Preparation of LiFePO4 Powders by Ultrasonic Spray Drying Method and Their Memory Effect
Abstract
:1. Introduction
2. Materials and Methods
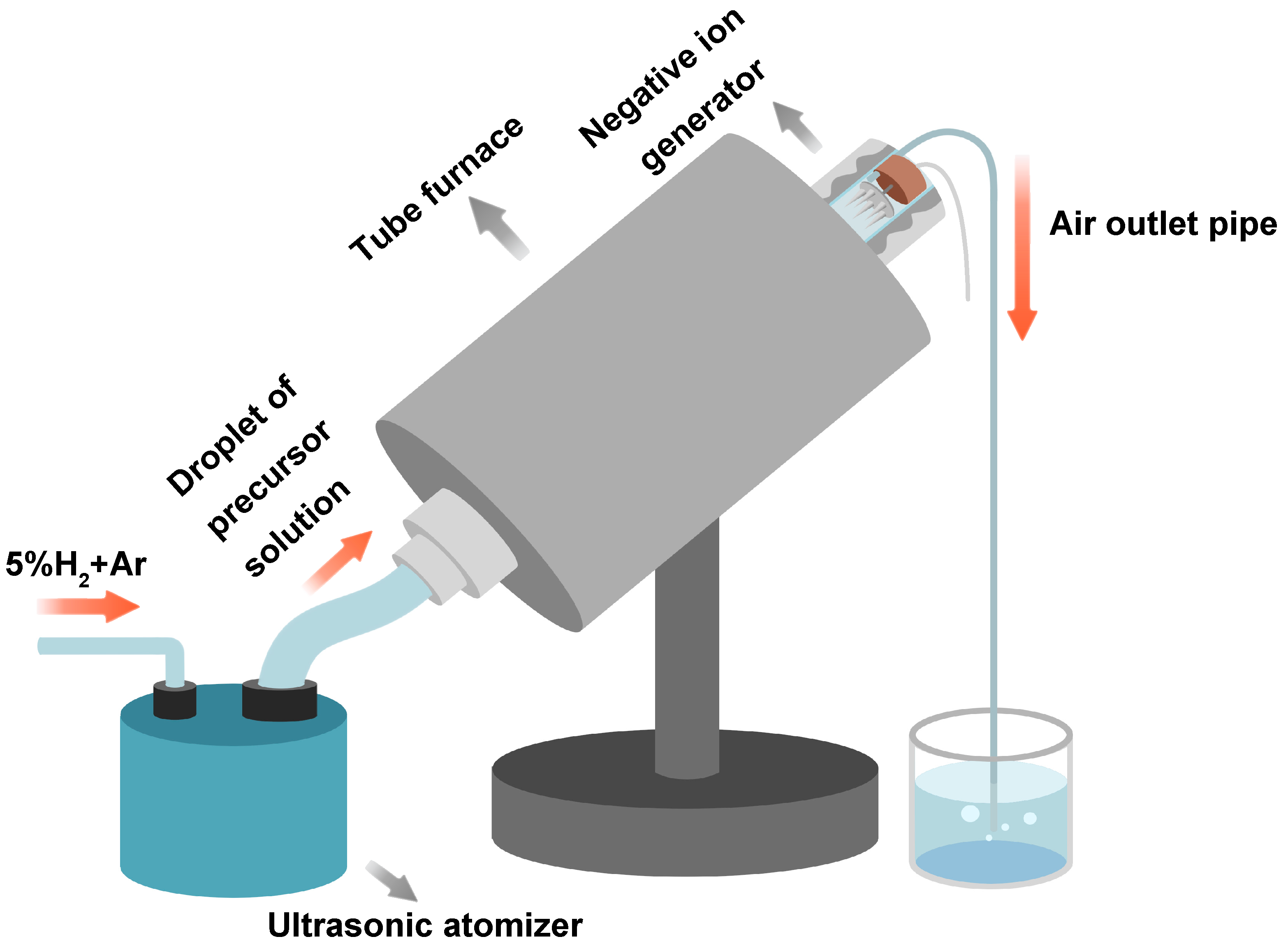
2.1. Preparation of LiFePO4 by Spray Drying Method
2.2. Characterization of Materials
2.3. Electrochemical Characterization
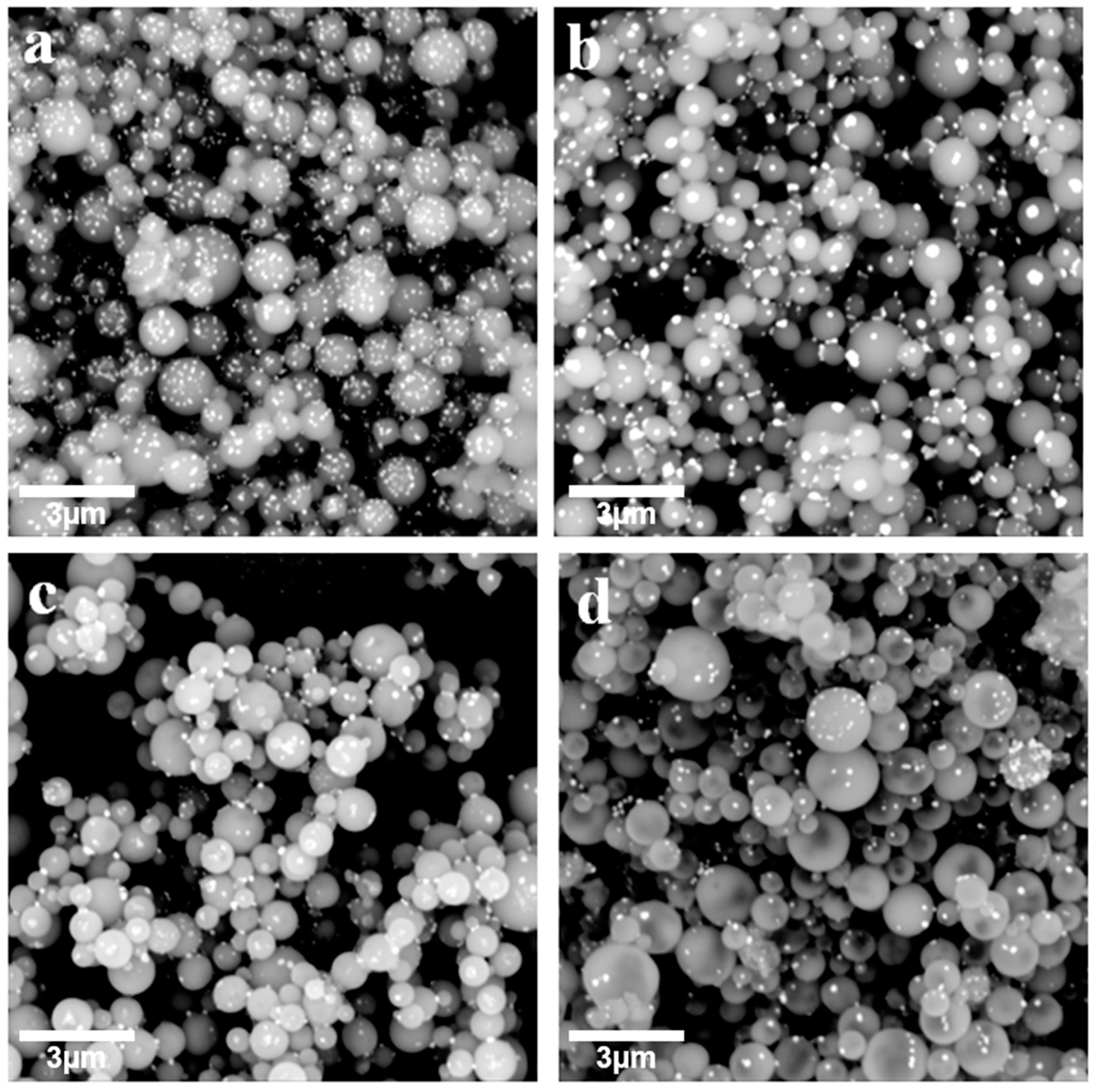
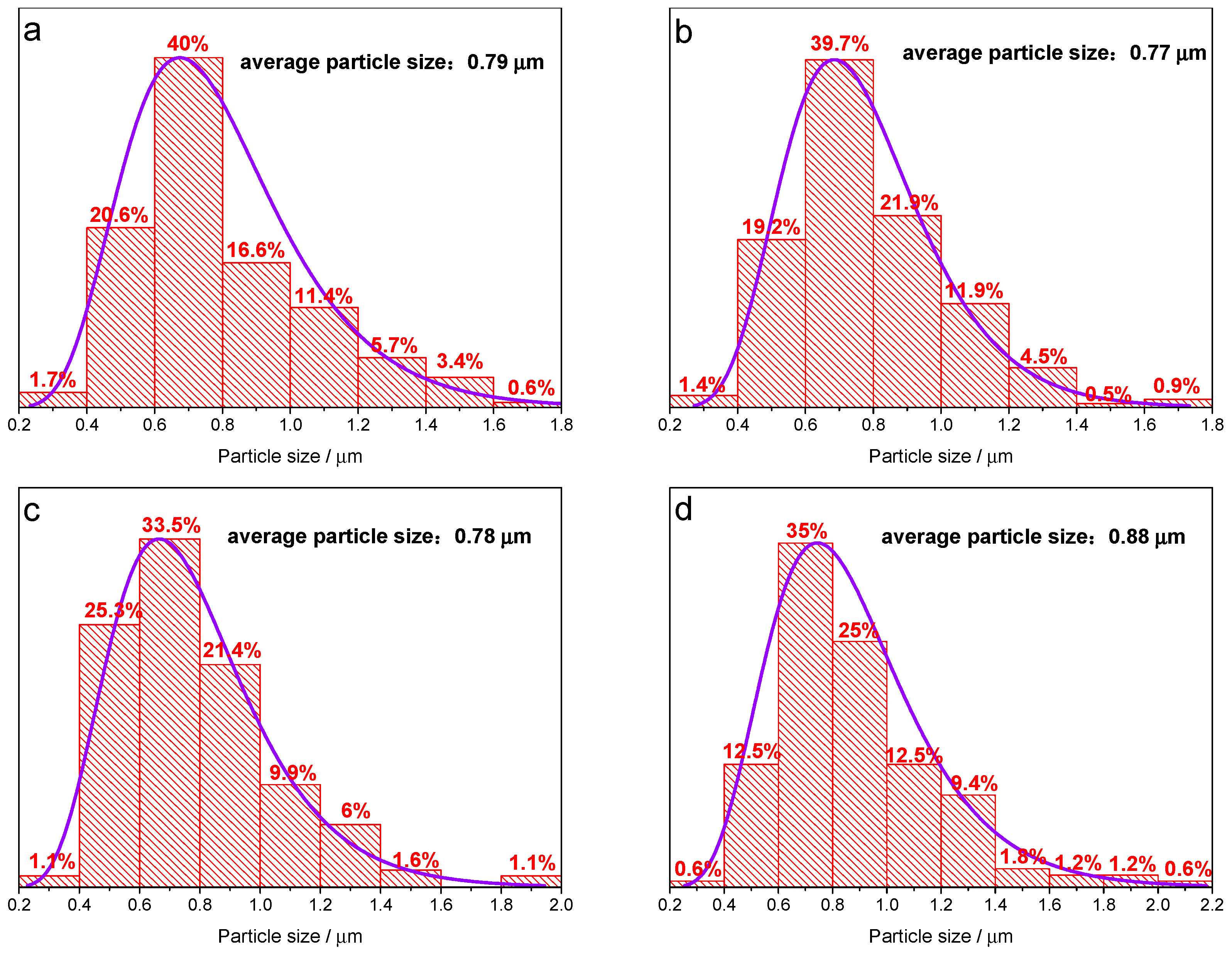
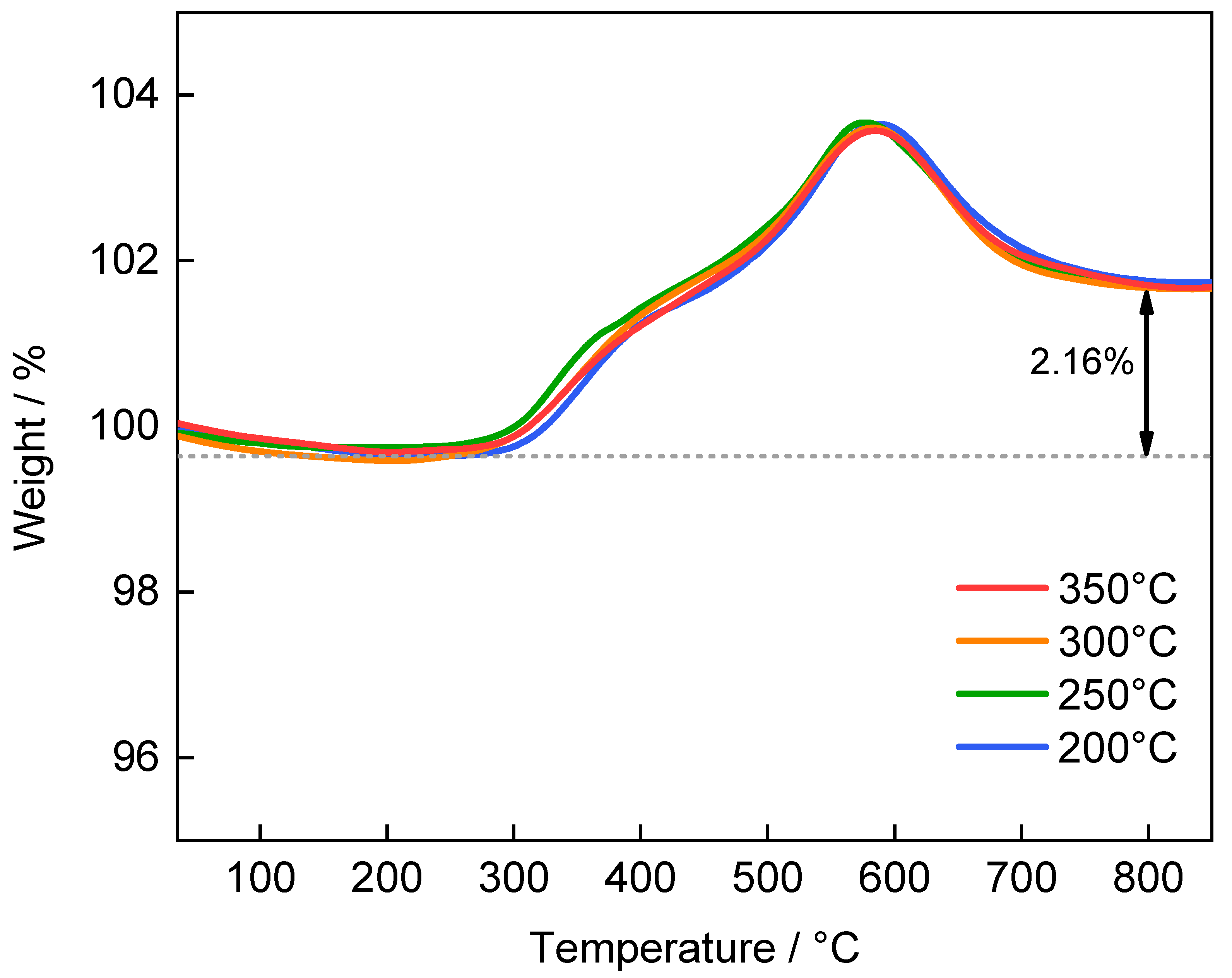
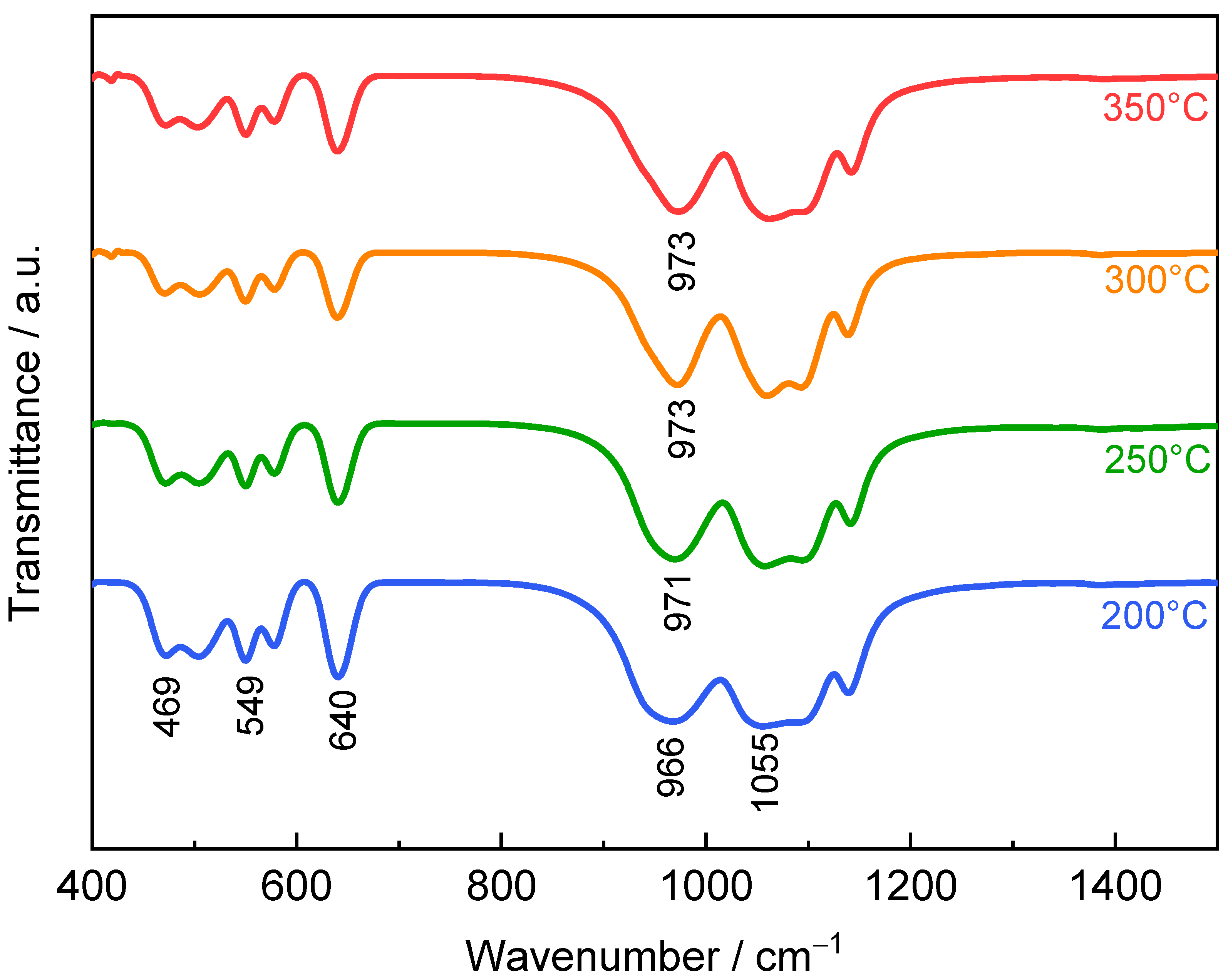
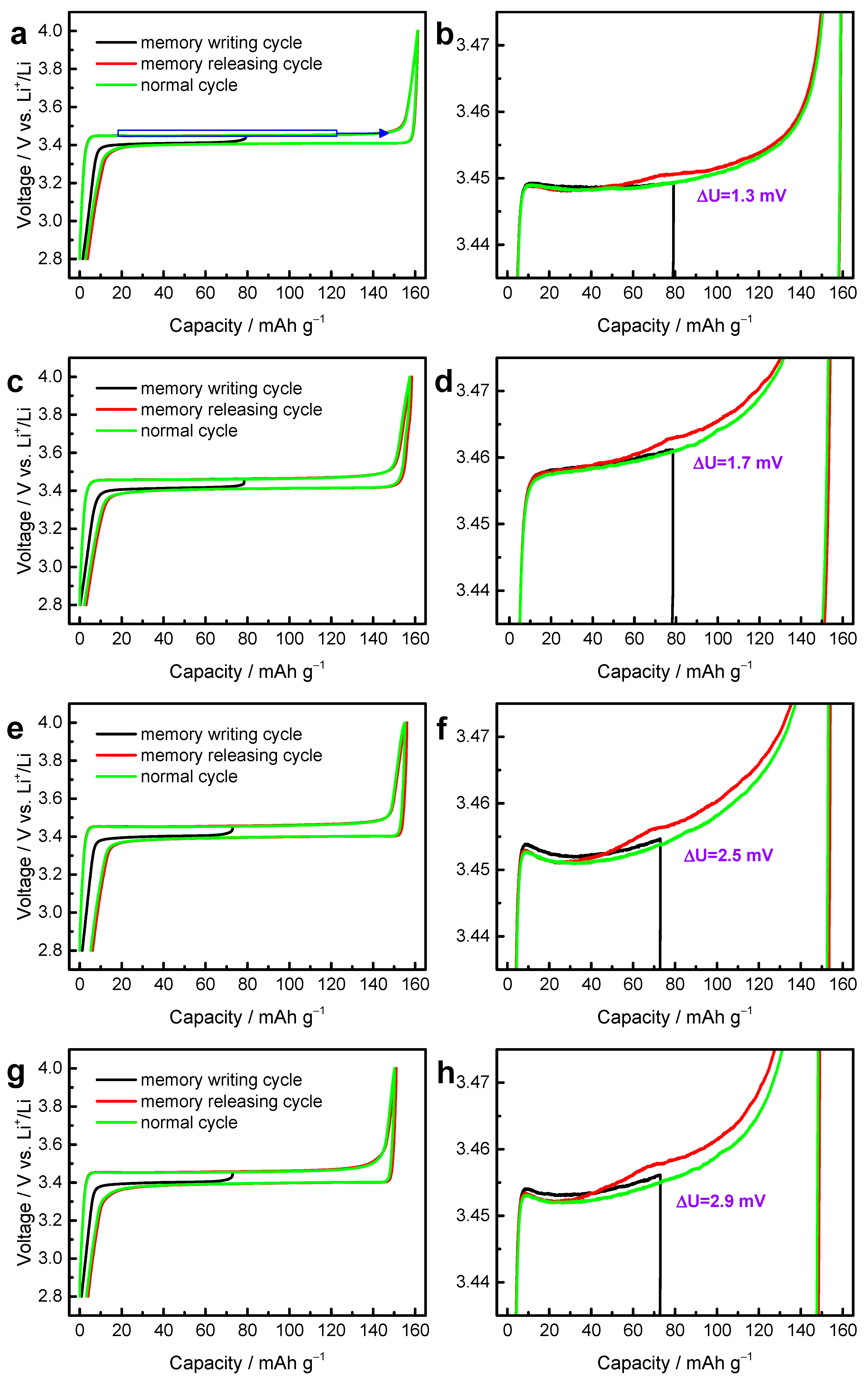
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, Z.; Zhang, J.; Kintner-Meyer, M.C.W.; Lu, X.; Choi, D.; Lemmon, J.P.; Liu, J. Electrochemical Energy Storage for Green Grid. Chem. Rev. 2011, 111, 3577–3613. [Google Scholar] [CrossRef]
- Liu, C.; Li, F.; Ma, L.-P.; Cheng, H.-M. Advanced Materials for Energy Storage. Adv. Mater. 2010, 22, E28–E62. [Google Scholar] [CrossRef] [PubMed]
- Song, H.-K.; Lee, K.T.; Kim, M.G.; Nazar, L.F.; Cho, J. Recent Progress in Nanostructured Cathode Materials for Lithium Secondary Batteries. Adv. Funct. Mater. 2010, 20, 3818–3834. [Google Scholar] [CrossRef]
- Hawkins, T.R.; Gausen, O.M.; Strømman, A.H. Environmental impacts of hybrid and electric vehicles—A review. Int. J. Life Cycle Assess. 2012, 17, 997–1014. [Google Scholar] [CrossRef]
- Fergus, J.W. Recent developments in cathode materials for lithium ion batteries. J. Power Sources 2010, 195, 939–954. [Google Scholar] [CrossRef]
- Marom, R.; Amalraj, S.F.; Leifer, N.; Jacob, D.; Aurbach, D. A review of advanced and practical lithium battery materials. J. Mater. Chem. 2011, 21, 9938–9954. [Google Scholar] [CrossRef]
