Recycled Steel Slag as a Porous Adsorbent to Filter Phosphorus-Rich Water with 8 Filtration Circles
Abstract
1. Introduction
2. Experimental
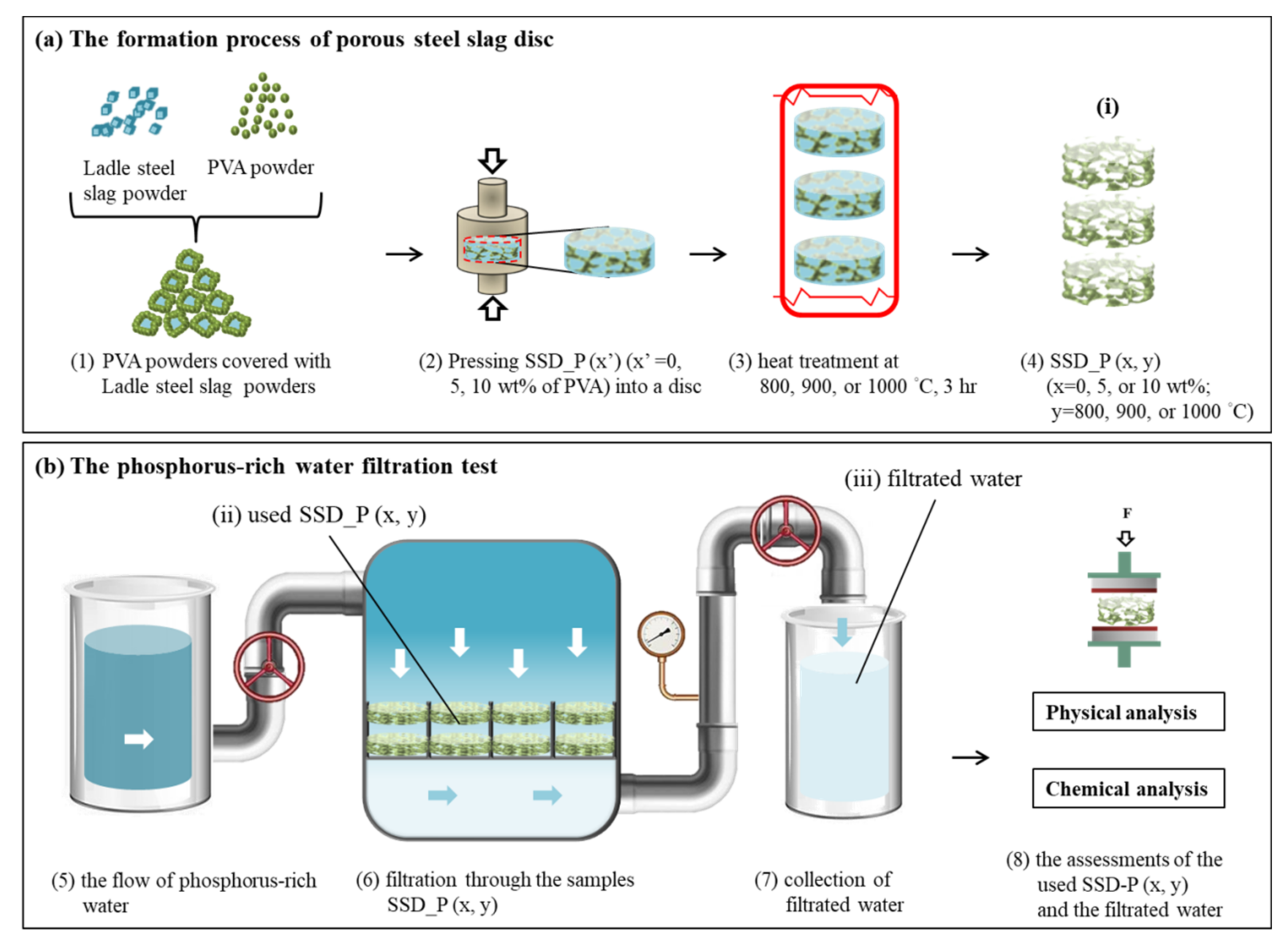
2.1. Preparation of Porous Steel Slag Disc SSD_P (x, y)
2.2. Quality Assessment of SSD_P (x, y)
2.3. Assessment of Filtering Efficiency
3. Results and Discussion
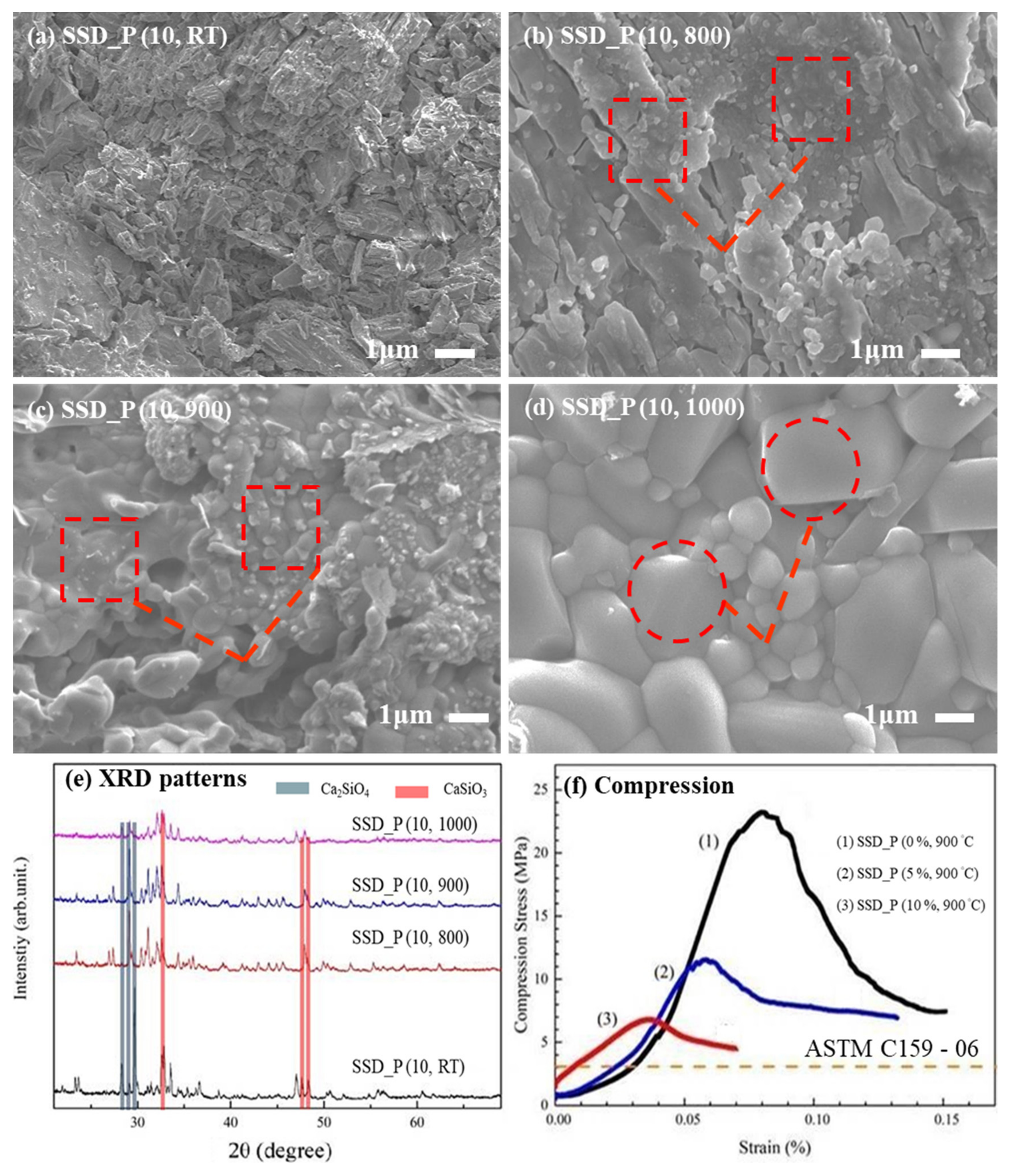
3.1. Physical Properties of SSD_P (x, y)
3.2. Chemical Composition of SSD_P (x, y)
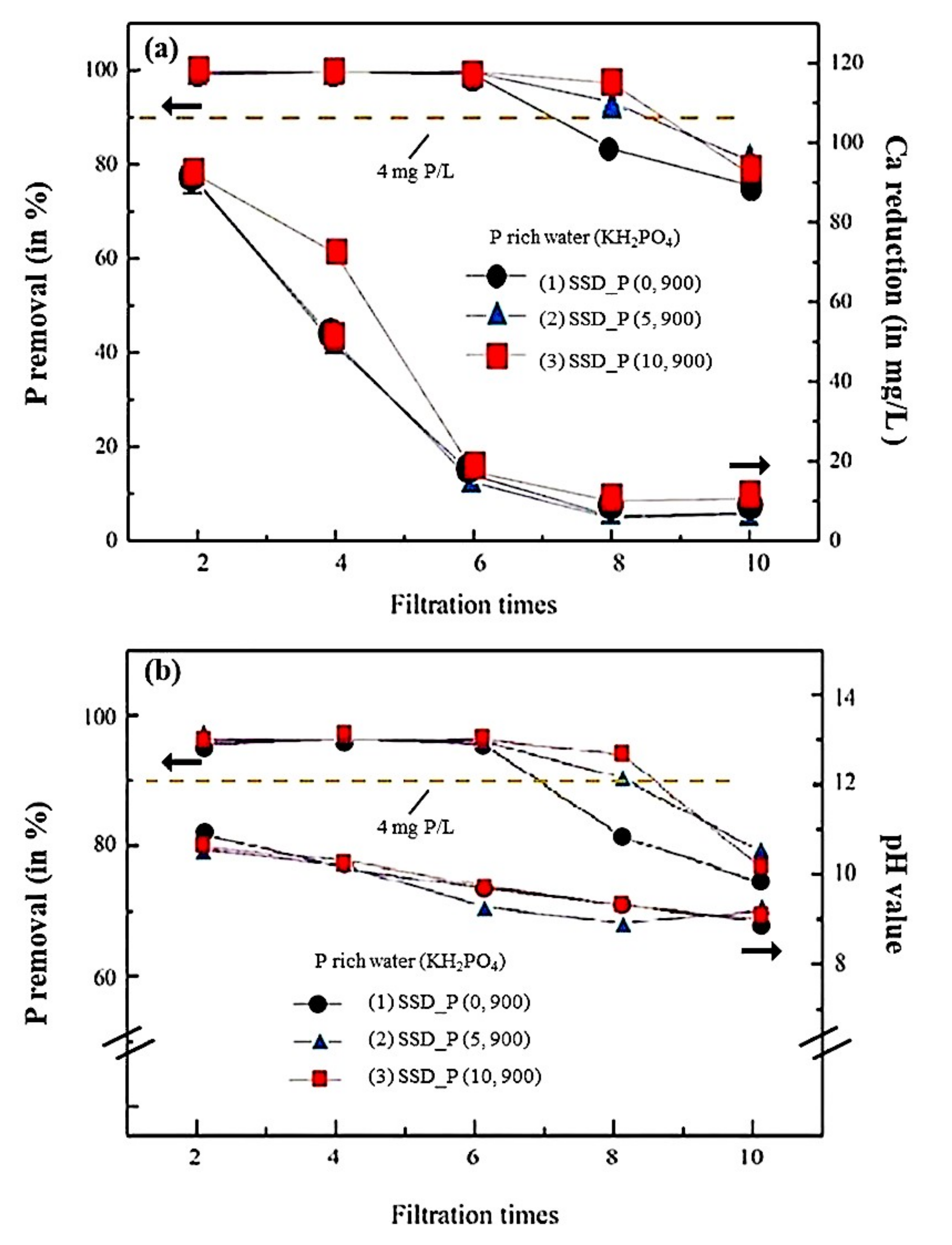
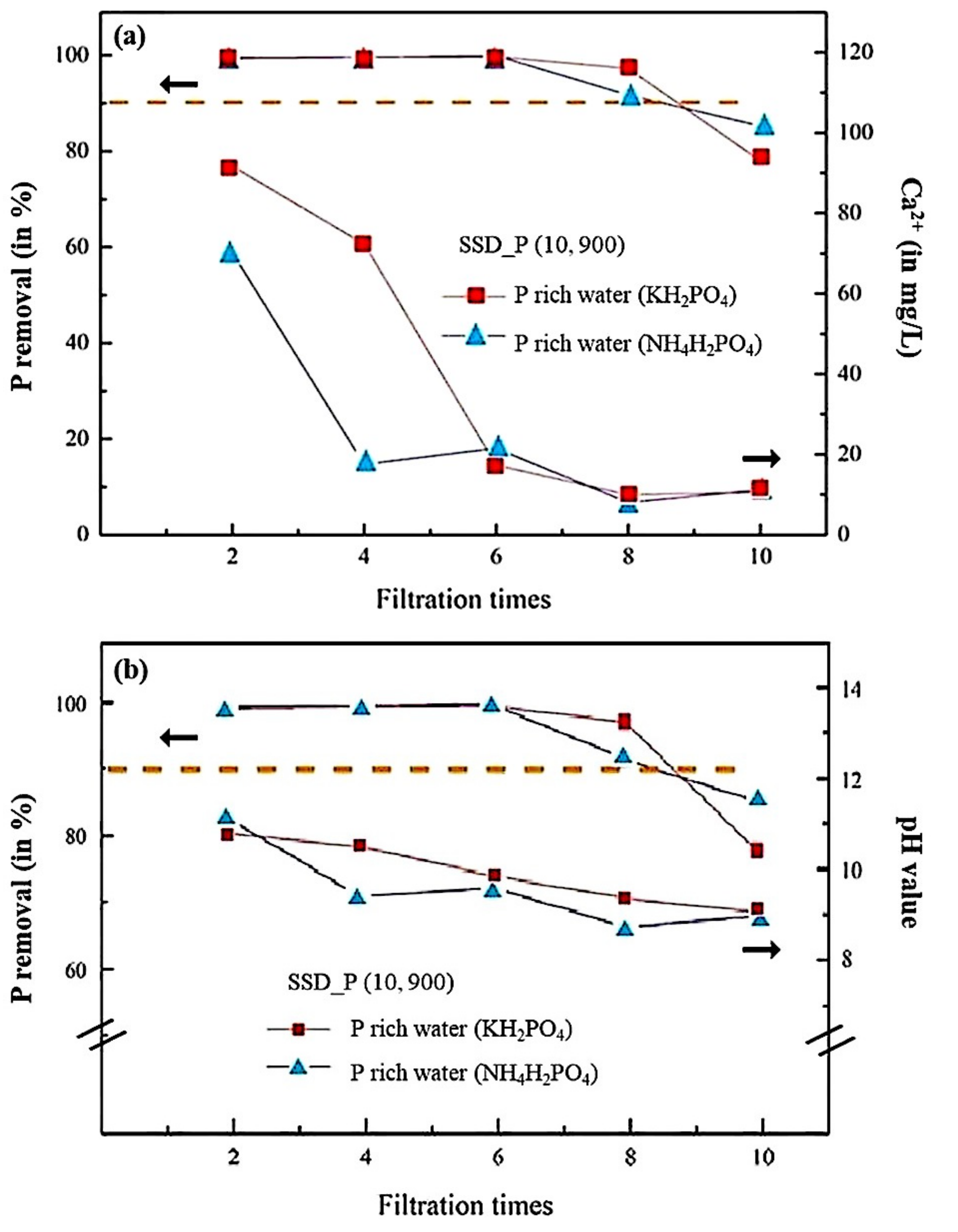
3.3. Filtering Performances of SSD_P (x, y) in the P-Rich Water (KH2PO4)
Filtering Performances of SSD_P (x, y) in the P-Rich Water (NH4H2PO4)
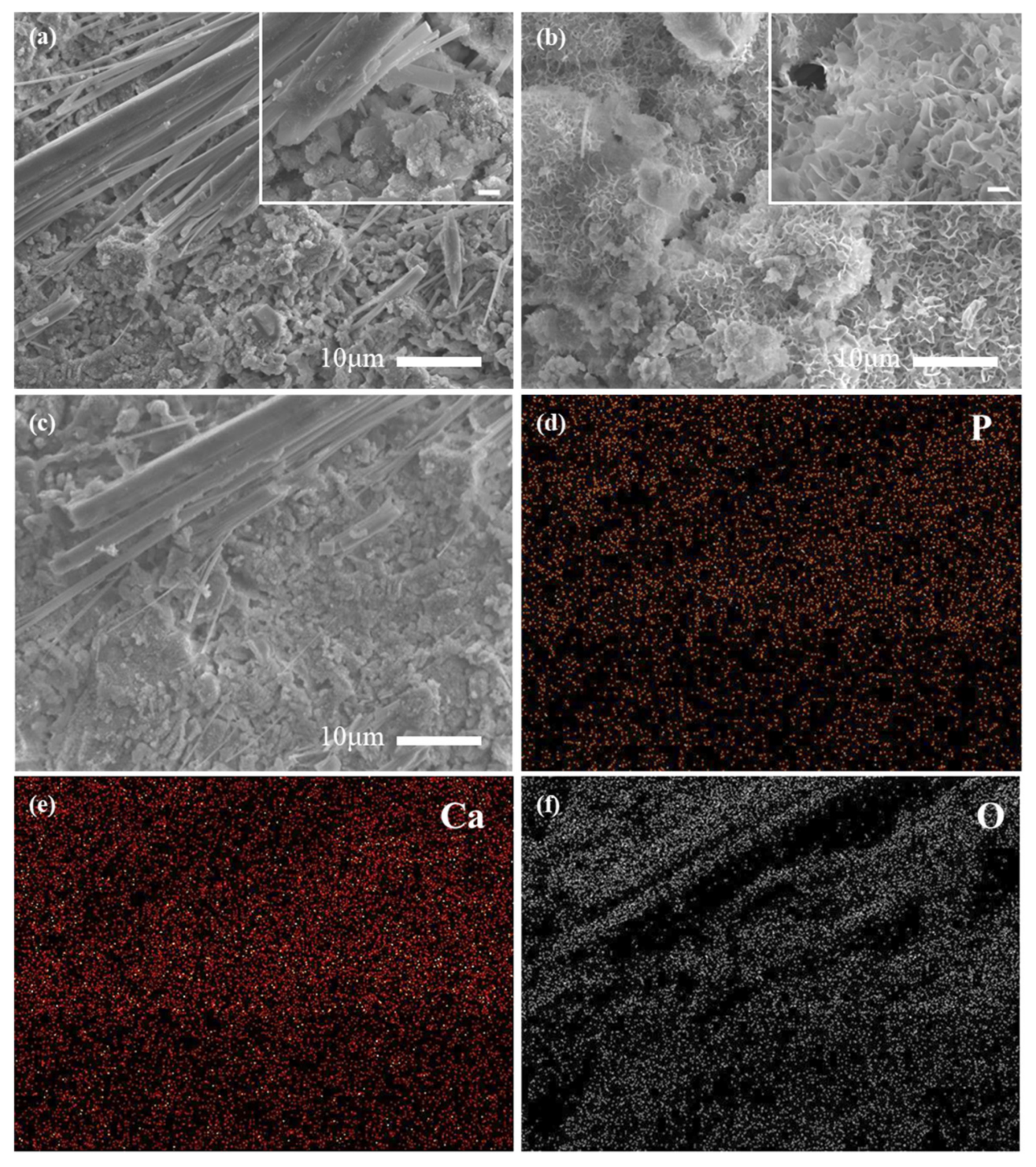
3.4. Proposed Reaction Mechanism and Perspective
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ferasso, M.; Beliaeva, T.; Kraus, S.; Clauss, T.; Ribeiro-Soriano, D. Circular economy business models: The state of research and avenues ahead. Bus. Strateg. Environ. 2020, 29, 3006–3024. [Google Scholar] [CrossRef]
- Hartley, K.; van Santen, R.; Kirchherr, J. Policies for transitioning towards a circular economy: Expectations from the European Union (EU). Resour. Conserv. Recycl. 2020, 155, 104634. [Google Scholar] [CrossRef]
- Sharma, S.; Basu, S.; Shetti, N.P.; Aminabhavi, T.M. Waste-to-energy nexus for circular economy and environmental protection: Recent trends in hydrogen energy. Sci. Total Environ. 2020, 713, 136633. [Google Scholar] [CrossRef] [PubMed]
