New Volatile Perfluorinated Amidine–Carboxylate Copper(II) Complexes as Promising Precursors in CVD and FEBID Methods
Abstract
1. Introduction
2. Materials and Methods
2.1. Instrumentation
2.2. DFT Calculations
2.3. Software
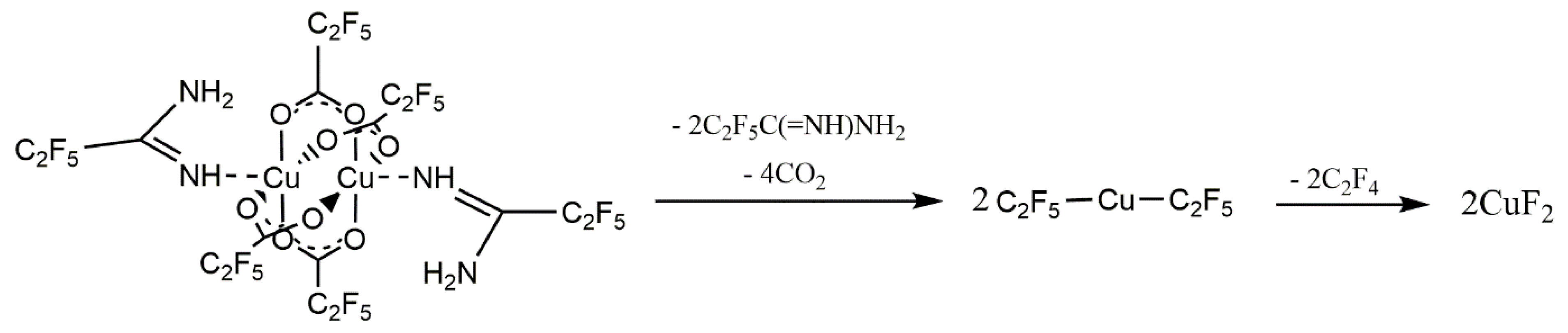
2.4. Synthesis of [Cu2(NH2(NH=)CC2F5))2(µ-O2CRF)4]) (1–4)
3. Results and Discussion
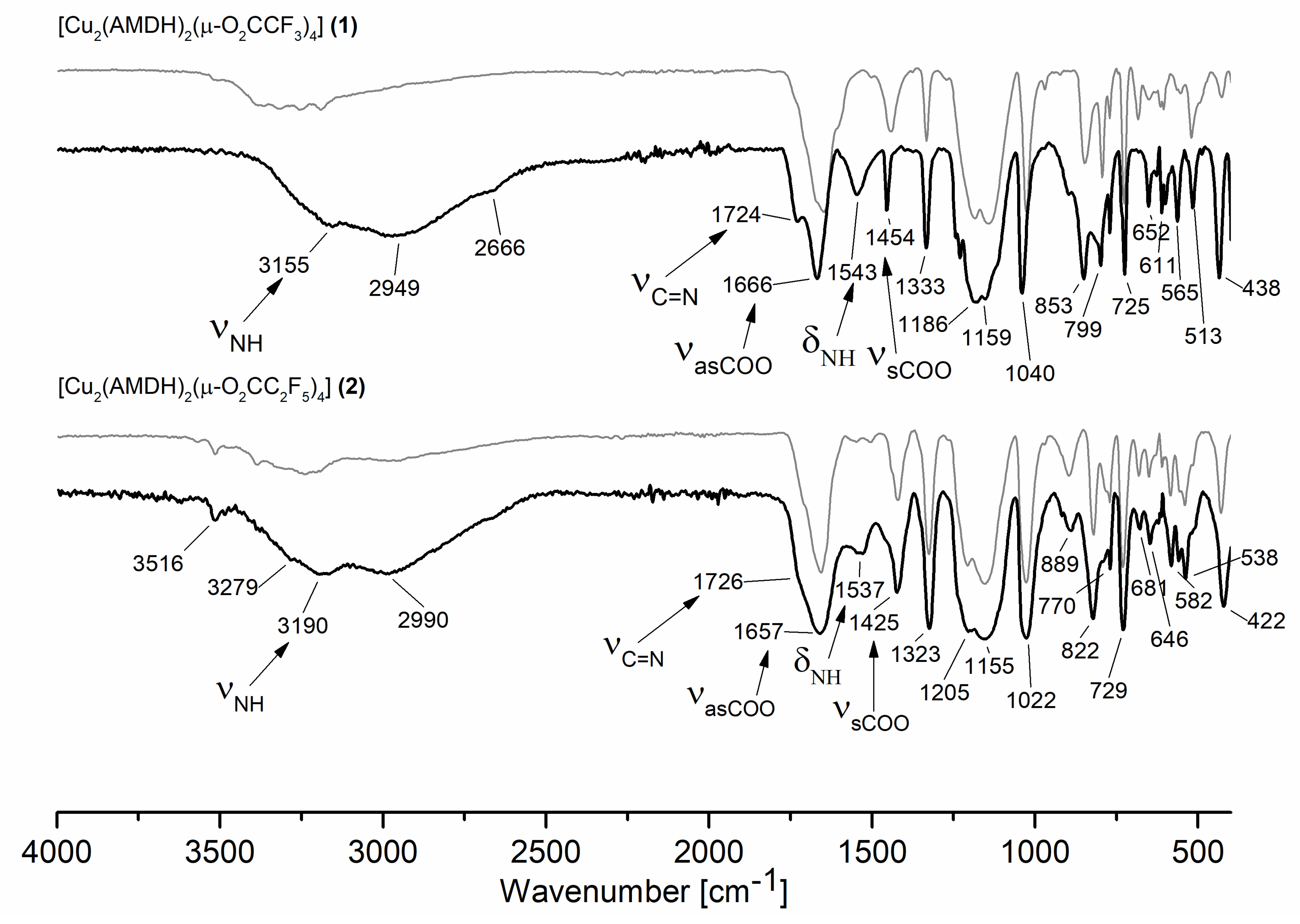
3.1. Infrared Spectra Analysis
3.2. DFT Calculations
3.3. Thermal Analysis
3.4. Mass Spectra Analysis
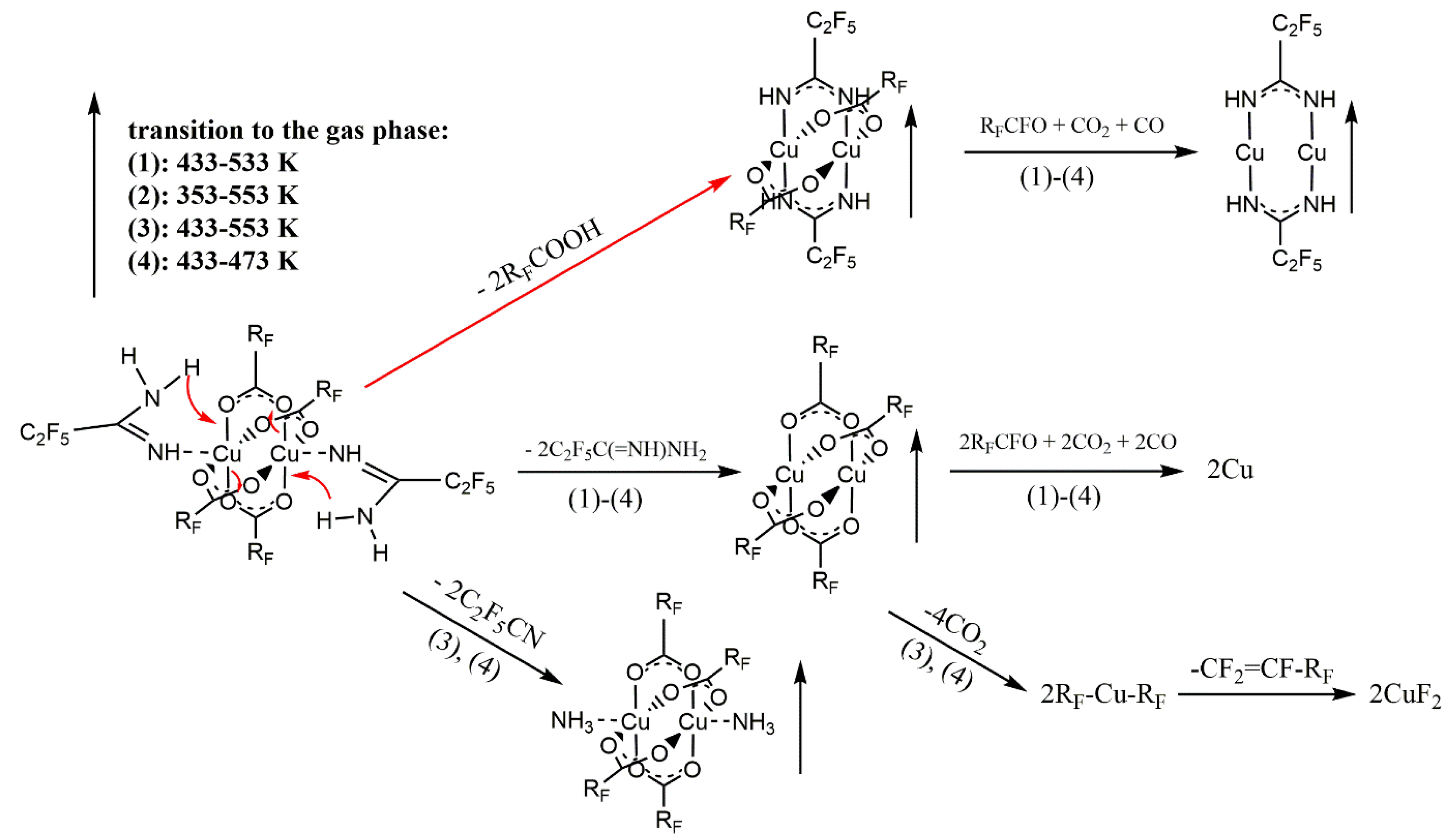
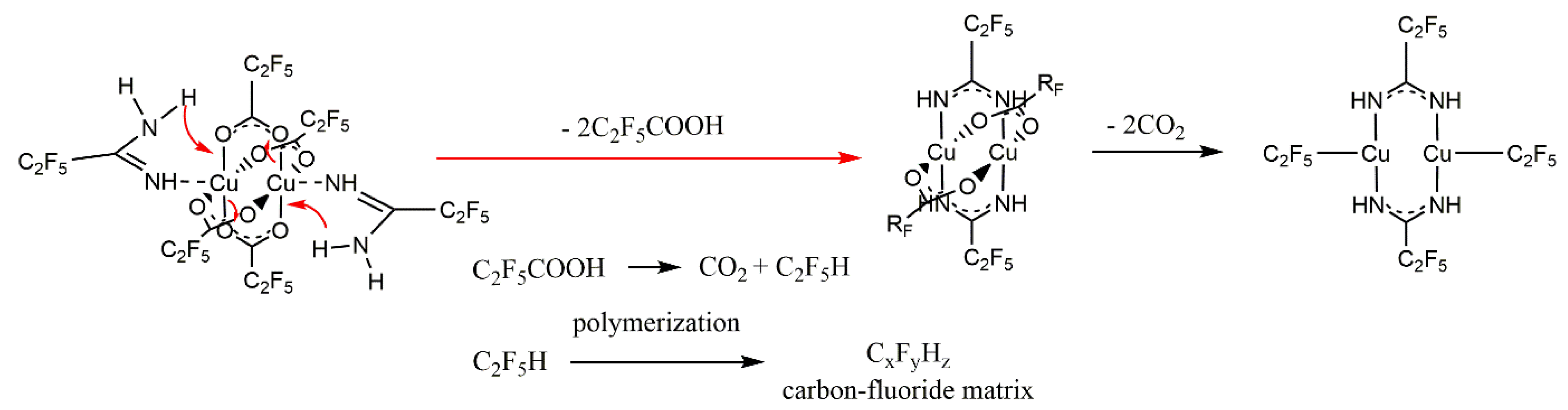
3.5. Variable Temperature Infrared Spectroscopy (VT IR)
3.6. Sublimation Experiments
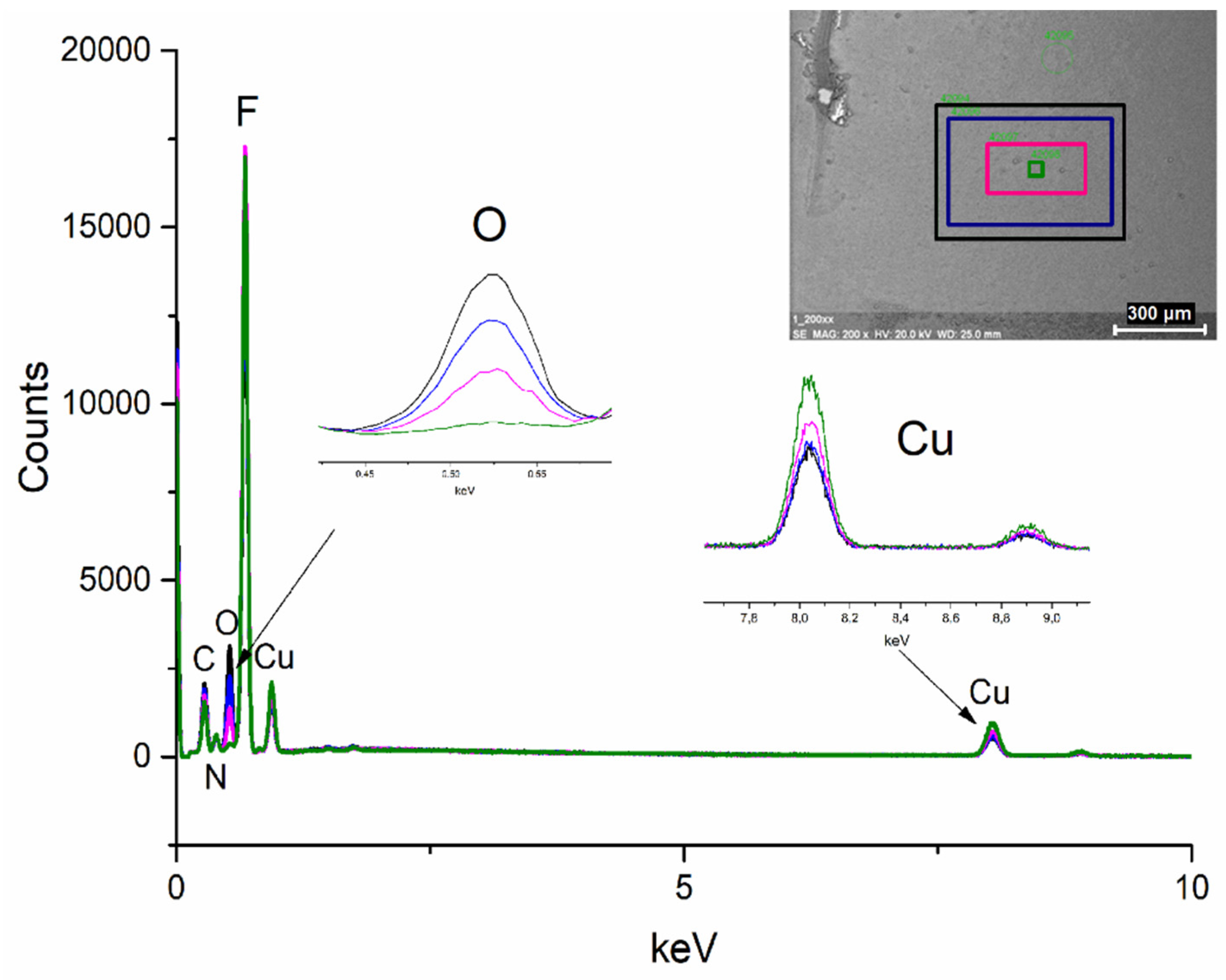
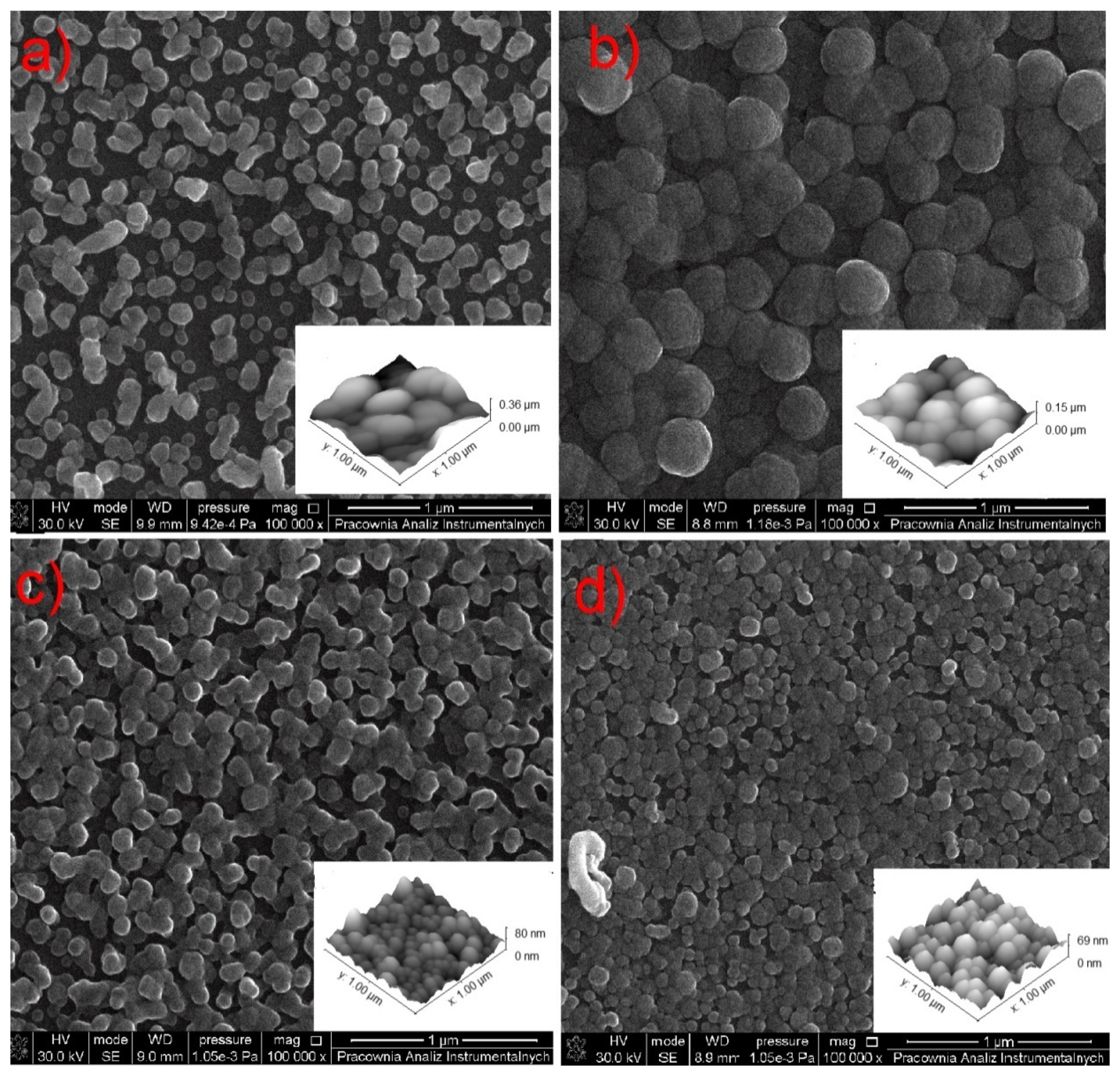
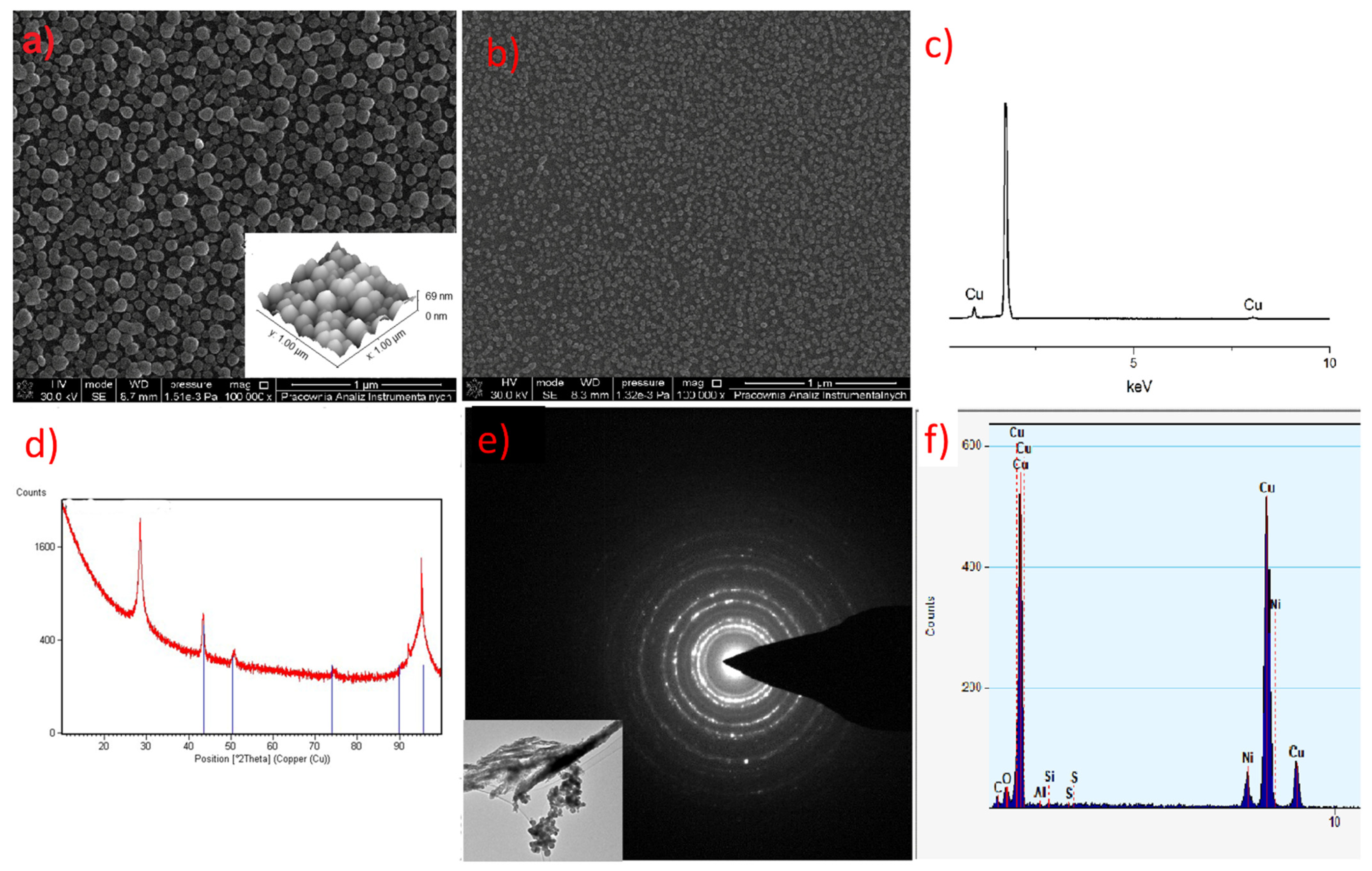
3.7. TEM and SEM Observations
3.8. CVD Preliminary Experiments
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Padnya, P.; Shibaeva, K.; Arsenyev, M.; Baryshnikova, S.; Terenteva, O.; Shiabiev, I.; Khannanov, A.; Boldyrev, A.; Gerasimov, A.; Grishaev, D.; et al. Catechol-Containing Schiff Bases on Thiacalixarene: Synthesis, Copper (II) Recognition, and Formation of Organic-Inorganic Copper-Based Materials. Molecules 2021, 26, 2334. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhong, X.; Hu, Y.; Li, B.; Sheng, Y.; Zhang, Y.; Weng, C.; Feng, M.; Han, H.; Wang, J. Organic-Inorganic Copper(II)-Based Material: A Low-Toxic, Highly Stable Light Absorber for Photovoltaic Application. J. Phys. Chem. Lett. 2017, 8, 1804–1809. [Google Scholar] [CrossRef]
- Wang, L.; Sun, H.; Sun, C.; Xu, D.; Tao, J.; Wei, T.; Zhang, Z.; Zhang, Y.; Wang, Z.; Bi, W. Lead-free, stable orange-red-emitting hybrid copper based organic-inorganic compounds. Dalton Trans. 2021, 50, 2766–2773. [Google Scholar] [CrossRef]
- Doniz Kettenmann, S.; Nossol, Y.; Louka, F.R.; Legrande, J.R.; Marine, E.; Fischer, R.C.; Mautner, F.A.; Hergl, V.; Kulak, N.; Massoud, S.S. Copper(II) Complexes with Tetradentate Piperazine-Based Ligands: DNA Cleavage and Cytotoxicity. Inorganics 2021, 9, 12. [Google Scholar] [CrossRef]
- Hussain, A.; AlAjmi, M.F.; Rehman, M.T.; Amir, S.; Husain, F.M.; Alsalme, A.; Siddiqui, M.A.; AlKhedhairy, A.A.; Khan, R.A. Copper(II) complexes as potential anticancer and Nonsteroidal anti-inflammatory agents: In vitro and in vivo studies. Sci. Rep. 2019, 9, 1–17. [Google Scholar] [CrossRef]
- Cao, M.G.; Wang, J.J.; Wang, Y.; Wang, X.F.; Li, J.X.; Chen, J.X.; Hu, B.Q.; Hu, D.D. Two cobalt(II) and copper(II) complexes with 2,4,5-tri(4-pyridyl)-imidazole and 5-hydroxyisophthalic acid as turn-on luminescence sensors for Mg2+, Ca2+ and SCN−ions. Z. Anorg. Allg. Chem. 2021, 647, 714–721. [Google Scholar] [CrossRef]