- Lu, L.; Han, X.; Li, J.; Hua, J.; Ouyang, M. A review on the key issues for lithium-ion battery management in electric vehicles. J. Power Sources 2013, 226, 272–288. [Google Scholar] [CrossRef]
- Whittingham, M.S. Lithium Batteries and Cathode Materials. Chem. Rev. 2004, 104, 4271–4302. [Google Scholar] [CrossRef]
- Padhi, A.K.; NanjundaSwamy, K.S.; Goodenough, J.B. Phospho-olivines as Positive-Electrode Materials for Rechargeable Lithium Batteries. J. Electrochem. Soc. 1997, 144, 1188–1194. [Google Scholar] [CrossRef]
- Takahashi, M. Reaction behavior of LiFePO4 as a cathode material for rechargeable lithium batteries. Solid State Ionics 2002, 148, 283–289. [Google Scholar] [CrossRef]
- Armand, M.; Tarascon, J.M. Building better batteries. Nature 2008, 451, 652–657. [Google Scholar] [CrossRef] [PubMed]
- Kang, B.; Ceder, G. Battery materials for ultrafast charging and discharging. Nat. Cell Biol. 2009, 458, 190–193. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Sun, X. Olivine LiFePO4: The remaining challenges for future energy storage. Energy Environ. Sci. 2015, 8, 1110–1138. [Google Scholar] [CrossRef]
- Chiu, K.-F.; Chen, C.-L. Electrochemical performance of magnetron sputter deposited LiFePO4-Ag composite thin film cathodes. Surf. Coatings Technol. 2010, 205, 1642–1646. [Google Scholar] [CrossRef]
- Swain, P.; Viji, M.; Mocherla, P.S.; Sudakar, C. Carbon coating on the current collector and LiFePO4 nanoparticles—Influence of sp and sp-like disordered carbon on the electrochemical properties. J. Power Sources 2015, 293, 613–625. [Google Scholar] [CrossRef]
- He, R.; Liu, Z.; Zhang, L.; Guo, R.; Yang, S. Electrochemical properties of V and Ti co-doping Li1.02FePO4/C material prepared by solid-state synthesis route. J. Alloys Compd. 2016, 662, 461–466. [Google Scholar] [CrossRef]