- Plaza, L.; Castellote, M.; Nevshupa, R.; Jimenez-Relinque, E. High-capacity adsorbents from stainless steel slag for the control of dye pollutants in water. Environ. Sci. Pollut. Res. 2021, 28. [Google Scholar] [CrossRef]
- Wu, F.; Yu, Q.; Gauvin, F.; Brouwers, H.J.H. A facile manufacture of highly adsorptive aggregates using steel slag and porous expanded silica for phosphorus removal. Resour. Conserv. Recycl. 2021, 166, 105238. [Google Scholar] [CrossRef]
- Lin, Y.; Yan, B.; Fabritius, T.; Shu, Q. Immobilization of chromium in stainless steel slag using low zinc electric arc furnace dusts. Metall. Mater. Trans. B 2020, 51, 763–775. [Google Scholar] [CrossRef]
- Chen, M.; Zhou, M.; Wu, S. Optimization of blended mortars using steel slag sand. J. Wuhan Univ. Technol. Sci. Ed. 2007, 22, 741–744. [Google Scholar] [CrossRef]
- Mulopo, J.; Mashego, M.; Zvimba, J.N. Recovery of calcium carbonate from steelmaking slag and utilization for acid mine drainage pre-treatment. Water Sci. Technol. 2012, 65, 2236–2241. [Google Scholar] [CrossRef] [PubMed]
- Dhoble, Y.N.; Ahmed, S. Review on the innovative uses of steel slag for waste minimization. J. Mater. Cycles Waste Manag. 2018, 20, 1373–1382. [Google Scholar] [CrossRef]
- Rohde, L.; Peres Núñez, W.; Augusto Pereira Ceratti, J. Electric arc furnace steel slag: Base material for low-volume roads. Transp. Res. Record. 2003, 1819, 201–207. [Google Scholar] [CrossRef]
- Wu, S.; Xue, Y.; Ye, Q.; Chen, Y. Utilization of steel slag as aggregates for stone mastic asphalt (SMA) mixtures. Build. Environ. 2007, 42, 2580–2585. [Google Scholar] [CrossRef]
