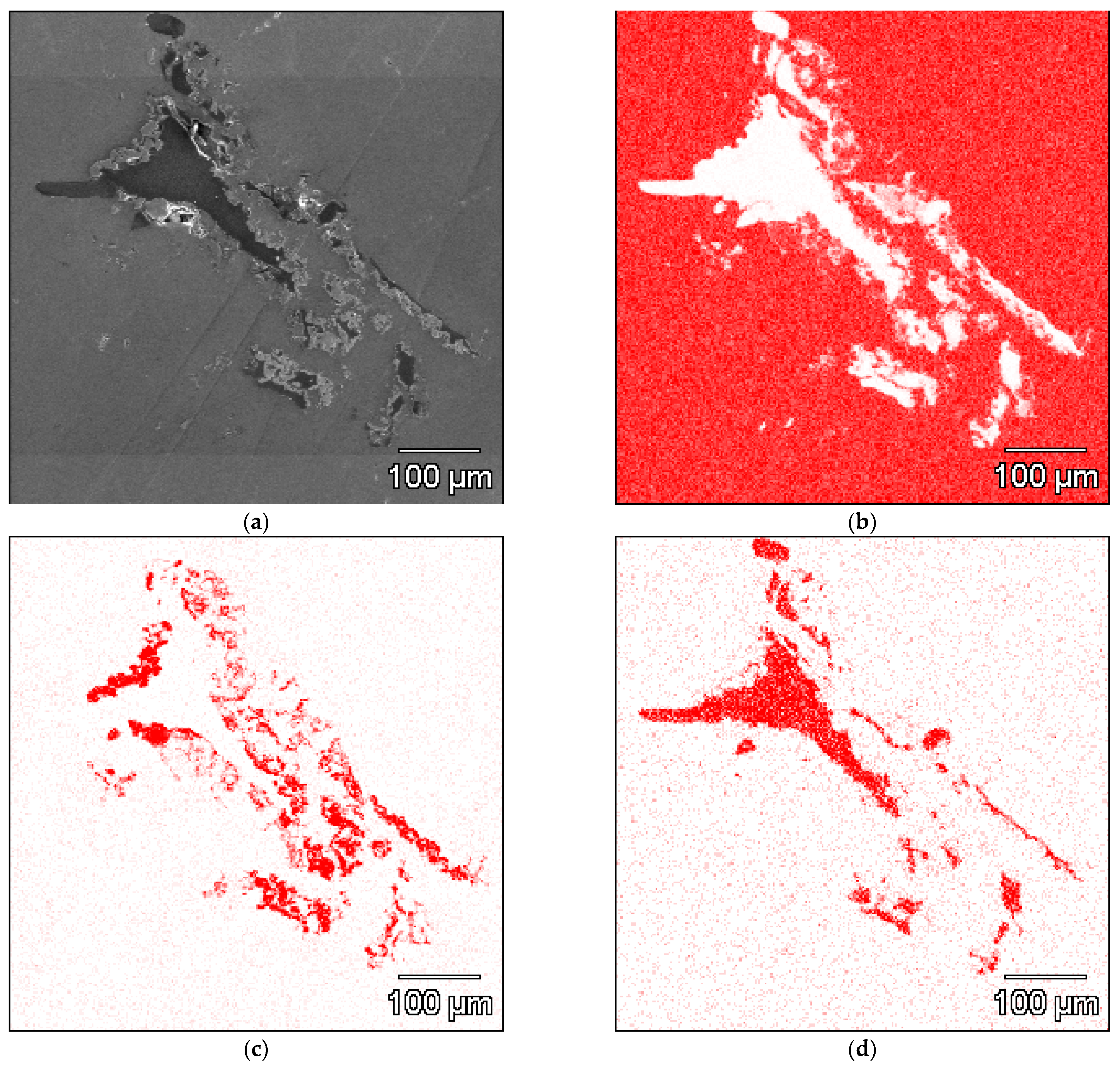
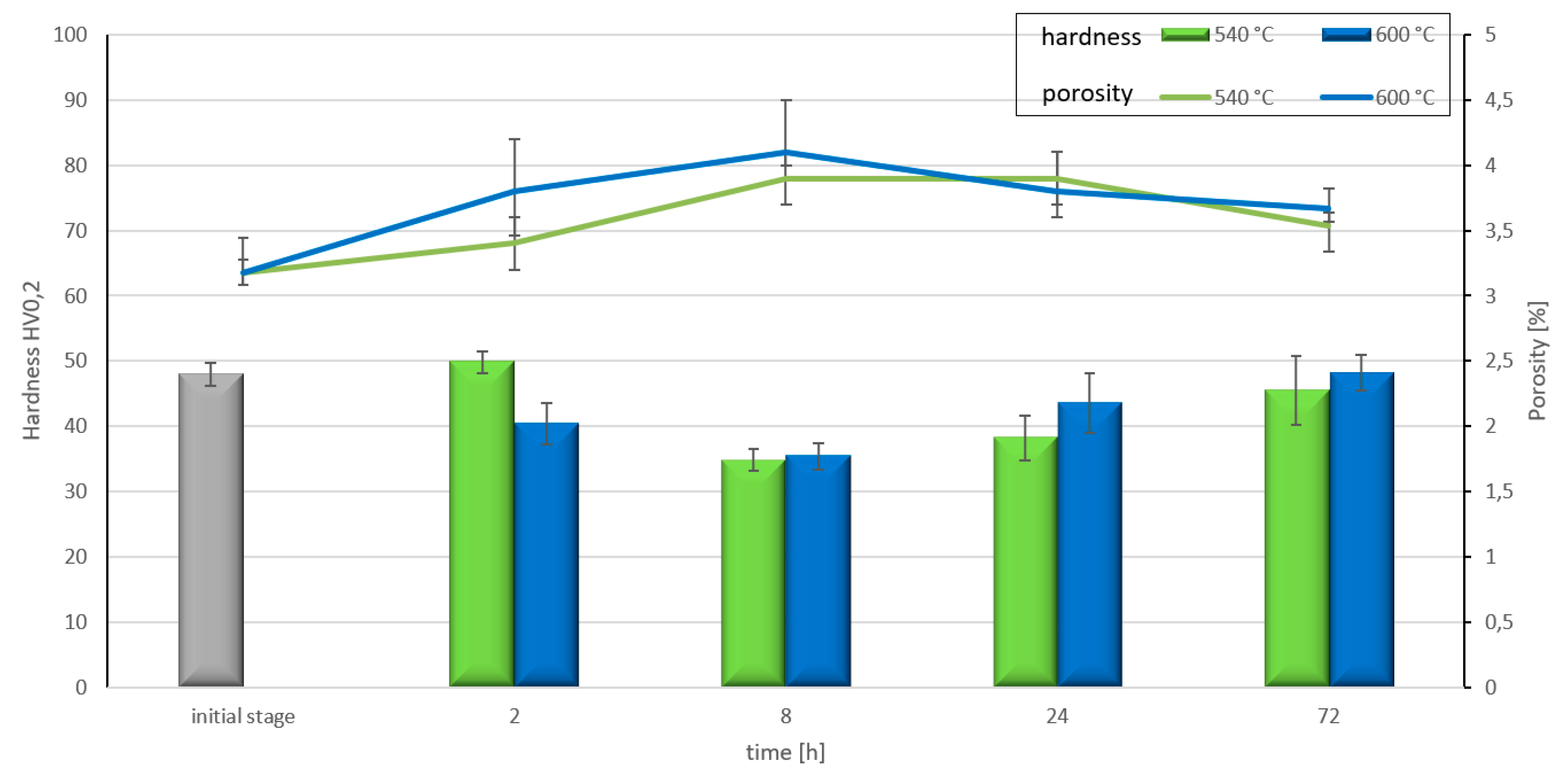
3. Results
4. Discussion
5. Conclusions
- -
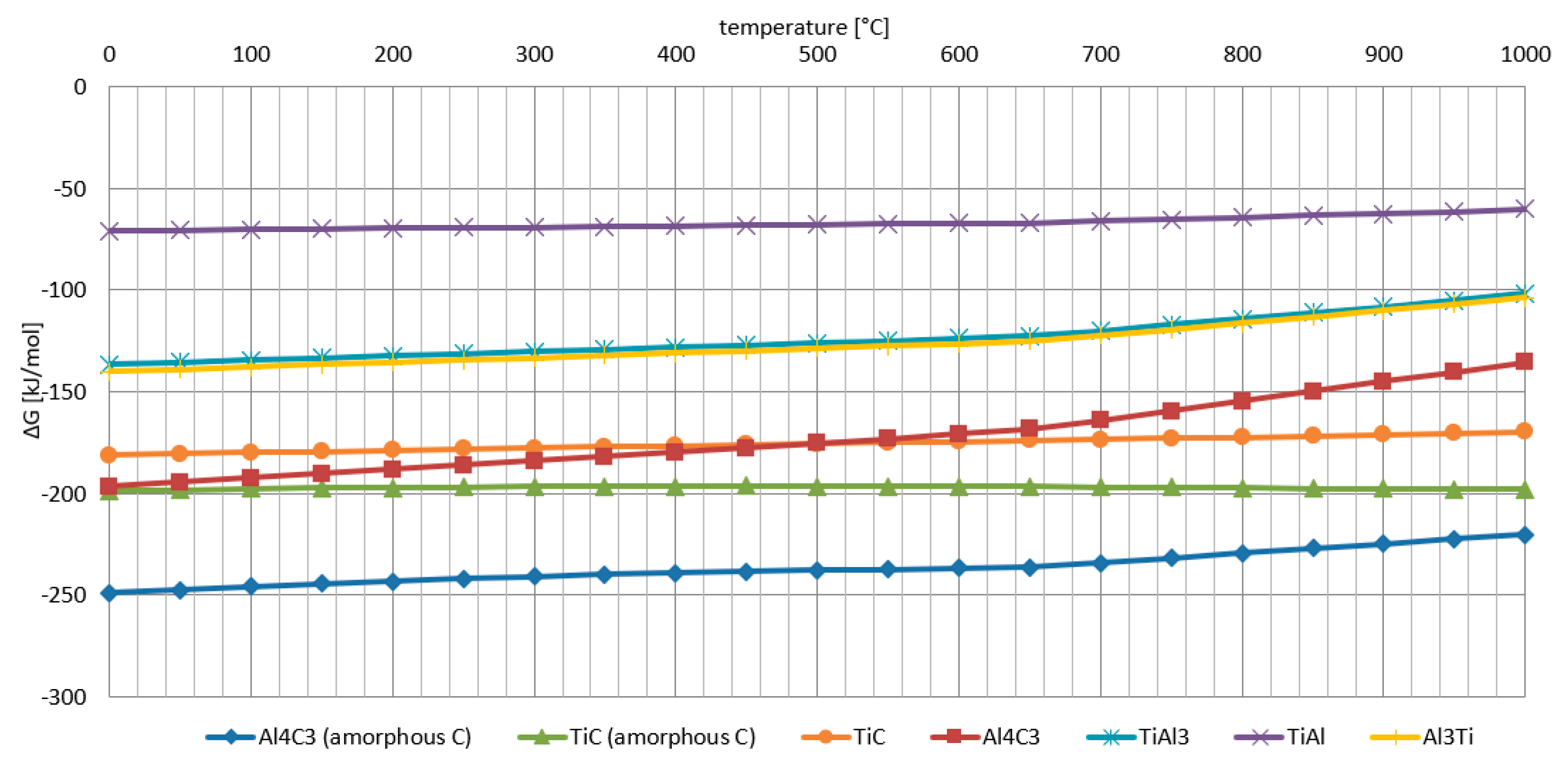
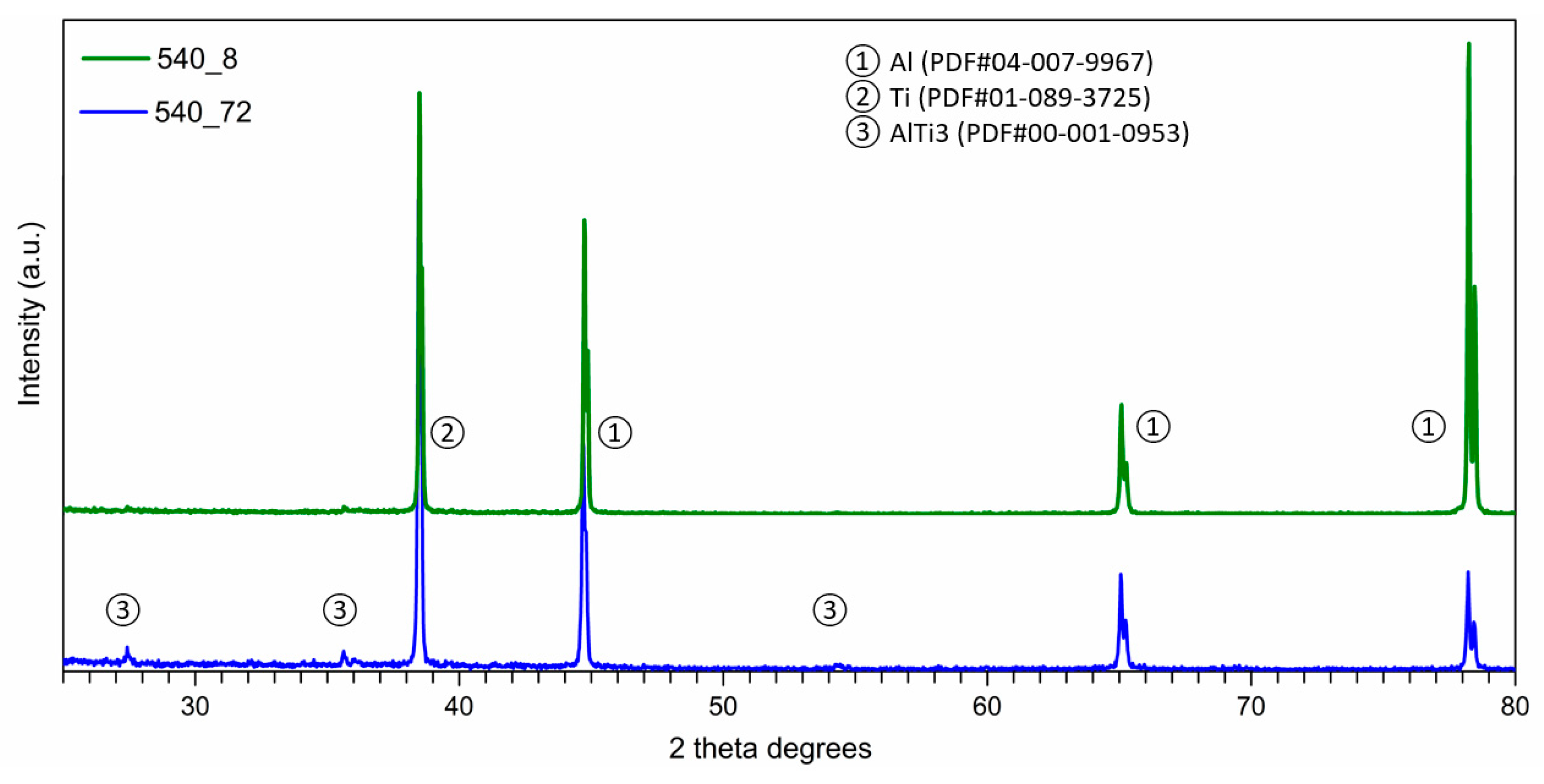
- The Al-Ti-C system revealed a tendency for the creation of new phases, especially in Al-Ti and Al-C systems. AlTi3 and Al4C3 phases were detected after TG investigation in the evaluated composites.
- -
- To control the phase composition of the material, a thin layer of Ti particles was applied on the surface of a C spatial structure. This solution limits contact between Al and C compounds and avoids the presence of an unwanted Al4C3 phase as a result.
- -
- Using titanium as a material for the creation of a protective layer, reactions (AlTi3) with the aluminium matrix, which benefit the material’s properties, are possible.
- -
- The optimal quality of protective Ti layer (tightness, continuity) can be achieved by adjustment of the compound’s ratio. The desired structure was achieved with reinforcement foam covered by suspension of 20 vol.% of Ti in PA resin.