- Yuan, H.; Wang, X.; Wu, Q.; Shu, H.; Yang, X. Effects of Ni and Mn doping on physicochemical and electrochemical performances of LiFePO4/C. J. Alloys Compd. 2016, 675, 187–194. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Zhang, M.; Li, Y.; Hu, Y.; Zhu, M.; Jin, H.; Li, W. Nano-sized LiFePO4/C composite with core-shell structure as cathode material for lithium ion battery. Electrochim. Acta 2015, 176, 689–693. [Google Scholar] [CrossRef]
- Bai, N.; Xiang, K.; Zhou, W.; Lu, H.; Zhao, X.; Chen, H. LiFePO4/carbon nanowires with 3D nano-network structure as potential high performance cathode for lithium ion batteries. Electrochim. Acta 2016, 191, 23–28. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Huo, Q.-Y.; Du, P.-P.; Wang, L.-Z.; Zhang, A.-Q.; Song, Y.-H.; Lv, Y.; Li, G.-Y. Advances in new cathode material LiFePO4 for lithium-ion batteries. Synth. Met. 2012, 162, 1315–1326. [Google Scholar] [CrossRef]
- Kang, H.-C.; Jun, D.-K.; Jin, B.; Jin, E.M.; Park, K.-H.; Gu, H.-B.; Kim, K.-W. Optimized solid-state synthesis of LiFePO4 cathode materials using ball-milling. J. Power Sources 2008, 179, 340–346. [Google Scholar] [CrossRef]
- Park, K.; Kang, K.; Lee, S.; Kim, G.; Park, Y.; Kim, H. Synthesis of LiFePO4 with fine particle by co-precipitation method. Mater. Res. Bull. 2004, 39, 1803–1810. [Google Scholar] [CrossRef]
- Park, K.; Son, J.; Chung, H.; Kim, S.; Lee, C.; Kim, H. Synthesis of LiFePO4 by co-precipitation and microwave heating. Electrochem. Commun. 2003, 5, 839–842. [Google Scholar] [CrossRef]
