Study on the Adsorption Properties of Graphene Oxide/Laponite RD/Chitosan Composites
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Preparation of the Modified Laponite RD
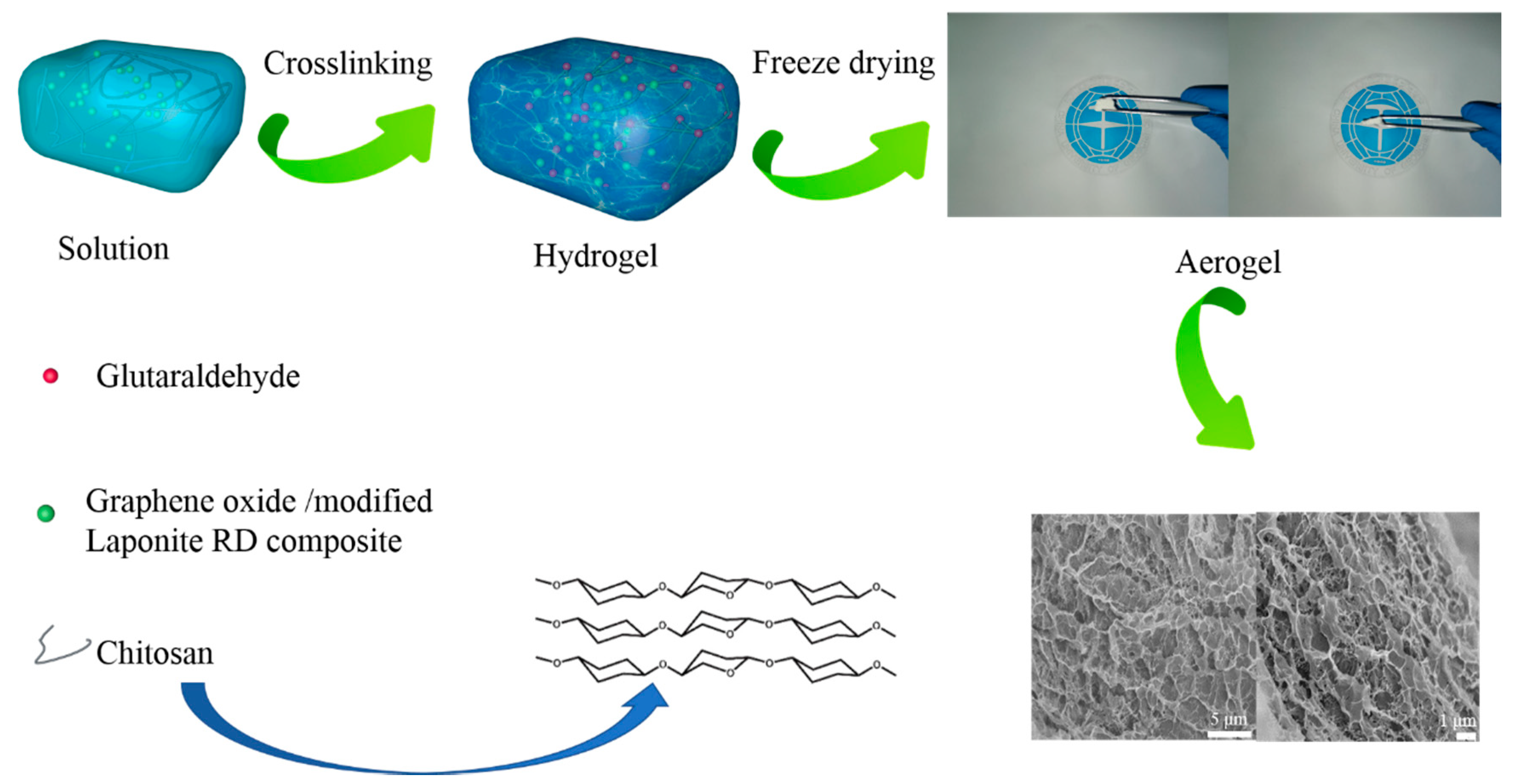
2.3. Preparation of the GO/Laponite RD/Chitosan Composite Material
2.4. Characterization
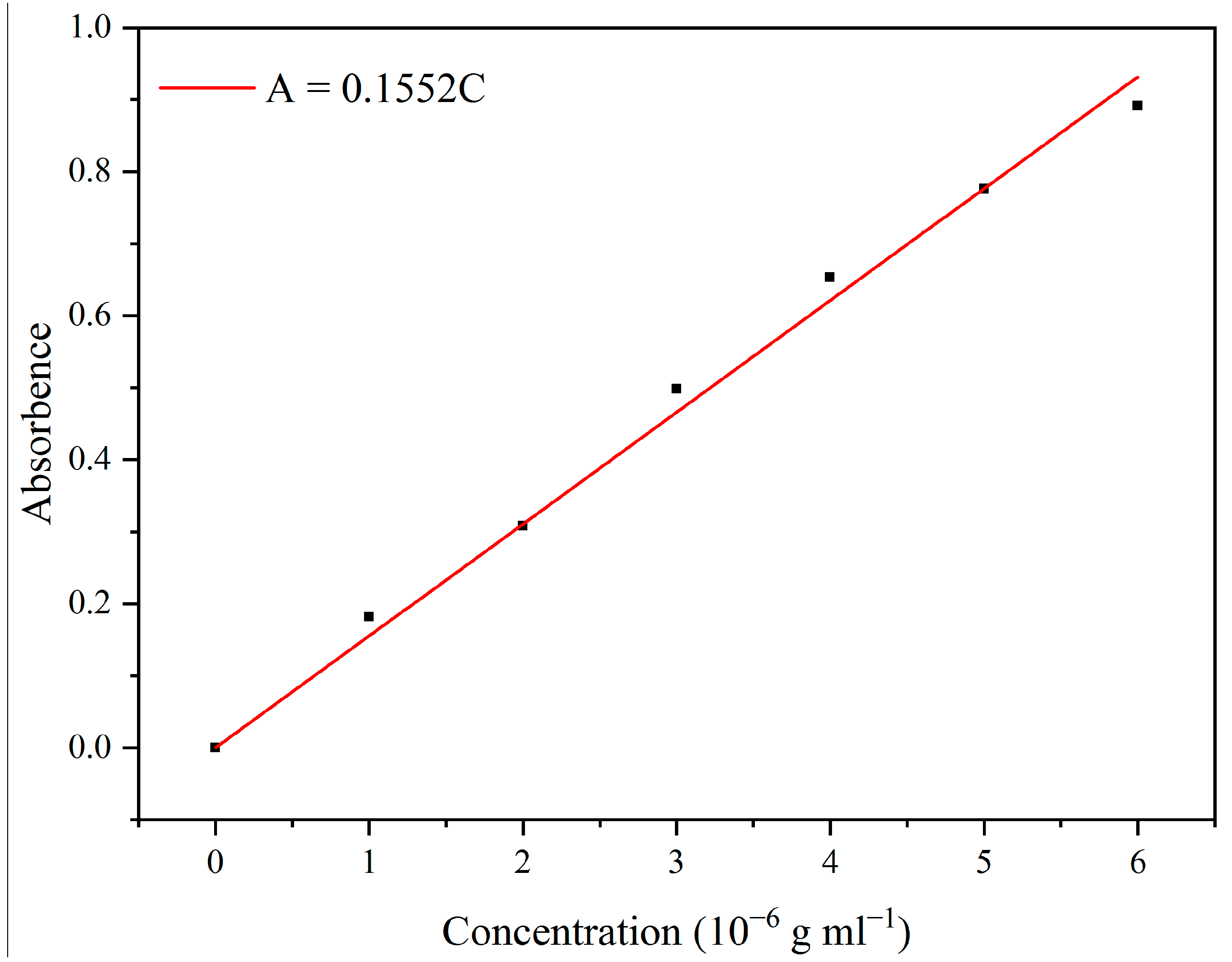
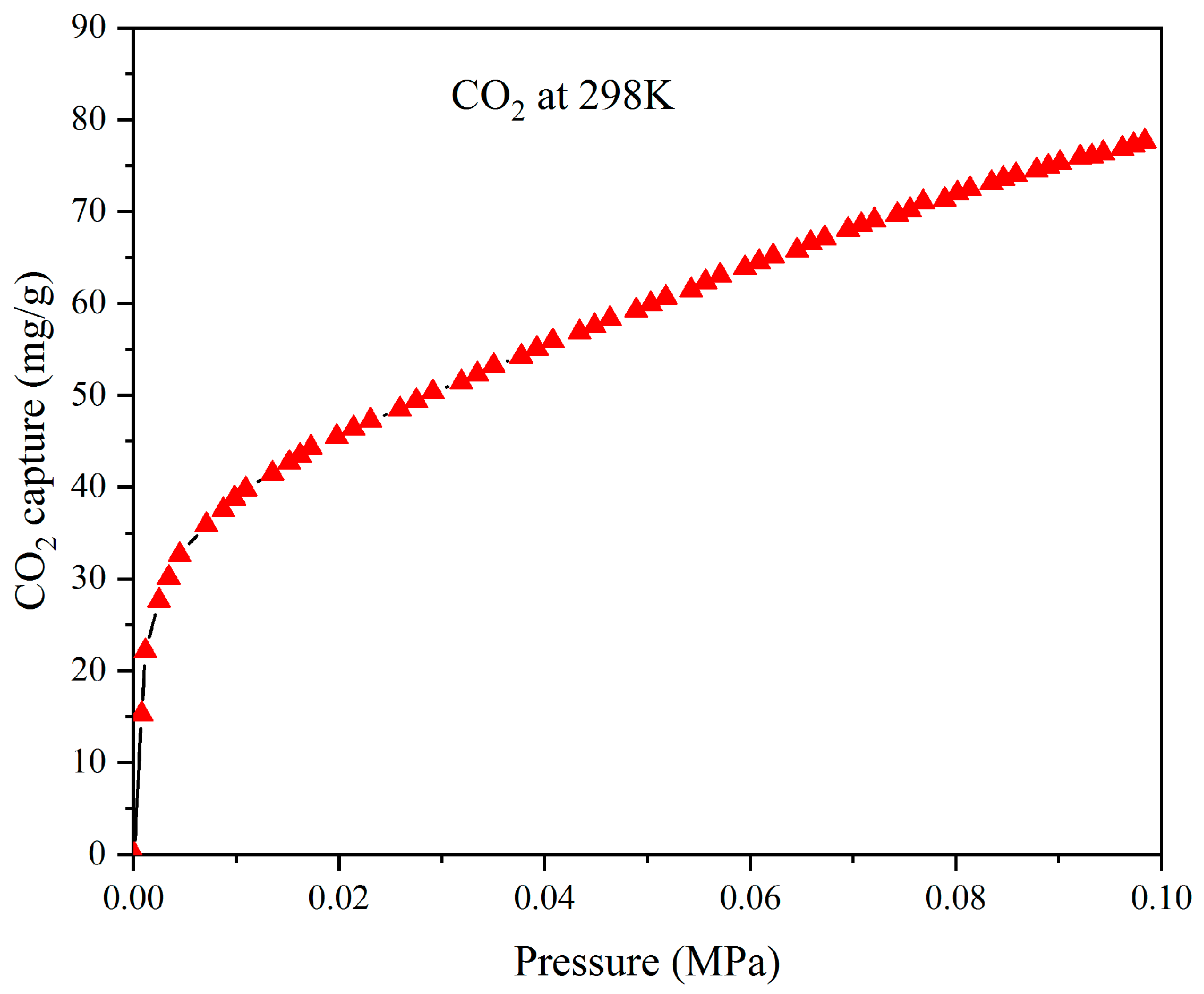
2.5. In Methylene Blue and CO2 Adsorption Studies
3. Results and Discussion
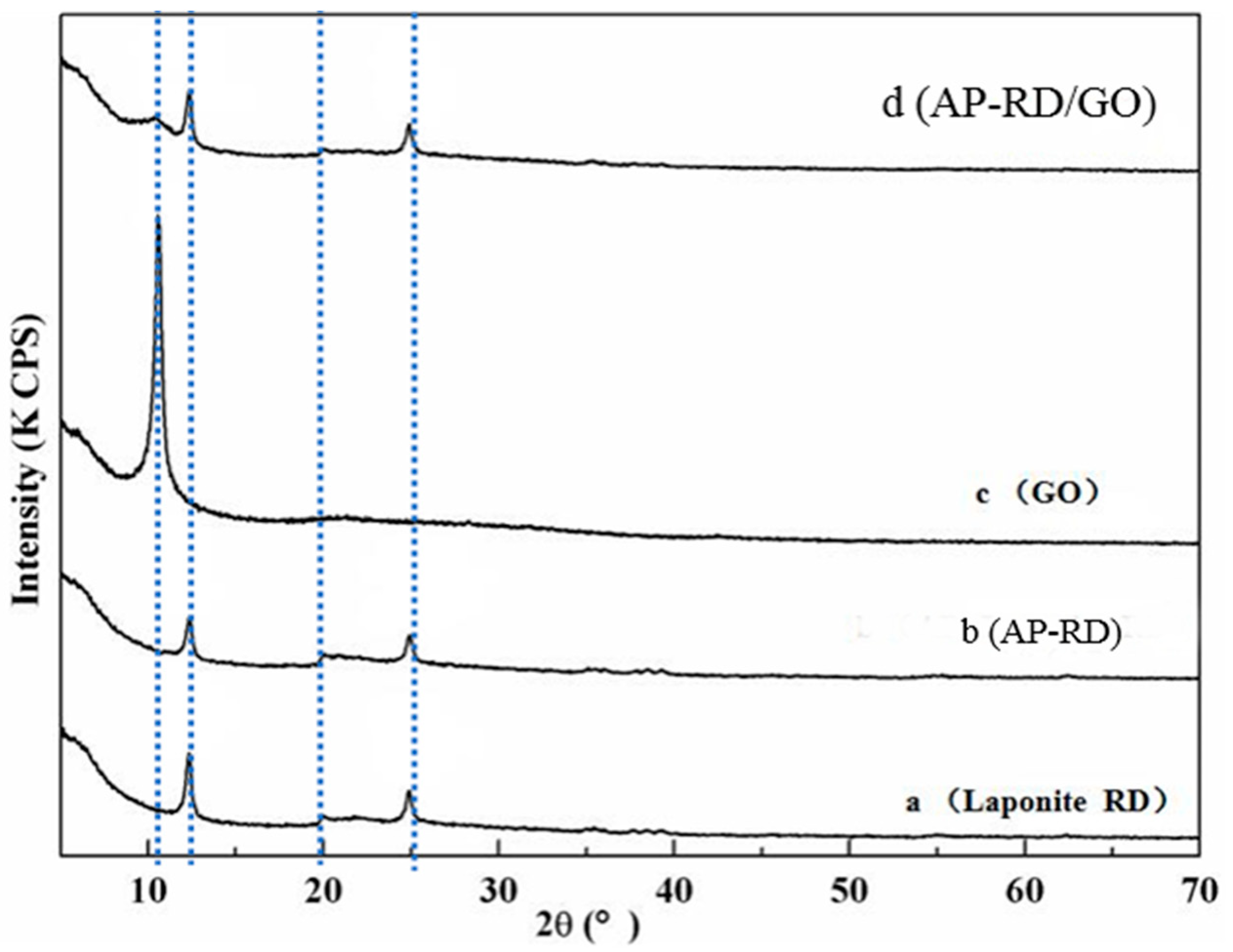
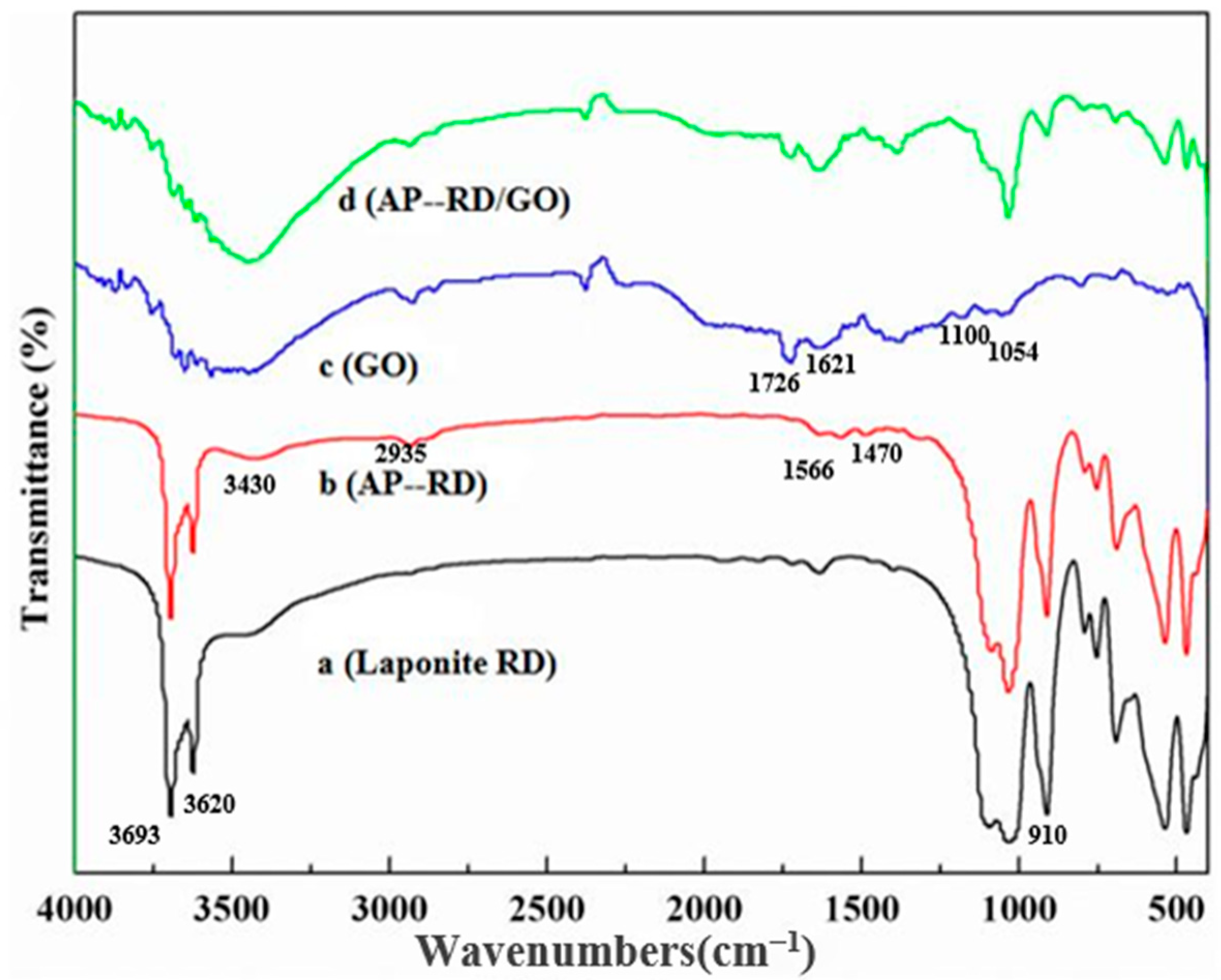
3.1. Study on Modified Laponite RD and GO
3.2. Construction and Structure of the GO/AP–RD/Chitosan Composite
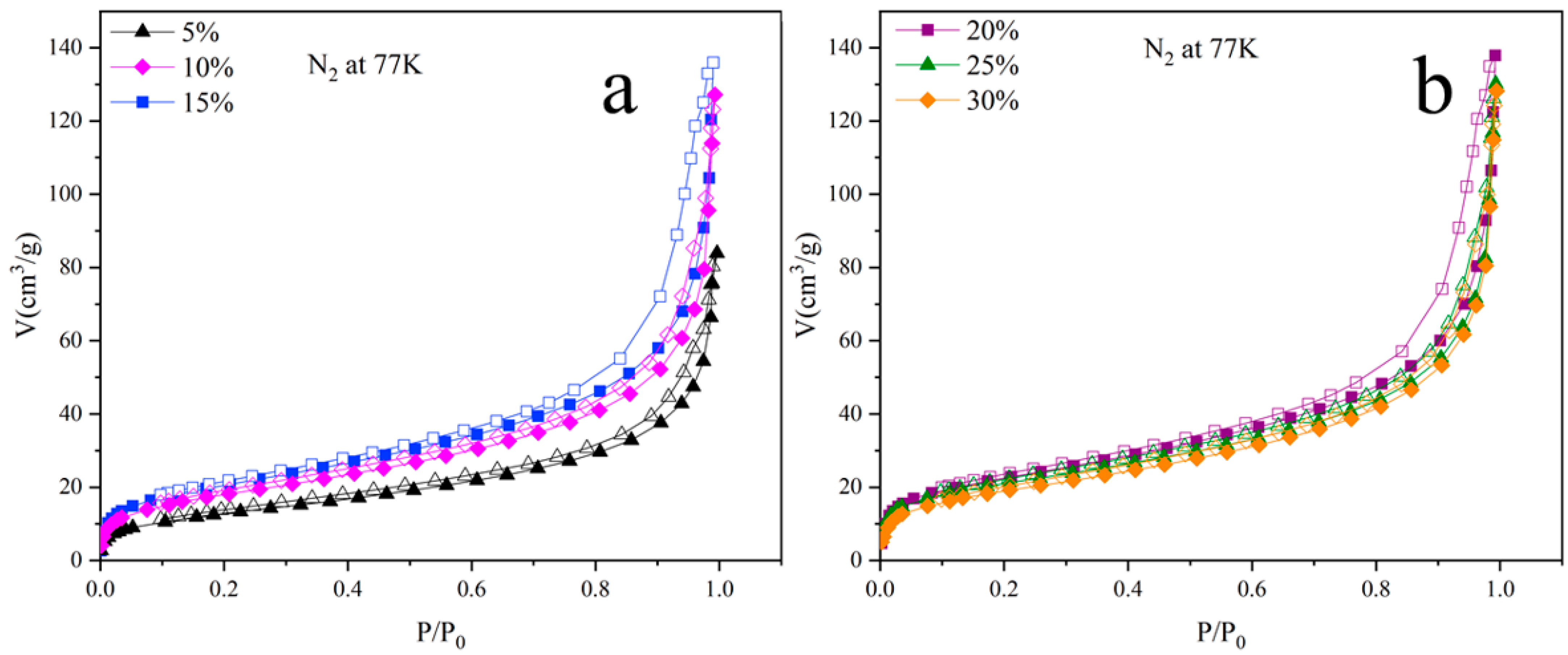
3.3. Adsorption Properties of Aerogels
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Harris, J.T.; McNeil, A.J. Localized hydrogels based on cellulose nanofibers and wood pulp for rapid removal of methylene blue. J. Polym. Sci. 2020, 58, 3042–3049. [Google Scholar] [CrossRef]
- He, H.; Zhuang, L.; Chen, S.; Liu, H. Solid amine adsorbent prepared by molecular imprinting and its carbon dioxide adsorption properties. Chem. Asian J. 2016, 11, 3055–3061. [Google Scholar] [CrossRef]
- Liu, F.; Chen, S.X.; Fu, W.H. Synthesis and CO2 adsorption behavior of amine-functionalized porous polyacrylonitrile resin. Acta Polym. Sin. 2018, 7, 886–892. [Google Scholar]