- Birat, J.P. Society, materials, and the environment: The case of steel. Metals 2020, 10, 331. [Google Scholar] [CrossRef]
- Lerede, D.; Bustreo, C.; Gracceva, F.; Saccone, M.; Savoldi, L. Techno-economic and environmental characterization of industrial technologies for transparent bottom-up energy modeling. Renew. Sustain. Energy Rev. 2021, 140, 110742. [Google Scholar] [CrossRef]
- Manchisi, J.; Matinde, E.; Rowson, N.A.; Simmons, M.J.H.; Simate, G.S.; Ndlovu, S.; Mwewa, B. Ironmaking and steelmaking slags as sustainable adsorbents for industrial effluents and wastewater treatment: A critical review of properties, performance, challenges and opportunities. Sustainability 2020, 12, 2118. [Google Scholar] [CrossRef]
- Tsakiridis, P.E.; Papadimitriou, G.D.; Tsivilis, S.; Koroneos, C. Utilization of steel slag for Portland cement clinker production. J. Hazard. Mater. 2008, 152, 805–811. [Google Scholar] [CrossRef] [PubMed]
- Gikas, G.D. Water quality of drainage canals and assessment of nutrient loads using QUAL2Kw. Environ. Process. 2014, 1, 369–385. [Google Scholar] [CrossRef]
- Akpor, O.B.; Muchie, B. Environmental and public health implications of wastewater quality. Afr. J. Biotechnol. 2011, 10, 2379–2387. [Google Scholar]
- Gubernat, S.; Masłoń, A.; Czarnota, J.; Koszelnik, P. Reactive materials in the removal of phosphorus compounds from wastewater—A review. Materials 2020, 13, 3377. [Google Scholar] [CrossRef] [PubMed]
- Vermeulen, J.; Whiteoak, K.; Nicholls, G.; Gerber, F.; McAndrew, K.; Cherrier, V.; Cunningham, E.; Kirhensteine, I.; Wolters, H.; Verweij, W.; et al. Fitness Check Evaluation of The Water Framework Directive and The Floods Directive—Final Evaluation Report; European Commission: Brussels, Belgium, 2019; p. 495. [Google Scholar]