- Jugović, D.; Uskoković, D. A review of recent developments in the synthesis procedures of lithium iron phosphate powders. J. Power Sources 2009, 190, 538–544. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Jiang, R.; Wang, X.; Huang, T.; Yu, A. The defect chemistry of LiFePO4 prepared by hydrothermal method at different pH values. J. Power Sources 2009, 194, 536–540. [Google Scholar] [CrossRef]
- Gao, F.; Tang, Z.; Xue, J. Preparation and characterization of nano-particle LiFePO4 and LiFePO4/C by spray-drying and post-annealing method. Electrochim. Acta 2007, 53, 1939–1944. [Google Scholar] [CrossRef]
- Konarova, M.; Taniguchi, I. Preparation of LiFePO4/C composite powders by ultrasonic spray pyrolysis followed by heat treatment and their electrochemical properties. Mater. Res. Bull. 2008, 43, 3305–3317. [Google Scholar] [CrossRef]
- Yang, M.-R.; Teng, T.-H.; Wu, S.-H. LiFePO4/carbon cathode materials prepared by ultrasonic spray pyrolysis. J. Power Sources 2006, 159, 307–311. [Google Scholar] [CrossRef]
- Teng, T.-H.; Yang, M.-R.; Wu, S.-H.; Chiang, Y.-P. Electrochemical properties of LiFe0.9Mg0.1PO4 / carbon cathode materials prepared by ultrasonic spray pyrolysis. Solid State Commun. 2007, 142, 389–392. [Google Scholar] [CrossRef]
- Jung, D.S.; Hwang, T.H.; Bin Park, S.; Choi, J.W. Spray Drying Method for Large-Scale and High-Performance Silicon Negative Electrodes in Li-Ion Batteries. Nano Lett. 2013, 13, 2092–2097. [Google Scholar] [CrossRef]
- Wan, C.; Wu, M.; Wu, D. Synthesis of spherical LiMn2O4 cathode material by dynamic sintering of spray-dried precursors. Powder Technol. 2010, 199, 154–158. [Google Scholar] [CrossRef]
- Sasaki, T.; Ukyo, Y.; Novák, P. Memory effect in a lithium-ion battery. Nat. Mater. 2013, 12, 569–575. [Google Scholar] [CrossRef] [PubMed]