- Kanso, H.; Ben Jrad, A.; Inguimbert, N.; Rammal, W.; Philouze, C.; Thomas, F.; Noguer, T.; Calas-Blanchard, C. Synthesis and Characterization of Bis-1,2,3-Triazole Ligand and its Corresponding Copper Complex for the Development of Electrochemical Affinity Biosensors. Chem. A Eur. J. 2021, 27, 1–10. [Google Scholar] [CrossRef]
- Guo, R.-F.; Yan, H.-T.; Liu, R.-X.; Li, H.-C.; Liu, Y.-C.; Chen, Z.-F.; Liang, H. Structural characterization and pharmacological assessment: In vitro/in vivo of a new copper(ii)-based derivative of enrofloxacin. Metallomics 2020, 12, 2145–2160. [Google Scholar] [CrossRef] [PubMed]
- Andrejević, T.P.; Aleksic, I.; Počkaj, M.; Kljun, J.; Milivojevic, D.; Stevanović, N.L.; Nikodinovic-Runic, J.; Turel, I.; Djuran, M.I.; Glišić, B. Tailoring copper(ii) complexes with pyridine-4,5-dicarboxylate esters for anti-: Candida activity. Dalton Trans. 2021, 50, 2627–2638. [Google Scholar] [CrossRef] [PubMed]
- Velo-Gala, I.; Barceló-Oliver, M.; Gil, D.M.; González-Pérez, J.M.; Castiñeiras, A.; Domínguez-Martín, A. Deciphering the H-Bonding Preference on Nucleoside Molecular Recognition through Model Copper(II) Compounds. Pharmaceuticals 2021, 14, 244. [Google Scholar] [CrossRef]
- Yang, Y.; Huang, Q.; Payne, G.F.; Sun, R.; Wang, X. A highly conductive, pliable and foldable Cu/cellulose paper electrode enabled by controlled deposition of copper nanoparticles. Nanoscale 2019, 11, 725–732. [Google Scholar] [CrossRef] [PubMed]
- Kwon, Y.T.; Kim, Y.S.; Lee, Y.; Kwon, S.; Lim, M.; Song, Y.; Choa, Y.H.; Yeo, W.H. Ultrahigh Conductivity and Superior Interfacial Adhesion of a Nanostructured, Photonic-Sintered Copper Membrane for Printed Flexible Hybrid Electronics. ACS Appl. Mater. Interfaces 2018, 10, 44071–44079. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, L.; Cui, K.; Ge, S.; Cheng, X.; Yan, M.; Yu, J.; Liu, H. Flexible electronics based on micro/nanostructured paper. Adv. Mater. 2018, 30, 1801588. [Google Scholar] [CrossRef]
- Chan, G.H.; Zhao, J.; Hicks, E.M.; Schatz, G.C.; Van Duyne, R.P. Plasmonic properties of copper nanoparticles fabricated by nanosphere lithography. Nano Lett. 2007, 7, 1947–1952. [Google Scholar] [CrossRef]
- Huang, C.-L.; Kumar, G.; Sharma, G.D.; Chen, F.-C. Plasmonic effects of copper nanoparticles in polymer photovoltaic devices for outdoor and indoor applications. Appl. Phys. Lett. 2020, 116, 253302. [Google Scholar] [CrossRef]
- Gu, Z.; Shen, H.; Shang, L.; Lv, X.; Qian, L.; Zheng, G. Nanostructured Copper-Based Electrocatalysts for CO 2 Reduction. Small Methods 2018, 2, 1800121. [Google Scholar] [CrossRef]