- Fu, F.; Wang, Q. Removal of heavy metal ions from wastewaters: A review. J. Environ. Manag. 2011, 92, 407–418. [Google Scholar] [CrossRef] [PubMed]
- De Gisi, S.; Lofrano, G.; Grassi, M.; Notarnicola, M. Characteristics and adsorption capacities of low-cost sorbents for wastewater treatment: A review. Sustain. Mater. Technol. 2016, 9, 10–40. [Google Scholar] [CrossRef]
- Saleem, J.; Bin Shahid, U.; Hijab, M.; Mackey, H.; McKay, G. Production and applications of activated carbons as adsorbents from olive stones. Biomass Convers. Biorefinery 2019, 9, 775–802. [Google Scholar] [CrossRef]
- Matsui, A.; Uchida, Y.I.; Kikuchi, N.; Miki, Y. Effects of temperature and oxygen potential on removal of sulfur from desulfurization slag. ISIJ Int. 2017, 57, 1012–1018. [Google Scholar] [CrossRef]
- Serjun, V.Z.; Mirtič, B.; Mladenovič, A. Evaluation of ladle slag as a potential material for building and civil engineering. Mater. Tehnol. 2013, 47, 543–550. [Google Scholar]
- Shi, C. Steel slag—Its production, processing, characteristics, and cementitious properties. J. Mater. Civ. Eng. 2004, 16, 230–236. [Google Scholar] [CrossRef]
- Barca, C.; Gérente, C.; Meyer, D.; Chazarenc, F.; Andrès, Y. Phosphate removal from synthetic and real wastewater using steel slags produced in Europe. Water Res. 2012, 46, 2376–2384. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, X.W.; Li, J.G.; Sun, X.D. Preparation and mechanical property of a novel 3D porous magnesium scaffold for bone tissue engineering. Mater. Sci. Eng. C 2014, 42, 362–367. [Google Scholar] [CrossRef] [PubMed]