- Madej, E.; La Mantia, F.; Schuhmann, W.; Ventosa, E. Impact of the Specific Surface Area on the Memory Effect in Li-Ion Batteries: The Case of Anatase TiO2. Adv. Energy Mater. 2014, 4, 1400829. [Google Scholar] [CrossRef]
- Li, D.; Sun, Y.; Liu, X.; Peng, R.; Zhou, H. Doping-induced memory effect in Li-ion batteries: The case of Al-doped Li4Ti5O12. Chem. Sci. 2015, 6, 4066–4070. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ulldemolins, M.; LE Cras, F.; Pecquenard, B. Memory effect highlighting in silicon anodes for high energy density lithium-ion batteries. Electrochem. Commun. 2013, 27, 22–25. [Google Scholar] [CrossRef]
- Guo, X.; Song, B.; Yu, G.; Wu, X.; Feng, X.; Li, D.; Chen, Y. Size-Dependent Memory Effect of the LiFePO4 Electrode in Li-Ion Batteries. ACS Appl. Mater. Interfaces 2018, 10, 41407–41414. [Google Scholar] [CrossRef] [PubMed]
- Jia, J.; Tan, C.; Liu, M.; Li, D.; Chen, Y. Relaxation-Induced Memory Effect of LiFePO4 Electrodes in Li-Ion Batteries. ACS Appl. Mater. Interfaces 2017, 9, 24561–24567. [Google Scholar] [CrossRef]
- Shi, W.; Wang, J.; Zheng, J.; Jiang, J.; Viswanathan, V.; Zhang, J.-G. Influence of memory effect on the state-of-charge estimation of large-format Li-ion batteries based on LiFePO4 cathode. J. Power Sources 2016, 312, 55–59. [Google Scholar] [CrossRef] [Green Version]
- Park, K.-Y.; Park, I.; Kim, H.; Yoon, G.; Gwon, H.; Cho, Y.; Yun, Y.S.; Kim, J.-J.; Lee, S.; Ahn, D.; et al. Lithium-excess olivine electrode for lithium rechargeable batteries. Energy Environ. Sci. 2016, 9, 2902–2915. [Google Scholar] [CrossRef] [Green Version]
- Ventosa, E.; Löffler, T.; La Mantia, F.; Schuhmann, W. Understanding memory effects in Li-ion batteries: Evidence of a kinetic origin in TiO 2 upon hydrogen annealing. Chem. Commun. 2016, 52, 11524–11526. [Google Scholar] [CrossRef] [Green Version]