- Luc, W.; Fu, X.; Shi, J.; Lv, J.J.; Jouny, M.; Ko, B.H.; Xu, Y.; Tu, Q.; Hu, X.; Wu, J.; et al. Two-dimensional copper nanosheets for electrochemical reduction of carbon monoxide to acetate. Nat. Catal. 2019, 2, 423–430. [Google Scholar] [CrossRef]
- Kuroda, K.; Keller, P.; Kawasaki, H. Mild synthesis of single-nanosized plasmonic copper nanoparticles and their catalytic reduction of methylene blue. Colloids Interface Sci. Commun. 2019, 31, 100187. [Google Scholar] [CrossRef]
- Gao, F.; Pang, H.; Xu, S.; Lu, Q. Copper-based nanostructures: Promising antibacterial agents and photocatalysts. Chem. Commun. 2009, 3571–3573. [Google Scholar] [CrossRef] [PubMed]
- Mahmoodi, S.; Elmi, A.; Hallaj-Nezhadi, S. Copper Nanoparticles as Antibacterial Agents. J. Mol. Pharm. Org. Process. Res. 2018, 6, 1–7. [Google Scholar] [CrossRef]
- Jain, A.; Chi, K.M.; Kodas, T.T.; Hampden-Smith, M.J.; Farr, J.D.; Paffett, M.F. Chemical Vapor Deposition of Copper from (Hexafluoroacetylacetonato)(alkyne)-copper(I) Complexes via Disproportionation. Chem. Mater. 1991, 3, 995–997. [Google Scholar] [CrossRef]
- Chi, Y.; Hsu, P.-F.; Liu, C.-S.; Ching, W.-L.; Chou, T.-Y.; Carty, A.J.; Peng, S.-M.; Lee, G.-H.; Chuang, S.-H. Fluorinated aminoalkoxide CuII complexes: New CVD precursors for deposition of copper metal. J. Mater. Chem. 2002, 12, 3541–3550. [Google Scholar] [CrossRef]
- Toh, B.H.W.; McNeill, D.W.; Gamble, H.S. Investigation of copper layers deposited by CVD using Cu(I)hfac(TMVS) precursor. J. Mater. Sci. Mater. Electron. 2005, 16, 437–443. [Google Scholar] [CrossRef]
- Córdoba, R.; Sesé, J.; De Teresa, J.M.M.; Ibarra, M.R.R.; Cordoba, R.; Sese, J. High-purity cobalt nanostructures grown by focused-electron-beam-induced deposition at low current. Microelectron. Eng. 2010, 87, 1550–1553. [Google Scholar] [CrossRef]
- Córdoba, R.; Sharma, N.; Kölling, S.; Koenraad, P.M.; Koopmans, B. High-purity 3D nano-objects grown by focused-electron-beam induced deposition. Nanotechnology 2016, 27, 355301. [Google Scholar] [CrossRef] [PubMed]
- Shawrav, M.M.; Taus, P.; Wanzenboeck, H.D.; Schinnerl, M.; Stöger-Pollach, M.; Schwarz, S.; Steiger-Thirsfeld, A.; Bertagnolli, E. Highly conductive and pure gold nanostructures grown by electron beam induced deposition. Sci. Rep. 2016, 6, 34003. [Google Scholar] [CrossRef]
- Kodas, T.T.; Hampden-Smith, M.J. The Chemistry of Metal CVD; Wiley-VCH: Weinheim, Germany, 1994. [Google Scholar]
- Huth, M.; Porrati, F.; Schwalb, C.; Winhold, M.; Sachser, R.; Dukic, M.; Adams, J.; Fantner, G. Focused electron beam induced deposition: A perspective. Beilstein J. Nanotechnol. 2012, 3, 597–619. [Google Scholar] [CrossRef] [PubMed]
- Utke, I.; Hoffmann, P.; Melngailis, J. Gas-assisted focused electron beam and ion beam processing and fabrication. J. Vac. Sci. Technol. B 2008, 26, 1197–1276. [Google Scholar] [CrossRef]