- Tang, G.; Liu, X.; Zhou, L.; Zhang, P.; Deng, D.; Jiang, H. Steel slag waste combined with melamine pyrophosphate as a flame retardant for rigid polyurethane foams. Adv. Powder Technol. 2020, 31, 279–286. [Google Scholar] [CrossRef]
- Kim, E.; Spooren, J.; Broos, K.; Nielsen, P.; Horckmans, L.; Geurts, R.; Vrancken, K.C.; Quaghebeur, M. Valorization of stainless steel slag by selective chromium recovery and subsequent carbonation of the matrix material. J. Clean. Prod. 2016, 117, 221–228. [Google Scholar] [CrossRef]
- Magrí, A.; Carreras-Sempere, M.; Biel, C.; Colprim, J. Recovery of phosphorus from waste water profiting from biological nitrogen treatment: Upstream, concomitant or downstream precipitation alternatives. Agronomy 2020, 10, 1039. [Google Scholar] [CrossRef]
- Pramanik, B.K.; Islam, M.A.; Asif, M.B.; Roychand, R.; Pramanik, S.K.; Shah, K.; Bhuiyan, M.; Hai, F. Emerging investigator series: Phosphorus recovery from municipal wastewater by adsorption on steelmaking slag preceding forward osmosis: An integrated process. Environ. Sci. Water Res. Technol. 2020, 6, 1559–1567. [Google Scholar] [CrossRef]
- Daneshgar, S.; Callegari, A.; Capodaglio, A.G.; Vaccari, D. The potential phosphorus crisis: Resource conservation and possible escape technologies: A review. Resources 2018, 7, 37. [Google Scholar] [CrossRef]
- Sengupta, S.; Nawaz, T.; Beaudry, J. Nitrogen and phosphorus recovery from wastewater. Curr. Pollut. Reports 2015, 1, 155–166. [Google Scholar] [CrossRef]