- Fritz, H.; Hofer, H.; Hammer, J. Adsorbing Material Comprised of Porous Functional Solid Incorporated in a Polymer Matrix. International Patent Application No. WO 03/055593 A1, 10 July 2003. [Google Scholar]
- Salles, F.; Ghoufi, A.; Maurin, G.; Bell, R.G.; Mellot-Draznieks, C.; Férey, G. Molecular dynamics simulations of breathing MOFs: Structural transformations of MIL-53 (Cr) upon thermal activation and CO2 adsorption. Angew. Chem. Int. Ed. 2008, 47, 8487–8491. [Google Scholar] [CrossRef]
- Zhuo, H.; Hu, Y.; Tong, X.; Zhong, L.; Peng, X.; Sun, R. Sustainable hierarchical porous carbon aerogel from cellulose for high-performance supercapacitor and CO2 capture. Ind. Crops Prod. 2016, 87, 229–235. [Google Scholar] [CrossRef]
- Zhang, S.; Cai, W.; Yu, J.; Ji, C.; Zhao, N. A facile one-pot cation-anion double hydrolysis approach to the synthesis of supported MgO/γ-Al2O3 with enhanced adsorption performance towards CO. Chem. Eng. J. 2017, 310, 216–225. [Google Scholar] [CrossRef]
- Dassanayake, R.; Gunathilake, C.; Abidi, N.; Jaroniec, M. Activated carbon derived from chitin aerogels: Preparation and CO2 adsorption. Cellulose 2018, 25, 1911–1920. [Google Scholar] [CrossRef]
- Cui, S.; Cheng, W.; Shen, X.; Fan, M.; Russell, A.T.; Wu, Z.; Yi, X. Mesoporous amine-modified SiO2 aerogel: A potential CO2 sorbent. Energy Environ. Sci. 2011, 4, 2070–2074. [Google Scholar] [CrossRef]