- Luisier, A.; Utke, I.; Bret, T.; Cicoira, F.; Hauert, R.; Rhee, S.-W.; Doppelt, P.; Hoffmann, P. Comparative Study of Cu Precursors for 3D Focused Electron Beam Induced Deposition. J. Electrochem. Soc. 2004, 151, C535–C537. [Google Scholar] [CrossRef][Green Version]
- Utke, I.; Luisier, A.; Hoffmann, P.; Laub, D.; Buffat, P.A. Focused-electron-beam-induced deposition of freestanding three-dimensional nanostructures of pure coalesced copper crystals. Appl. Phys. Lett. 2002, 81, 3245–3247. [Google Scholar] [CrossRef]
- Haverkamp, C.; Sarau, G.; Polyakov, M.N.; Utke, I.; dos Santos, M.V.P.; Christiansen, S.; Höflich, K. A novel copper precursor for electron beam induced deposition. Beilstein J. Nanotechnol. 2018, 9, 1220–1227. [Google Scholar] [CrossRef]
- Berger, L.; Jurczyk, J.; Madajska, K.; Edwards, T.E.J.; Szymańska, I.; Hoffmann, P.; Utke, I. High-Purity Copper Structures from a Perfluorinated Copper Carboxylate Using Focused Electron Beam Induced Deposition and Post-Purification. ACS Appl. Electron. Mater. 2020, 2, 1989–1996. [Google Scholar] [CrossRef]
- Reilly, W.L.; Brown, H.C. Reactions of Perfluoronitriles. I. Synthesis of Derivatives of Perfluoroamidines, N-Substituted Perfluoroamidines and Perfluorothioamides. J. Am. Chem. Soc. 1956, 78, 6032–6034. [Google Scholar] [CrossRef]
- Hagen, D.J.; Pemble, M.E.; Karppinen, M. Atomic layer deposition of metals: Precursors and film growth. Appl. Phys. Rev. 2019, 6, 041309. [Google Scholar] [CrossRef]
- Krisyuk, V.V.; Aloui, L.; Prud’homme, N.; Sarapata, B.; Senocq, F.; Samélor, D.; Vahlas, C. CVD of pure copper films from a novel amidinate precursor. ECS Trans. 2009, 25, 581–586. [Google Scholar] [CrossRef]
- Krisyuk, V.; Aloui, L.; Prud’Homme, N.; Sysoev, S.; Senocq, F.; Saḿlor, D.; Vahlas, C. CVD of pure copper films from amidinate precursor. Electrochem. Solid State Lett. 2011, 14, D26–D29. [Google Scholar] [CrossRef]
- Chua, D.; Kim, S.B.; Li, K.; Gordon, R. Low temperature chemical vapor deposition of cuprous oxide thin films using a copper(i) amidinate precursor. ACS Appl. Energy Mater. 2019, 2, 7750–7756. [Google Scholar] [CrossRef]
- Li, Z.; Barry, S.T.; Gordon, R.G. Synthesis and characterization of copper(I) amidinates as precursors for atomic layer deposition (ALD) of copper metal. Inorg. Chem. 2005, 44, 1728–1735. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.; Daniele, S. Metal-Organic Derivatives with Fluorinated Ligands as Precursors for Inorganic Nanomaterials. Chem. Rev. 2015, 115, 8379–8448. [Google Scholar] [CrossRef]