- Toby, B.H. EXPGUI, a graphical user interface forGSAS. J. Appl. Crystallogr. 2001, 34, 210–213. [Google Scholar] [CrossRef] [Green Version]
- Yu, F.; Zhang, J.; Yang, Y.; Song, G. Porous micro-spherical aggregates of LiFePO4/C nanocomposites: A novel and simple template-free concept and synthesis via sol–gel-spray drying method. J. Power Sources 2010, 195, 6873–6878. [Google Scholar] [CrossRef]
- Yang, G.; Jiang, C.; He, X.; Ying, J.; Gao, J.; Wan, C. Synthesis of Size-controllable LiFePO4/C Cathode Material by Controlled Crystallization. J. New Mater. Electrochem. Syst. 2012, 15, 75–78. [Google Scholar] [CrossRef]
- Liu, T.; Zhao, L.; Wang, D.; Zhu, J.; Wang, B.; Guo, C. Carbon-coated single-crystalline LiFePO4 nanocomposites for high-power Li-ion batteries: The impact of minimization of the precursor particle size. RSC Adv. 2014, 4, 10067–10075. [Google Scholar] [CrossRef]
- Yu, J.; Hu, J.; Li, J. One-pot synthesis and electrochemical reactivity of carbon coated LiFePO4 spindles. Appl. Surf. Sci. 2012, 263, 277–283. [Google Scholar] [CrossRef]
- Wang, X.; Feng, Z.; Huang, J.; Deng, W.; Li, X.; Zhang, H.; Wen, Z. Graphene-decorated carbon-coated LiFePO4 nanospheres as a high-performance cathode material for lithium-ion batteries. Carbon 2018, 127, 149–157. [Google Scholar] [CrossRef]
- Fisher, C.A.J.; Prieto, V.M.H.; Islam, M.S. Lithium Battery Materials LiMPO4 (M = Mn, Fe, Co, and Ni): Insights into Defect Association, Transport Mechanisms, and Doping Behavior. Chem. Mater. 2008, 20, 5907–5915. [Google Scholar] [CrossRef]
- Wang, L.; He, X.; Sun, W.; Wang, J.; Li, Y.; Fan, S. Crystal Orientation Tuning of LiFePO4 Nanoplates for High Rate Lithium Battery Cathode Materials. Nano Lett. 2012, 12, 5632–5636. [Google Scholar] [CrossRef] [PubMed]