- Sengupta, S.; Pandit, A. Selective removal of phosphorus from wastewater combined with its recovery as a solid-phase fertilizer. Water Res. 2011, 45, 3318–3330. [Google Scholar] [CrossRef] [PubMed]
- Egle, L.; Rechberger, H.; Krampe, J.; Zessner, M. Phosphorus recovery from municipal wastewater: An integrated comparative technological, environmental and economic assessment of P recovery technologies. Sci. Total Environ. 2016, 571, 522–542. [Google Scholar] [CrossRef] [PubMed]
- Rosyidah, N.D.; Kusairi, S.; Taufiq, A.; Affriyenni, Y. Profile of students’ critical thinking processes on the topics of Hydrostatic Pressure and Archimedes’ principle. J. Phys. Conf. Ser. 2020, 1511, 012081. [Google Scholar] [CrossRef]
- Mankoula, A.F.; Tawfik, W.; Gagnon, J.E.; Fryer, B.J.; El-Mekawy, F.; Shaheen, M.E. ICMMS-2: Assessment of heavy metals content in the agricultural soils of Kafr El-Zayat Egypt using laser ablation inductively coupled plasma mass spectrometry and inductively coupled plasma optical emission. Egypt. J. Chem. 2021, 64, 1167–1177. [Google Scholar]
- ASTM C159-06(2016). Standard Specification for Vitrified Clay Filter Blocks; ASTM International: West Conshohocken, PA, USA, 2016; Volume 14, pp. 1–12. [Google Scholar]
- Faye, T.; Tamburello, A.; Vegarud, G.E.; Skeie, S. Survival of lactic acid bacteria from fermented milks in an in vitro digestion model exploiting sequential incubation in human gastric and duodenum juice. J. Dairy Sci. 2012, 95, 558–566. [Google Scholar] [CrossRef] [PubMed]