- Szymańska, I.B. Gaseous phase studies of new copper(II) carboxylate complexes with tert-butylamine as potential precursors for chemical vapor deposition (CVD). Polyhedron 2013, 50, 200–207. [Google Scholar] [CrossRef]
- Piszczek, P.; Szymańska, I.B.; Talik, E.; Heimann, J. Deposition of thin copper layers using copper(II) carboxylate complexes with tert-butylamine as new CVD precursors. Chem. Vap. Depos. 2013, 19, 251–259. [Google Scholar] [CrossRef]
- Brown, D.J.; Chisholm, M.H.; Gallucci, J.C. Amidinate–carboxylate complexes of dimolybdenum and ditungsten: M2(O2CR)2((NiPr)2CR’)2. Preparations, molecular and electronic structures and reactions. J. Chem. Soc. Dalton Trans. 2008, 1615–1624. [Google Scholar] [CrossRef] [PubMed]
- Mendoza, I.; Curado, N.; Carrasco, M.; Álvarez, E.; Peloso, R.; Rodríguez, A.; Carmona, E. Synthesis and structure of mixed carboxylate-aminopyridinate and -amidinate complexes of dimolybdenum and ditungsten. Inorganica Chim. Acta 2015, 424, 120–128. [Google Scholar] [CrossRef]
- Munzeiwa, W.A.; Nyamori, V.O.; Omondi, B. Zn(II) and Cu(II) unsymmetrical formamidine complexes as effective initiators for ring-opening polymerization of cyclic esters. Appl. Organomet. Chem. 2018, 32, e4247. [Google Scholar] [CrossRef]
- Szłyk, E.; Szymańska, I. Studies of new volatile copper(I) complexes with triphenylphosphite and perfluorinated carboxylates. Polyhedron 1999, 18, 2941–2948. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. G16_C01; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Zhao, Y.; Truhlar, D.G. The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: Two new functionals and systematic testing of four M06-class functionals and 12 other function. Theor. Chem. Acc. 2008, 120, 215–241. [Google Scholar] [CrossRef]
- John Wiley & Sons. SpectraBase. Available online: https://spectrabase.com/ (accessed on 29 May 2021).
- Barry, S.T. Amidinates, guanidinates and iminopyrrolidinates: Understanding precursor thermolysis to design a better ligand. Coord. Chem. Rev. 2013, 257, 3192–3201. [Google Scholar] [CrossRef]
- John Wiley & Sons. SpectraBase Compound ID=JSNx6EfxlY2 SpectraBase Spectrum ID=3ZkQWtxcvyC. Available online: https://spectrabase.com/spectrum/3ZkQWtxcvyC (accessed on 29 May 2021).
- Majima, T.; Sugita, K.; Nakao, A.; Matsunuma, S.; Abe, K. Synthesis of thin layer of fluorinated polymer by laser CVD—direct synthesis of perfluoropolyether thin film on surface by oxidative polymerization of a perfluoropropene-oxygen mixture using an ArF excimer laser. J. Photopolym. Sci. Technol. 1993, 6, 421–428. [Google Scholar] [CrossRef]
- John Wiley & Sons. SpectraBase Compound ID=8A1dEqurmmH SpectraBase Spectrum ID=G4CBuKNSKtQ. Available online: https://spectrabase.com/spectrum/G4CBuKNSKtQ (accessed on 29 May 2021).