- Jensen, K.M.Ø.; Christensen, M.; Gunnlaugsson, H.P.; Lock, N.; Bøjesen, E.D.; Proffen, T.; Iversen, B.B. Defects in Hydrothermally Synthesized LiFePO4and LiFe1-xMnxPO4Cathode Materials. Chem. Mater. 2013, 25, 2282–2290. [Google Scholar] [CrossRef]
- Zou, Y.; Chen, S.; Yang, X.; Ma, N.; Xia, Y.; Yang, D.; Guo, S. Suppressing Fe-Li Antisite Defects in LiFePO4/Carbon Hybrid Microtube to Enhance the Lithium Ion Storage. Adv. Energy Mater. 2016, 6, 1601549. [Google Scholar] [CrossRef]
- Liu, H.; Choe, M.-J.; Enrique, R.A.; Orvañanos, B.; Zhou, L.; Liu, T.; Thornton, K.; Grey, C.P. Effects of Antisite Defects on Li Diffusion in LiFePO4 Revealed by Li Isotope Exchange. J. Phys. Chem. C 2017, 121, 12025–12036. [Google Scholar] [CrossRef]
- Sundarayya, Y.; Swamy, K.K.; Sunandana, C. Oxalate based non-aqueous sol–gel synthesis of phase pure sub-micron LiFePO4. Mater. Res. Bull. 2007, 42, 1942–1948. [Google Scholar] [CrossRef]
- Lu, Y.; Shi, J.; Guo, Z.; Tong, Q.; Huang, W.; Li, B. Synthesis of LiFe1−xNixPO4/C composites and their electrochemical performance. J. Power Sources 2009, 194, 786–793. [Google Scholar] [CrossRef]
- Shu, H.; Wang, X.; Wu, Q.; Hu, B.; Yang, X.; Wei, Q.; Liang, Q.; Bai, Y.; Zhou, M.; Wu, C.; et al. Improved electrochemical performance of LiFePO4/C cathode via Ni and Mn co-doping for lithium-ion batteries. J. Power Sources 2013, 237, 149–155. [Google Scholar] [CrossRef]
- Radhamani, A.; Karthik, C.; Ubic, R.; Rao, M.R.; Sudakar, C. Suppression of antisite defects in fluorine-doped LiFePO4. Scr. Mater. 2013, 69, 96–99. [Google Scholar] [CrossRef]