- -
- The optimum conditions for new phase creation in Al-Ti systems are high temperature (higher than 0.8 of the melting point of aluminium) and prolonged treatment duration (minimum 24 hours). With these parameters, the behaviour of Al-Ti-C composites in real-time working conditions (high temperature and load) can be better understood.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Venkata Ravi Kumar, S.; Muniappan, A.; Mohanavel, V. Investigation of SiC and AL2O3—Reinforced with Aluminium Composites—A Review. Mater. Today Proc. 2020. [Google Scholar] [CrossRef]
- Moustafa, E. Effect of Multi-Pass Friction Stir Processing on Mechanical Properties for AA2024/Al2O3 Nanocomposites. Materials 2017, 10, 1053. [Google Scholar] [CrossRef]
- Ubaid, F.; Matli, P.R.; Shakoor, R.A.; Parande, G.; Manakari, V.; Amer Mohamed, A.M.; Gupta, M. Using B4C Nanoparticles to Enhance Thermal and Mechanical Response of Aluminum. Materials 2017, 10, 621. [Google Scholar] [CrossRef]
- Huang, G.; Hou, W.; Shen, Y. Evaluation of the microstructure and mechanical properties of WC particle reinforced aluminum matrix composites fabricated by friction stir processing. Mater. Charact. 2018, 138, 26–37. [Google Scholar] [CrossRef]
- Zhang, C.; Zeng, Y.-P.; Yao, D.; Yin, J.; Zuo, K.; Xia, Y.; Liang, H. The improved mechanical properties of Al matrix composites reinforced with oriented β-Si3N4 whisker. J. Mater. Sci. Technol. 2019, 35, 1345–1353. [Google Scholar] [CrossRef]
- Manghnani, S.; Shekhawat, D.; Goswami, C.; Patnaik, T.K.; Singh, T. Mechanical and Tribological Characteristics of Si3N4 Reinforced Aluminium Matrix Composites: A Short Review. Mater. Today Proc. 2020, 44, 4059–4064. [Google Scholar] [CrossRef]
- Alladi, A.; Aluri, M.; Maddela, N.; Abbadi, C.R. Recent Progress of CNTs Reinforcement with Metal Matrix Composites Using Friction Stir Processing. Mater. Today Proc. 2021, 44, 1731–1738. [Google Scholar] [CrossRef]
- Brodova, I.; Petrova, A.; Shirinkina, I.; Rasposienko, D.; Yolshina, L.; Muradymov, R.; Razorenov, S.; Shorokhov, E. Mechanical properties of submicrocrystalline aluminium matrix composites reinforced by “in situ” graphene through severe plastic deformation processes. J. Alloys Compd. 2021, 859, 158387. [Google Scholar] [CrossRef]
- Mohamad, S.; Liza, S.; Yaakob, Y. Strengthening of the mechanical and tribological properties of composite oxide film formed on aluminum alloy with the addition of graphite. Surf. Coat. Technol. 2020, 403, 126435. [Google Scholar] [CrossRef]
- Myalski, J.; Śleziona, J. Glassy carbon particles as component to modification of tribological properties. J. Mater. Process. Technol. 2006, 175, 291–298. [Google Scholar] [CrossRef]