- John Wiley & Sons. SpectraBase Compound ID=n8DC1v4dvL SpectraBase Spectrum ID=Big2BLmUPf2. Available online: https://spectrabase.com/spectrum/Big2BLmUPf2 (accessed on 29 May 2021).
- John Wiley & Sons. SpectraBase Compound ID=4G3cv2HmDfG SpectraBase Spectrum ID=89U74D3zedG. Available online: https://spectrabase.com/spectrum/89U74D3zedG (accessed on 29 May 2021).
- Shurvell, H.F.; Bulmer, J.T. The infrared and Raman spectra of pentafluoropropionitrile. J. Fluor. Chem. 1972, 1, 391–406. [Google Scholar] [CrossRef]
- John Wiley & Sons. SpectraBase Compound ID=COBj6FpC1Cb SpectraBase Spectrum ID=4dr5ESO3mVP. Available online: https://spectrabase.com/spectrum/4dr5ESO3mVP (accessed on 29 May 2021).
- Run Nielsen, J.; Claassen, H.H.; Smith, D.C. Infrared and Raman Spectra of Fluorinated Ethylenes. V. Hexafluoropropene. J. Chem. Phys. 1952, 20, 1916–1919. [Google Scholar] [CrossRef]
- U.S. Secretary of Commerce on Behalf of the United States of America. Perfluoro-n-Pentanoic Acid. Available online: https://webbook.nist.gov/cgi/cbook.cgi?ID=C2706903&Mask=80 (accessed on 29 May 2021).
- Yuan, Q.; Zhou, T.; Li, L.; Zhang, J.; Liu, X.; Ke, X.; Zhang, A. Hydrogen bond breaking of TPU upon heating: Understanding from the viewpoints of molecular movements and enthalpy. RSC Adv. 2015, 5, 31153–31165. [Google Scholar] [CrossRef]
- John Wiley & Sons. SpectraBase Compound ID=JSNx6EfxlY2 SpectraBase Spectrum ID=C0XmNpe0v68. Available online: https://spectrabase.com/spectrum/C0XmNpe0v68 (accessed on 29 May 2021).
- Stoffels, W.W.; Stoffels, E.; Tachibana, K. Polymerization of fluorocarbons in reactive ion etching plasmas. J. Vac. Sci. Technol. A Vac. Surf. Films 1998, 16, 87–95. [Google Scholar] [CrossRef]
- Rijs, N.J.; O’Hair, R.A.J.J. Forming trifluoromethylmetallates: Competition between decarboxylation and C-F bond activation of group 11 trifluoroacetate complexes, [CF 3CO 2ML]. Dalton Trans. 2012, 41, 3395–3406. [Google Scholar] [CrossRef] [PubMed]