- Hermassi, M.; Valderrama, C.; Moreno, N.; Font, O.; Querol, X.; Batis, N.; Cortina, J.L. Powdered Ca-activated zeolite for phosphate removal from treated waste-water. J. Chem. Technol. Biotechnol. 2016, 91, 1962–1971. [Google Scholar] [CrossRef]
- Drizo, A.; Cummings, J.; Weber, D.; Twohig, E.; Druschel, G.; Bourke, B. New evidence for rejuvenation of phosphorus retention capacity in EAF steel slag. Environ. Sci. Technol. 2008, 42, 6191–6197. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Yang, C.; Jia, Y.; Chen, H.; Gu, X. Dual removal of phosphate and ammonium from high concentrations of aquaculture wastewaters using an efficient two-stage infiltration system. Sci. Total Environ. 2018, 635, 936–946. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wu, B.; Zhou, T.; Chai, X. Preferential removal of phosphorus using modified steel slag and cement combination for its implications in engineering applications. Environ. Technol. Innov. 2018, 10, 264–274. [Google Scholar] [CrossRef]
- Erauskin-Tolosa, A.; Zubeltzu-Jaka, E.; Heras-Saizarbitoria, I.; Boiral, O. ISO 14001, EMAS and environmental performance: A meta-analysis. Bus. Strat. Environ. 2020, 29, 1145–1159. [Google Scholar] [CrossRef]

| PVA | Volume (cm2) | Bulk Density (g/cm2) | Porosity (%) | |||
|---|---|---|---|---|---|---|
| (wt%) | Average | Deviation | Average | Deviation | Average | Deviation |
| 0 | 0.6828 | 0.0055 | 1.4526 | 0.0112 | 28.9474 | 0.4520 |
| 5 | 0.6477 | 0.0034 | 1.4692 | 0.0151 | 36.1800 | 0.2975 |
| 10 | 0.6150 | 0.0040 | 1.4659 | 0.0043 | 42.8706 | 0.3903 |