- Ahamad, N.; Mohammad, A.; Gupta, P. Wear characteristics of Al matrix reinforced with Al2O3-carbon hybrid metal matrix composites. Mater. Today Proc. 2020, 38, 63–68. [Google Scholar] [CrossRef]
- Fallahdoost, H.; Nouri, A.; Azimi, A. Dual functions of TiC nanoparticles on tribological performance of Al/graphite composites. J. Phys. Chem. Solids 2016, 93, 137–144. [Google Scholar] [CrossRef]
- Nayim, S.M.T.I.; Hasan, M.Z.; Seth, P.P.; Gupta, P.; Thakur, S.; Kumar, D.; Jamwal, A. Effect of CNT and TiC Hybrid Reinforcement on the Micro-Mechano-Tribo Behaviour of Aluminium Matrix Composites. Mater. Today Proc. 2020, 21, 1421–1424. [Google Scholar] [CrossRef]
- Zhang, W.; Du, Y.; Zhang, P.; Wang, Y. Air-isolated stir casting of homogeneous Al-SiC composite with no air entrapment and Al4C3. J. Mater. Process. Technol. 2019, 271, 226–236. [Google Scholar] [CrossRef]
- Rodríguez-Reyes, M.; Pech-Canul, M.; Rendón-Angeles, J.; López-Cuevas, J. Limiting the development of Al4C3 to prevent degradation of Al/SiCp composites processed by pressureless infiltration. Compos. Sci. Technol. 2006, 66, 1056–1062. [Google Scholar] [CrossRef]
- Hyla, I.; Jerzy, M.; Józef, Ś.; Stanisław, W. Sposób Wytwarzania Węgla Szklistego. 166256, 8 May 1992. [Google Scholar]
- Myalski, J.; Olszowka-Myalska, A.; Hekner, B. Sposób Otrzymywania Otwartokomórkowych Pianek Węglowych. 234409, 4 June 2016. [Google Scholar]
- Okamoto, H. C-Ti (Carbon-Titanium). J. Phase Equilib. 1998, 19, 89. [Google Scholar] [CrossRef]
- Mitra, R.; Wanhill, R.J.H. Structural Intermetallics. In Aerospace Materials and Material Technologies; Prasad, N., Wanhill, R., Eds.; Indian Institute of Metals Series; Springer: Singapore, 2017; pp. 229–245. [Google Scholar]
- Kosova, N.; Sachkov, V.; Kurzina, I.; Pichugina, A.; Vladimirov, A.; Kazantseva, L.; Sachkova, A. The Preparation of the Ti-Al Alloys Based on Intermetallic Phases. In Proceedings of the 2nd International Symposium on Fundamental Aspects of Rare-earth Elements Mining and Separation and Modern Materials Engineering (REES-2015), Altay, Russia, 7–15 September 2015; Volume 112. [Google Scholar]
- Bandyopadhyay, D.; Sharma, R.C.; Chakraborti, N. The Ti-Co-C system (Titanium-Cobalt-Carbon). J. Phase Equilib. 2000, 21, 179–185. [Google Scholar] [CrossRef]
- Emamian, A.; Corbin, S.F.; Khajepour, A. In-Situ Deposition of Metal Matrix Composite in Fe-Ti-C System Using Laser Cladding Process. In Metal, Ceramic and Polymeric Composites for Various Uses; IntechOpen: London, UK, 2011. [Google Scholar]