- Lacko, M.; Papp, P.; Szymańska, I.B.; Szłyk, E.; Matejčík, Š. Electron interaction with copper(II) carboxylate compounds. Beilstein J. Nanotechnol. 2018, 9, 384–398. [Google Scholar] [CrossRef]
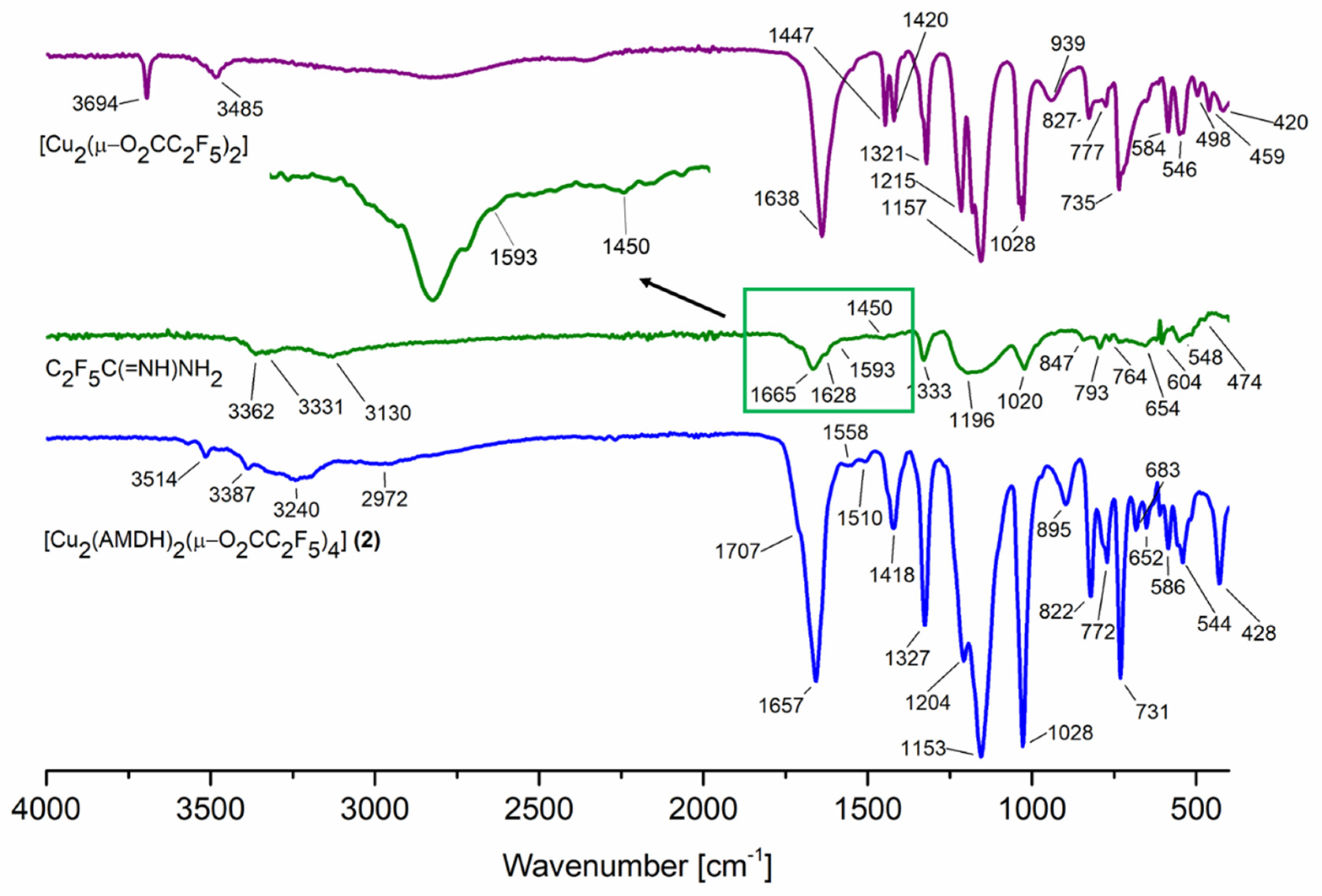
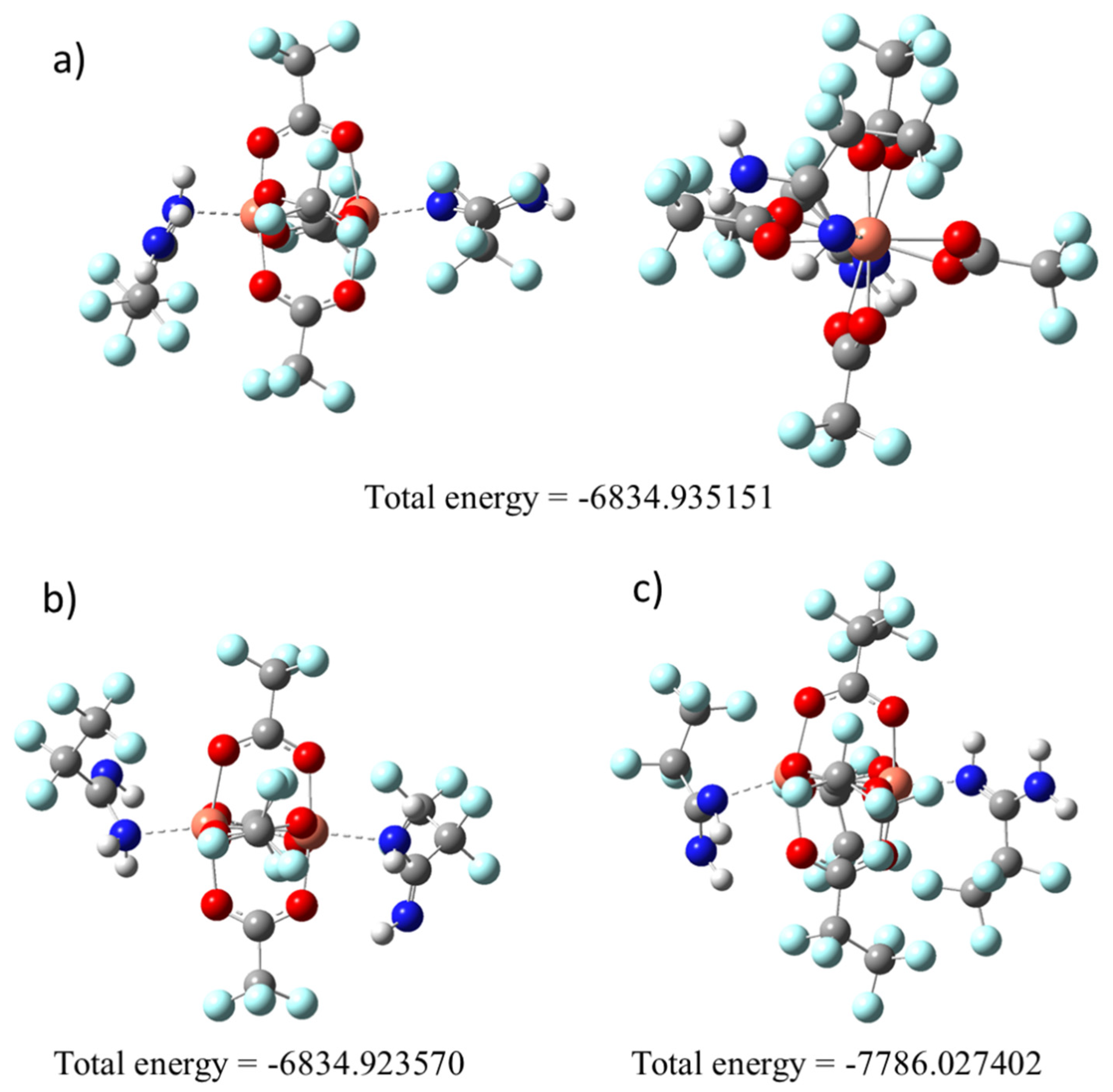


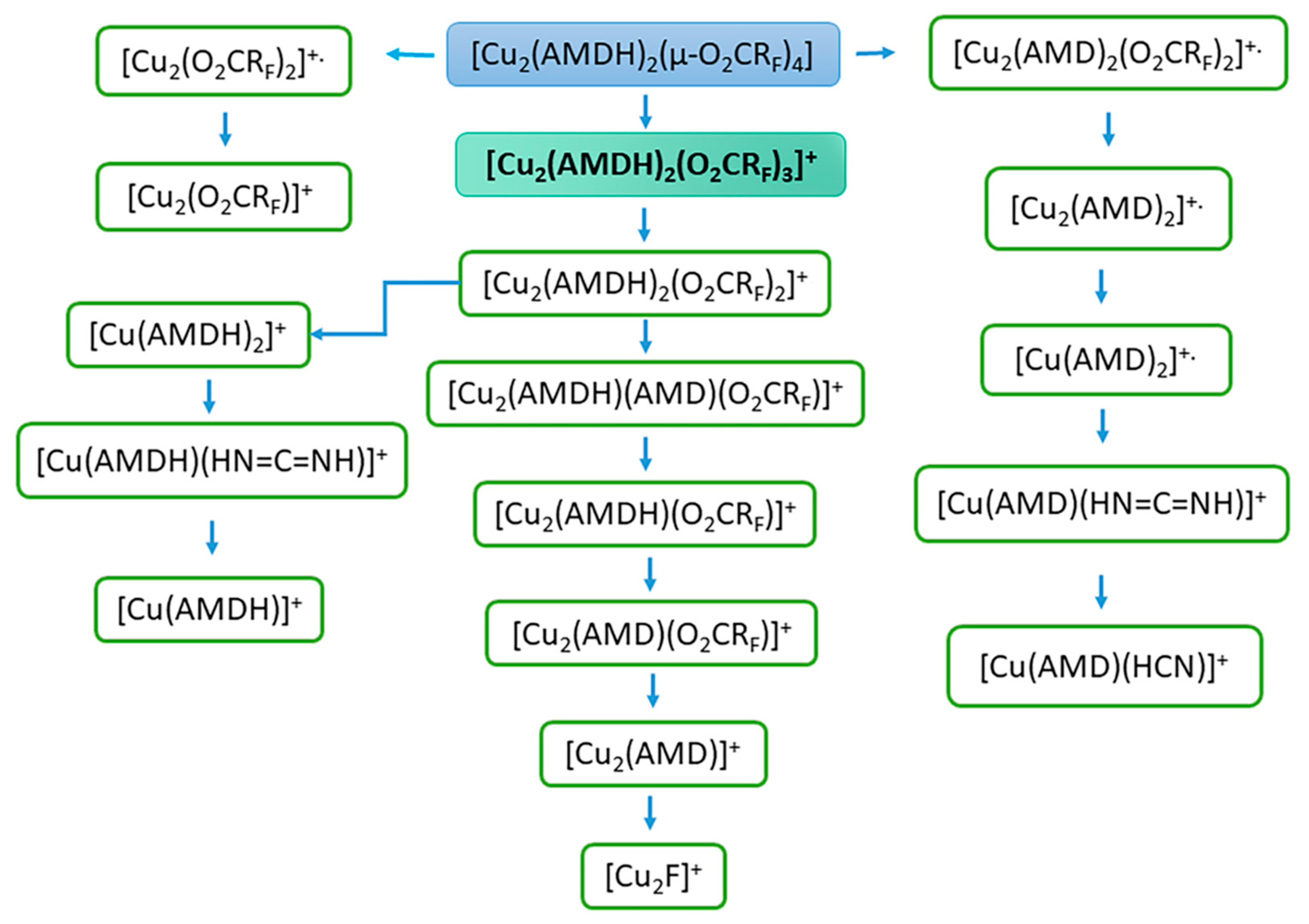
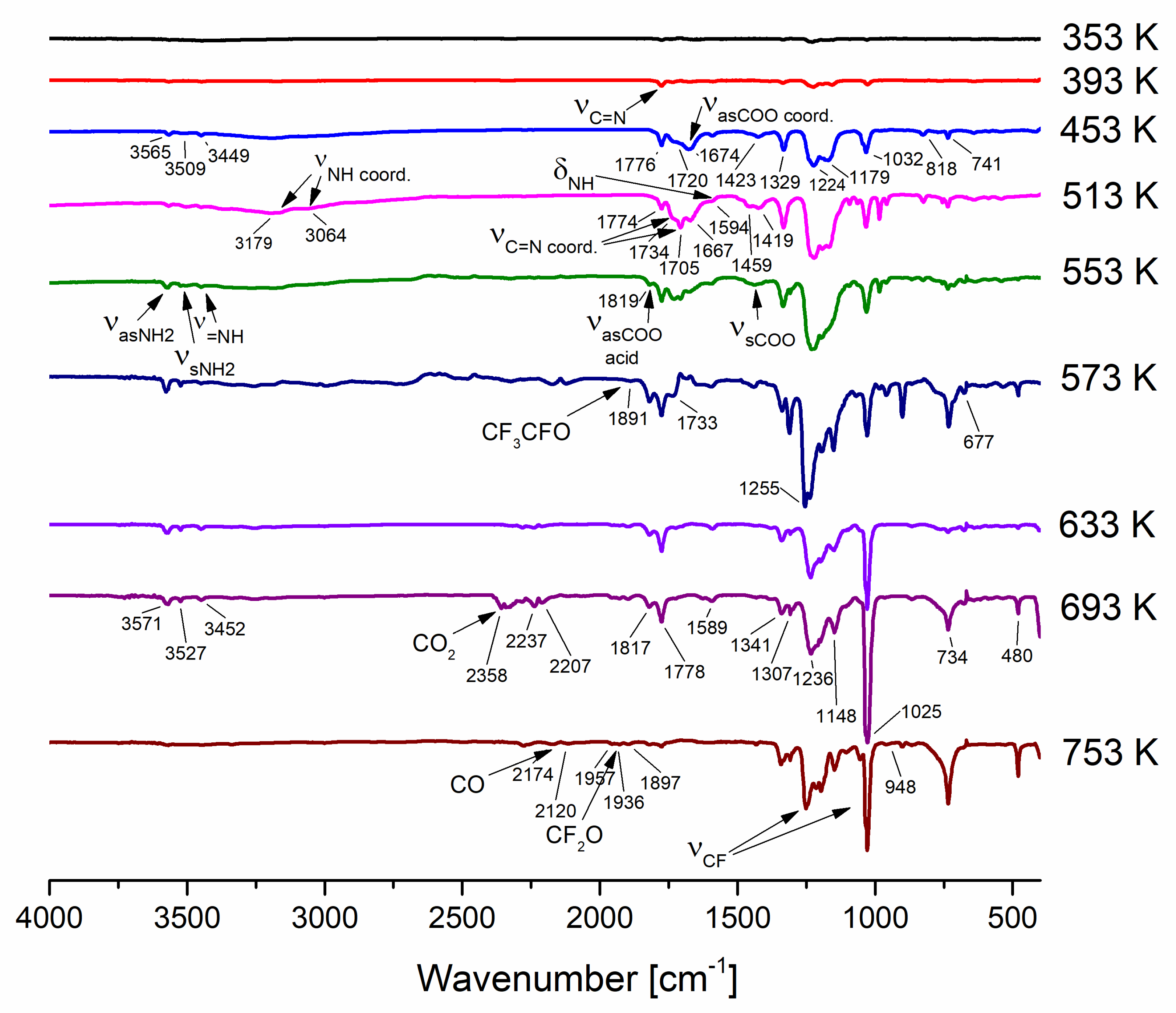




| Compound | νasNH2 | ν=NH | δNH2 | νN=C–N | νasCOO | νsCOO | ΔCOO |
|---|---|---|---|---|---|---|---|
| [Cu2(AMDH)2(µ-O2CCF3)4] (1) | 3380 | 3009 | 1597 | 1507 | 1649 | 1443 | 206 |
| [Cu2(AMDH)2(µ-O2CC2F5)4] (2) | 3390 | 3240 | 1603 | 1510 | 1657 | 1418 | 239 |
| [Cu2(AMDH)2(µ-O2CC3F7)4] (3) | 3387 | 3012 | 1606 | 1504 | 1675 | 1411 | 264 |
| [Cu2(AMDH)2(µ-O2CC4F9)4] (4) | 3390 | 3202 | 1608 | 1502 | 1672 | 1413 | 259 |
| AMDH | 3362 | 3130 | 1593 | 1450 | - | - | - |
| Complex | Temperature (K) | Residue Cu2O (%) | |||
|---|---|---|---|---|---|
| Ti | Tm | Tf | Found | Calc. | |
| [Cu2(AMDH)2(µ-O2CCF3)4] (1) | 391 544 | 471 573 | 492 586 | 13.0 | 15.8 |
| [Cu2(AMDH)2(µ-O2CC2F5)4] (2) | 364 | 449 | 556 | 3.59 | 13.0 |
| [Cu2(AMDH)2(µ-O2CC3F7)4] (3) | 337 | 499 | 551 | 1.96 | 11.0 |
| [Cu2(AMDH)2(µ-O2CC4F9)4] (4) | 338 | 513 | 555 | 4.64 | 9.52 |
| Fragments | m/z | Temperature [K] | ||||
|---|---|---|---|---|---|---|
| 327 | 353 | 398 | 433 | 529 | ||
| [Cu2(AMDH)2(O2CC2F5)3]+ | 939 | 8 | 24 | 7 | 3 | - |
| [Cu2(AMDH)(HN=C=NH)(O2CC2F5)3]+ | 819 | <1 | 2 | <1 | - | - |
| [Cu2(AMDH)2(O2CC2F5)2]+ | 776 | 1 | 3 | 4 | 7 | - |
| [Cu2(AMD)2(O2CC2F5)2]+. | 774 | <1 | 1 | 1 | - | - |
| [Cu2(AMD)3]+ | 609 | 2 | 7 | 2 | 1 | - |
| [Cu(AMDH)(AMD)(O2CC2F5)]+ | 549 | 9 | 30 | 10 | 4 | - |
| [Cu2(O2CC2F5)2]+. | 452 | 1 | 4 | 3 | 3 | 18 |
| [Cu2(AMDH)(O2CC2F5)]+ | 451 | 2 | 5 | 3 | 4 | <1 |
| [Cu2(AMD)(O2CC2F5)]+ | 450 | 2 | 4 | 4 | 8 | <1 |