- Saeed, A.M.; Rewatkar, P.M.; Majedi Far, H.; Taghvaee, T.; Donthula, S.; Mandal, C.; Sotiriou-Leventis, C.; Leventis, N. Selective CO2 sequestration with monolithic bimodal micro/macroporous carbon aerogels derived from stepwise pyrolytic decomposition of polyamide-polyimide-polyurea random copolymers. ACS Appl. Mater. Interfaces 2017, 9, 13520–13536. [Google Scholar] [CrossRef]
- Gao, X.-D.; Huang, Y.-D.; Zhang, T.-T.; Wu, Y.-Q.; Li, X.-M. Amphiphilic SiO2 hybrid aerogel: An effective absorbent for emulsified wastewater. J. Mater. Chem. A 2017, 5, 12856–12862. [Google Scholar] [CrossRef]
- Baetens, R.; Jelle, B.P.; Gustavsen, A. Aerogel insulation for building applications: A state-of-the-art review. Energy Build. 2011, 43, 761–769. [Google Scholar] [CrossRef] [Green Version]
- Akimov, Y.K. Fields of application of aerogels. Instrum. Exp. Tech. 2003, 46, 287–299. [Google Scholar] [CrossRef]
- Duan, J.; Liang, X.; Cao, Y.; Wang, S.; Zhang, L. High strength chitosan hydrogels with biocompatibility via new avenue based on constructing nanofibrous architecture. Macromolecules 2015, 48, 2706–2714. [Google Scholar] [CrossRef]
- Kumar, M.N.R. A review of chitin and chitosan applications. React. Funct. Polym. 2000, 46, 1–27. [Google Scholar] [CrossRef]
- Liu, P. Polymer modified clay minerals: A review. Appl. Clay Sci. 2007, 38, 64–76. [Google Scholar] [CrossRef]
- Tan, C.; Fung, B.M.; Newman, J.K.; Vu, C. Organic aerogels with very high impact strength. Adv. Mater. 2001, 13, 644–646. [Google Scholar] [CrossRef]