- Shah Ismail, M.I.; Okamoto, Y.; Ok, A. Micro-Welding of Super Thermal Conductive Composite by Pulsed Nd:YAG Laser. In Nd YAG Laser; InTech: London, UK, 2012. [Google Scholar]
- Li, P.; Kandalova, E.; Nikitin, V. In Situ Synthesis of Al-TiC in Aluminum Melt. Mater. Lett. 2005, 59, 2545–2548. [Google Scholar] [CrossRef]
- Bandyopadhyay, D.; Sharma, R.C.; Chakraborti, N. The Ti-Al-C system (Titanium-Aluminum-Carbon). J. Phase Equilib. 2000, 21, 195–198. [Google Scholar] [CrossRef]
- Peng, L.; Wang, J.; Li, H.; Zhao, J.; He, L. Synthesis and microstructural characterization of Ti-Al3Ti metal-intermetallic laminate (MIL) composites. Scr. Mater. 2005, 52, 243–248. [Google Scholar] [CrossRef]
- Witusiewicz, V.; Hallstedt, B.; Bondar, A.; Hecht, U.; Sleptsov, S.; Velikanova, T. Thermodynamic description of the Al-C-Ti system. J. Alloys Compd. 2015, 623, 480–496. [Google Scholar] [CrossRef]
- Dębski, A.; Dębski, R.; Gasior, W. New Features of Entall Database: Comparison of Experimental and Model Formation Enthalpies. Arch. Metall. Mater. 2014, 59, 1337–1343. [Google Scholar] [CrossRef]
- Sadeghi, E.; Karimzadeh, F.; Abbasi, M. Thermodynamic analysis of Ti-Al-C intermetallics formation by mechanical alloying. J. Alloys Compd. 2013, 576, 317–323. [Google Scholar] [CrossRef]
- Myalski, J.; Hekner, B. Glassy Carbon Foams as Skeleton Reinforcement in Polymer Composite. Compos. Theory Pract. 2017, 17, 41–46. [Google Scholar]
- Hekner, B.; Jerzy, M. Correlations between Stereological Parameters of Carbon Component and Tribological Properties of Heterophase Composites Al-Al2O3+C. Compos. Thery Pract. 2016, 16, 67–73. [Google Scholar]
- Školáková, A.; Salvetr, P.; Leitner, J.; Lovaši, T.; Novák, P. Formation of Phases in Reactively Sintered TiAl3 Alloy. Molecules 2020, 25, 1912. [Google Scholar] [CrossRef]
- Karantzalis, A.E.; Wyatt, S.; Kennedy, A.R. The Mechanical Properties of Al-Tic Metal Matrix Composites Fabricated by a Flux-Casting Technique Mater. Sci. Eng. A 1997, 237, 200–206. [Google Scholar] [CrossRef]
- Yang, B.; Gan, G.; Yang, L.; Sun, M.; Zhang, H.; Fang, Z.Z. Microstructural characterization and wear behavior of in situ TiC/7075 composites synthesized by displacement reactions and spray forming. Mater. Sci. Eng. A 2011, 528, 5649–5655. [Google Scholar] [CrossRef]
- Rai, R.N.; Datta, G.L.; Chakraborty, M.; Chattopadhyay, A.B. A study on the machinability behaviour of Al-TiC composite prepared by in situ technique. Mater. Sci. Eng. A 2006, 428, 34–40. [Google Scholar] [CrossRef]
- Hekner, B.; Myalski, J.; Pawlik, T.; Sopicka-Lizer, M. Effect of Carbon in Fabrication Al-SiC Nanocomposites for Tribological Application. Materials 2017, 10, 679. [Google Scholar] [CrossRef]