| [Cu(AMDH)2]+ | 387 | 4 | 14 | 9 | - | - |
| [Cu(AMD)2]+. | 385 | 6 | 18 | 7 | - | - |
| [Cu2(O2CC2F5)]+ | 289 | 2 | 5 | 5 | 10 | 50 |
| [Cu(AMDH)(HN=C=NH)]+ | 267 | 4 | 10 | 6 | 3 | - |
| [Cu(AMD)(HN=C=NH)]+ | 266 | 1 | 3 | 1 | 1 | - |
| [AMDH]+. | 162 | 9 | 39 | 7 | 8 | - |
| [NH2CC2F5]+ | 147 | 9 | 34 | 11 | 23 | 12 |
| [Cu2F]+ | 145 | - | <1 | 1 | 2 | 13 |
| [C2F4CN]+ | 126 | 62 | 100 | 66 | 75 | 51 |
| [C2F5]+ | 119 | 86 | 1 | 100 | 1 | 100 |
| [CF2CN]+ | 76 | 80 | 1 | 85 | 100 | 67 |
| [Cu]+ | 63 | - | 1 | 2 | 2 | 9 |
| [CO2H]+ | 45 | 40 | 1 | 45 | 90 | 24 |
| [CO2]+. | 44 | 100 | 75 | 76 | 90 | 1 |
| [HN=C=NH]+. | 42 | 5 | 7 | 2 | 2 | 2 |
| [F2]+. | 38 | 9 | 3 | 3 | 2 | 1 |
| Precursor | |
|---|---|
| Precursor mass (m) [mg] | 100 |
| Vaporization temperature (TV) [K] | 393 (2), 453 (2), (3) |
| Deposition temperature (TD) [K] | 573–633 |
| Carrier gas | Ar |
| Substrates | Si(111) |
| Deposition time (t) [min] | 60 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Madajska, K.; Szymańska, I.B. New Volatile Perfluorinated Amidine–Carboxylate Copper(II) Complexes as Promising Precursors in CVD and FEBID Methods. Materials 2021, 14, 3145. https://doi.org/10.3390/ma14123145
Madajska K, Szymańska IB. New Volatile Perfluorinated Amidine–Carboxylate Copper(II) Complexes as Promising Precursors in CVD and FEBID Methods. Materials. 2021; 14(12):3145. https://doi.org/10.3390/ma14123145
Chicago/Turabian StyleMadajska, Katarzyna, and Iwona Barbara Szymańska. 2021. "New Volatile Perfluorinated Amidine–Carboxylate Copper(II) Complexes as Promising Precursors in CVD and FEBID Methods" Materials 14, no. 12: 3145. https://doi.org/10.3390/ma14123145
APA StyleMadajska, K., & Szymańska, I. B. (2021). New Volatile Perfluorinated Amidine–Carboxylate Copper(II) Complexes as Promising Precursors in CVD and FEBID Methods. Materials, 14(12), 3145. https://doi.org/10.3390/ma14123145