- Petricevic, R.; Glora, M.; Möginger, A.; Fricke, J. Skin formation on RF aerogel sheets. J. Non-Cryst. Solids 2001, 285, 272–276. [Google Scholar] [CrossRef]
- Zhang, L.; Zeng, Y.; Cheng, Z. Removal of heavy metal ions using chitosan and modified chitosan: A review. J. Mol. Liq. 2016, 214, 175–191. [Google Scholar] [CrossRef]
- Harreld, J.H.; Sakamoto, J.; Dunn, B. Non-hydrolytic sol–gel synthesis and electrochemical characterization of tin-based oxide aerogels. J. Power Sources 2003, 115, 19–26. [Google Scholar] [CrossRef]
- Manandhar, S.; Roder, P.B.; Hanson, J.L.; Lim, M.; Smith, B.E.; Mann, A.; Pauzauskie, P.J. Rapid sol–gel synthesis of nanodiamond aerogel. J. Mater. Res. 2014, 29, 2905–2911. [Google Scholar] [CrossRef]
- Gash, A.E.; Tillotson, T.M.; Satcher, J.H., Jr.; Hrubesh, L.W.; Simpson, R.L. New sol–gel synthetic route to transition and main-group metal oxide aerogels using inorganic salt precursors. J. Non-Cryst. Solids 2001, 285, 22–28. [Google Scholar] [CrossRef] [Green Version]
- Chen, M.; Li, Z.; Li, J.; Li, J.; Li, Q.; Zhang, L. The extraction of uranium using graphene aerogel loading organic solution. Talanta 2017, 166, 284–291. [Google Scholar] [CrossRef]
- Worsley, M.A.; Pauzauskie, P.J.; Olson, T.Y.; Biener, J.; Satcher, J.H., Jr.; Baumann, T.F. Synthesis of graphene aerogel with high electrical conductivity. J. Am. Chem. Soc. 2010, 132, 14067–14069. [Google Scholar] [CrossRef] [PubMed]