- Chlubny, L.; Lis, J.; Chabior, K.; Chachlowska, P.; Kapusta, C. Processing and Properties of Max Phases-Based Materials Using SHS Technique. Arch. Metall. Mater. 2015, 60, 859–863. [Google Scholar] [CrossRef]
- Singh, J. Fabrication characteristics and tribological behavior of Al/SiC/Gr hybrid aluminum matrix composites: A review. Friction 2016, 4, 191–207. [Google Scholar] [CrossRef]
- Hekner, B.; Myalski, J.; Valle, N.; Botor-Probierz, A.; Sopicka-Lizer, M.; Wieczorek, J. Friction and wear behavior of Al-SiC(n) hybrid composites with carbon addition. Compos. Eng. 2017, 108, 291–300. [Google Scholar] [CrossRef]
- Park, K.; Kim, D.; Kim, K.; Cho, S.; Kwon, H. Behavior of Intermetallic Compounds of Al-Ti Composite Manufactured by Spark Plasma Sintering. Materials 2019, 12, 331. [Google Scholar] [CrossRef]
- Yonetken, A.; Çakmakkaya, M.; Oğuz, Y. Production and Characterization of Al-TiC Composite Materials. In Proceedings of the 1st International Conference on Engineering and Natural Sciences, Skopje, Macedonia, 15–19 May 2015. [Google Scholar]
- Ghasali, E.; Fazili, A.; Alizadeh, M.; Shirvanimoghaddam, K.; Ebadzadeh, T. Evaluation of Microstructure and Mechanical Properties of Al-TiC Metal Matrix Composite Prepared by Conventional, Microwave and Spark Plasma Sintering Methods. Materials 2017, 10, 1255. [Google Scholar] [CrossRef] [PubMed]
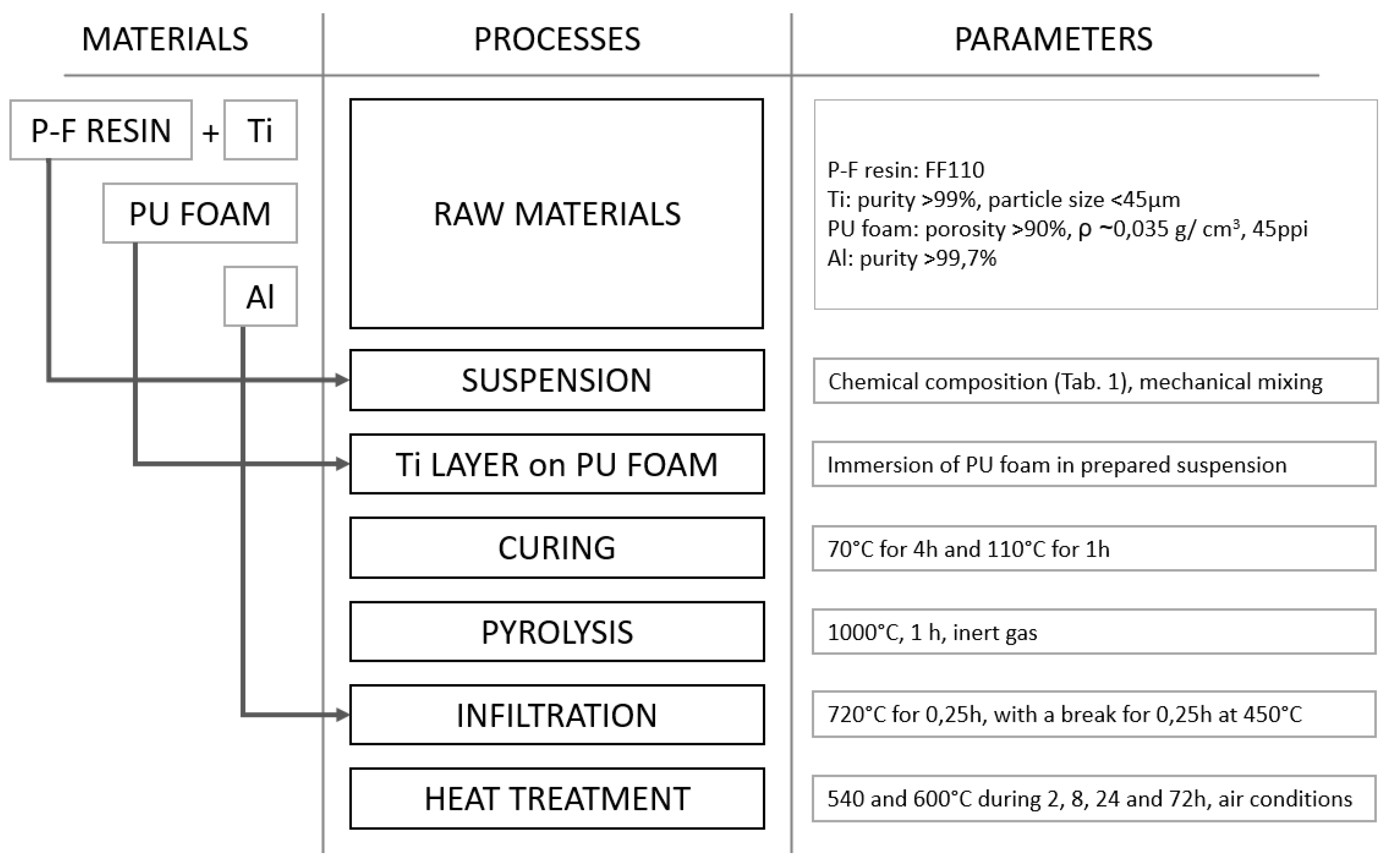



| Volume Ratio (%) | Ti | 5 | 10 | 15 | 20 | 25 | 30 | 40 |
| P-F resin | 95 | 90 | 85 | 80 | 75 | 70 | 60 | |
| mass (g) | Ti | 8.98 | 17.96 | 26.94 | 35.92 | 44.90 | 53.88 | 71.84 |
| P-F resin | 45.60 | 43.20 | 40.80 | 38.40 | 36.00 | 33.60 | 28.80 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hekner, B.; Myalski, J.; Wrześniowski, P.; Maciąg, T. Al Matrix Composites Reinforced by Ti and C Dedicated to Work at Elevated Temperature. Materials 2021, 14, 3114. https://doi.org/10.3390/ma14113114
Hekner B, Myalski J, Wrześniowski P, Maciąg T. Al Matrix Composites Reinforced by Ti and C Dedicated to Work at Elevated Temperature. Materials. 2021; 14(11):3114. https://doi.org/10.3390/ma14113114
Chicago/Turabian StyleHekner, Bartosz, Jerzy Myalski, Patryk Wrześniowski, and Tomasz Maciąg. 2021. "Al Matrix Composites Reinforced by Ti and C Dedicated to Work at Elevated Temperature" Materials 14, no. 11: 3114. https://doi.org/10.3390/ma14113114
APA StyleHekner, B., Myalski, J., Wrześniowski, P., & Maciąg, T. (2021). Al Matrix Composites Reinforced by Ti and C Dedicated to Work at Elevated Temperature. Materials, 14(11), 3114. https://doi.org/10.3390/ma14113114