- Qin, X.; Wang, J.; Xie, J.; Li, F.; Wen, L.; Wang, X. Hydrothermally synthesized LiFePO4 crystals with enhanced electrochemical properties: Simultaneous suppression of crystal growth along [010] and antisite defect formation. Phys. Chem. Chem. Phys. 2012, 14, 2669–2677. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, S.-I.; Kobayashi, G.; Ohoyama, K.; Kanno, R.; Yashima, M.; Yamada, A. Experimental visualization of lithium diffusion in LixFePO4. Nat. Mater. 2008, 7, 707–711. [Google Scholar] [CrossRef]
- Malik, R.; Burch, D.; Bazant, M.; Ceder, G. Particle Size Dependence of the Ionic Diffusivity. Nano Lett. 2010, 10, 4123–4127. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Xiao, Y.; Tang, H.; Wang, H.; Wang, Z.; Liu, C.; Zeng, H.; Huang, Q.; Ren, Y.; Wang, C.; et al. Tuning Li-Ion Diffusion in α-LiMn1–xFexPO4 Nanocrystals by Antisite Defects and Embedded β-Phase for Advanced Li-Ion Batteries. Nano Lett. 2017, 17, 4934–4940. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Graetz, J. Study of Antisite Defects in Hydrothermally Prepared LiFePO4 by in Situ X-ray Diffraction. ACS Appl. Mater. Interfaces 2011, 3, 1380–1384. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lan, T.; Guo, X.; Li, D.; Chen, Y. Preparation of LiFePO4 Powders by Ultrasonic Spray Drying Method and Their Memory Effect. Materials 2021, 14, 3193. https://doi.org/10.3390/ma14123193
Lan T, Guo X, Li D, Chen Y. Preparation of LiFePO4 Powders by Ultrasonic Spray Drying Method and Their Memory Effect. Materials. 2021; 14(12):3193. https://doi.org/10.3390/ma14123193
Chicago/Turabian StyleLan, Tu, Xiaolong Guo, De Li, and Yong Chen. 2021. "Preparation of LiFePO4 Powders by Ultrasonic Spray Drying Method and Their Memory Effect" Materials 14, no. 12: 3193. https://doi.org/10.3390/ma14123193