- Cui, S.; Suo, H.; Jing, F.; Yu, S.; Xue, J.; Shen, X.; Lin, B.; Jiang, S.; Liu, Y. Facile preparation of ZrCO composite aerogel with high specific surface area and low thermal conductivity. J. Sol-Gel Sci. Technol. 2018, 86, 383–390. [Google Scholar] [CrossRef]
- Pekala, R.; Farmer, J.; Alviso, C.; Tran, T.; Mayer, S.; Miller, J.; Dunn, B. Carbon aerogels for electrochemical applications. J. Non-Cryst. Solids 1998, 225, 74–80. [Google Scholar] [CrossRef]
- Zhu, X.; Zhou, B.; Zhong, Y.; Du, A.; Chen, K.; Li, Y.; Zhang, Z.; Shen, J.; Wu, G.; Ni, X. Design and fabrication of a CH/CRF dual-layer perturbation target for ICF hydrodynamic experiments. Nucl. Fusion 2011, 51, 083044. [Google Scholar] [CrossRef]
- Song, J.H.; Lee, H.J.; Kim, J.H. Synthesis of resorcinol/formaldehyde organic aerogels by low temperature supercritical drying process. Korean J. Mater. Res. 1996, 6, 1082–1089. [Google Scholar]
- Xi, J.; Li, Y.; Zhou, E.; Liu, Y.; Gao, W.; Guo, Y.; Ying, J.; Chen, Z.; Chen, G.; Gao, C. Graphene aerogel films with expansion enhancement effect of high-performance electromagnetic interference shielding. Carbon 2018, 135, 44–51. [Google Scholar] [CrossRef]
- Aaltonen, O.; Jauhiainen, O. The preparation of lignocellulosic aerogels from ionic liquid solutions. Carbohydr. Polym. 2009, 75, 125–129. [Google Scholar] [CrossRef]