| Element Mode (%) | Oxide Mode (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Sample 1 | Sample 2 | Sample 3 | Sample 4 | Sample 1 | Sample 2 | Sample 3 | Sample 4 | ||
| Ca | 64.68 | ✓ | ✓ | ✓ | CaO | 74.54 | ✓ | ✓ | ✓ |
| Si | 32.95 | ✓ | N.D. | N.D. | SiO2 | 21.37 | ✓ | N.D. | N.D. |
| Mg | 1 | N.D. | N.D. | N.D. | MgO | 0.9 | N.D. | N.D. | N.D. |
| S | 0.99 | N.D. | N.D. | N.D. | SO3 | 1.5 | N.D. | N.D. | N.D. |
| Cr | 0.18 | N.D. | N.D. | N.D. | Cr2O3 | 0.37 | N.D. | N.D. | N.D. |
| Ti | 0.1 | N.D. | N.D. | N.D. | TiO2 | 0.27 | N.D. | N.D. | N.D. |
| Fe | 0.1 | N.D. | N.D. | N.D. | Fe2O3 | 0.39 | N.D. | N.D. | N.D. |
| P | - | N.D. | ✓ | ✓ | P2O5 | - | N.D. | ✓ | ✓ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, H.; Peng, Y.-L.; Whang, L.-M.; Liao, J.-D. Recycled Steel Slag as a Porous Adsorbent to Filter Phosphorus-Rich Water with 8 Filtration Circles. Materials 2021, 14, 3187. https://doi.org/10.3390/ma14123187
Lee H, Peng Y-L, Whang L-M, Liao J-D. Recycled Steel Slag as a Porous Adsorbent to Filter Phosphorus-Rich Water with 8 Filtration Circles. Materials. 2021; 14(12):3187. https://doi.org/10.3390/ma14123187
Chicago/Turabian StyleLee, Han, Yen-Ling Peng, Liang-Ming Whang, and Jiunn-Der Liao. 2021. "Recycled Steel Slag as a Porous Adsorbent to Filter Phosphorus-Rich Water with 8 Filtration Circles" Materials 14, no. 12: 3187. https://doi.org/10.3390/ma14123187
APA StyleLee, H., Peng, Y.-L., Whang, L.-M., & Liao, J.-D. (2021). Recycled Steel Slag as a Porous Adsorbent to Filter Phosphorus-Rich Water with 8 Filtration Circles. Materials, 14(12), 3187. https://doi.org/10.3390/ma14123187