- Rao, A.P.; Pajonk, G. Hydrophobic and physical properties of the ambient pressure dried silica aerogels with sodium silicate precursor using various surface modification agents. Appl. Surf. Sci. 2007, 253, 6032–6040. [Google Scholar] [CrossRef]
- Pan, Y.; He, S.; Gong, L.; Cheng, X.; Li, C.; Li, Z.; Liu, Z.; Zhang, H. Low thermal-conductivity and high thermal stable silica aerogel based on MTMS/water-glass co-precursor prepared by freeze drying. Mater. Des. 2017, 113, 246–253. [Google Scholar] [CrossRef]
- Wu, G.L.H.C.L.; Xing, Z.Y. Preparation of silica aerogels by non supercritical drying. Acta Phys. Chim. Sin. 2003, 6. [Google Scholar] [CrossRef]
- Schwertfeger, F.; Frank, D.; Schmidt, M. Hydrophobic waterglass based aerogels without solvent exchange or supercritical drying. J. Non-Cryst. Solids 1998, 225, 24–29. [Google Scholar] [CrossRef]
- Shi, F.; Wang, L.; Liu, J. Synthesis and characterization of silica aerogels by a novel fast ambient pressure drying process. Mater. Lett. 2006, 60, 3718–3722. [Google Scholar] [CrossRef]
- Ding, M.; Jiang, H.-L. One-step assembly of a hierarchically porous phenolic resin-type polymer with high stability for CO2 capture and conversion. Chem. Commun. 2016, 52, 12294–12297. [Google Scholar] [CrossRef]
- Sai, H.; Fu, R.; Xing, L.; Xiang, J.; Li, Z.; Li, F.; Zhang, T. Surface modification of bacterial cellulose aerogels’ web-like skeleton for oil/water separation. ACS Appl. Mater. Interfaces 2015, 7, 7373–7381. [Google Scholar] [CrossRef] [PubMed]
- Neskoromnaya, E.A.; Burakov, A.E.; Melezhik, A.V.; Babkin, A.V.; Burakova, I.; Kurnosov, D.A.; Tkachev, A.G. Synthesis and evaluation of adsorption properties of reduced graphene oxide hydro-and aerogels modified by iron oxide nanoparticles. Inorg. Mater. Appl. Res. 2020, 11, 467–475. [Google Scholar] [CrossRef]
- Hu, X.-S.; Liang, R.; Sun, G. Super-adsorbent hydrogel for removal of methylene blue dye from aqueous solution. J. Mater. Chem. A 2018, 6, 17612–17624. [Google Scholar] [CrossRef]
- Kang, S.; Zhao, Y.; Wang, W.; Zhang, T.; Chen, T.; Yi, H.; Rao, F.; Song, S. Removal of methylene blue from water with montmorillonite nanosheets/chitosan hydrogels as adsorbent. Appl. Surf. Sci. 2018, 448, 203–211. [Google Scholar] [CrossRef]
- Soleimani, K.; Tehrani, A.D.; Adeli, M. Preparation of new GO-based slide ring hydrogel through a convenient one-pot approach as methylene blue absorbent. Carbohydr. Polym. 2018, 187, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Bera, R.; Ansari, M.; Alam, A.; Das, N. Triptycene, phenolic-OH, and Azo-functionalized porous organic polymers: Efficient and selective CO2 capture. ACS Appl. Polym. Mater. 2019, 1, 959–968. [Google Scholar] [CrossRef]
- Zou, L.; Sun, Y.; Che, S.; Yang, X.; Wang, X.; Bosch, M.; Wang, Q.; Li, H.; Smith, M.; Yuan, S.; et al. Porous organic polymers for post-combustion carbon capture. Adv. Mater. 2017, 29, 1700229. [Google Scholar] [CrossRef] [PubMed]





| Samples | Z-Average (d·nm) | PDI | Zeta Potential (mV) * |
|---|---|---|---|
| Laponite RD | 236 | 0.804 | 2.7 |
| AP–RD | 264 | 0.852 | 11.9 |
| GO | 1955 | 0.791 | −20.9 |
| Sample | BET-Specific Surface Area (m2/g) | BJH Adsorption Cumulative Volume (cm3/g) | BJH Median Pore Width (nm) |
|---|---|---|---|
| AP–RD/GO–CT (5%) | 47 | 0.13 | 2.32 |
| AP–RD/GO–CT (10%) | 66 | 0.19 | 2.80 |
| AP–RD/GO–CT (15%) | 75 | 0.20 | 2.22 |
| AP–RD/GO–CT (20%) | 81 | 0.28 | 2.33 |
| AP–RD/GO–CT (25%) | 78 | 0.24 | 2.45 |
| AP–RD/GO–CT (30%) | 70 | 0.24 | 2.61 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, W.; Ma, R.; Liu, Z.; Yang, G.; Chen, T. Study on the Adsorption Properties of Graphene Oxide/Laponite RD/Chitosan Composites. Materials 2021, 14, 3224. https://doi.org/10.3390/ma14123224
Du W, Ma R, Liu Z, Yang G, Chen T. Study on the Adsorption Properties of Graphene Oxide/Laponite RD/Chitosan Composites. Materials. 2021; 14(12):3224. https://doi.org/10.3390/ma14123224
Chicago/Turabian StyleDu, Wenjie, Rui Ma, Zhiyan Liu, Gang Yang, and Tao Chen. 2021. "Study on the Adsorption Properties of Graphene Oxide/Laponite RD/Chitosan Composites" Materials 14, no. 12: 3224. https://doi.org/10.3390/ma14123224
APA StyleDu, W., Ma, R., Liu, Z., Yang, G., & Chen, T. (2021). Study on the Adsorption Properties of Graphene Oxide/Laponite RD/Chitosan Composites. Materials, 14(12), 3224. https://doi.org/10.3390/ma14123224






