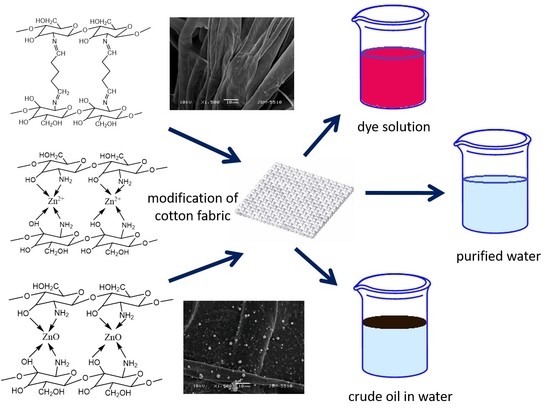
Pollutants Sorbent Made of Cotton Fabric Modified with Chitosan-Glutaraldehyde and Zinc Oxide Particles
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Reactive Dye Drimarene Rot K-7B and Crude Oil and Oil Products
2.3. Preparation of Composite Materials
2.3.1. The Preparation of Different Solution
- 1)
- The chitosan (2.7% w/v) was dissolved in water under stirring, with the gradual addition of glacial acetic acid (1% v/v) to obtain a clear viscous solution;
- 2)
- The chitosan (2.7% w/v) and Zn(NO3)2x6H2O (2.7% w/v) were dissolved in water with the gradual addition of glacial acetic acid (1% v/v) to obtain a clear viscous solution;
- 3)
- Zn(NO3)2x6H2O was dissolved in water. The quantity of Zn2+ was 20% owf (on weight of fabric).
2.3.2. The Fabric Treatment
- 1)
- Sample Ch
- 2)
- Sample ChZn
- 3)
- Sample ZnChZn
2.4. Analysis
2.5. Sorbent Properties of the Composite Materials
2.5.1. The Sorption of Crude Oil and Oil Products
2.5.2. Reusability of ChZn Composite Material
2.5.3. Discoloration of the Reactive Dye Drimaren Rot K-7B Water Solution
3. Results and Discussion
Morphological Properties of Composites
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Atanasova, D.; Staneva, D.; Grabchev, I. Textile with a hydrogel and iron oxide nanoparticles forwastewat er treatment after reactive dyeing. J. Appl. Polym. Sci. 2020, 138, e49954. [Google Scholar]
- Sharma, S.; Bhattacharya, A. Drinking water contamination and treatment techniques. Appl. Water Sci. 2017, 7, 1043–1067. [Google Scholar] [CrossRef]
- Satır, I.T.; Sayin, F.; Gedikbey, T.; Akar, S.T. A novel sorbent for removal of reactive textile dye: TDPA-KCl. Environ. Sci. Pollut. Res. 2019, 26, 23279–23291. [Google Scholar] [CrossRef] [PubMed]
- Hoanga, A.T.; Bui, X.L.; Pham, X.D. The Efficient Lignocellulose-Based Sorbent for Oil Spill Treatment from Polyurethane and Agricultural Residue of Vietnam. Energ. Source Part A 2018, 40, 929–939. [Google Scholar] [CrossRef]
- Idris, J.; Eyu, G.D.; Mansor, A.M.; Ahmad, Z.; Chukwuekezie, C.S. A Preliminary Study of Biodegradable Waste as Sorbent Material for Oil-Spill Cleanup. Sci. World J. 2014, 2014, 638687. [Google Scholar] [CrossRef]
- Escudero-Oñate, C.; Martínez-Francés, E. A review of chitosan-based materials for the removal of organic pollution from water and bioaugmentation. In Chitin-Chitosan—Myriad Functionalities in Science and Technology; Dongre, R., Ed.; IntechOpen: London, UK, 2018; pp. 71–87. [Google Scholar]
- Mir, S.H.; Nagahara, L.A.; Thundat, T.; Mokarian-Tabari, P.; Furukawa, H.; Khosla, A. Review—Organic-Inorganic Hybrid Functional Materials: An Integrated Platform for Applied Technologies. J. Electrochem. Soc. 2018, 165, B3137–B3156. [Google Scholar] [CrossRef]
- Toteva, V.; Staneva, D. Preparation and application of composite material as sorbent for oil spills. In Proceedings of the 3rd International Conference on High Technology for Sustainable Development (HiTech), Sofia, Bulgaria, 8–9 October 2020. [Google Scholar]
- Luxbacher, T.; Pušić, T.; Bukšek, H.; Petrinić, I. The zeta potential of textile fabrics: A review. Tekstil 2016, 65, 346–351. [Google Scholar]
- Houshyar, S.; Amirshahi, S.H. Treatment of Cotton with Chitosan and Its Effect on Dyeability with Reactive Dyes. Iran. Polym. J. 2002, 1, 295–301. [Google Scholar]
- Li, X.; Yu, Z.; Chen, Q.; Yang, S.; Li, F.; Yang, Y. Chitosan-coated filter paper with superhydrophilicity for treatment of oily wastewater in acidic and alkaline environments. Mater. Technol. 2019, 34, 213–223. [Google Scholar] [CrossRef]
- Yadollahi, M.; Farhoudian, S.; Barkhordari, S.; Gholamali, I.; Farhadnejad, H.; Motasadizadeh, H. Facile synthesis of chitosan/ZnO bio-nanocomposite hydrogel beads as drug delivery systems. Int. J. Biol. Macromol. 2016, 82, 273–278. [Google Scholar] [CrossRef]
- The Impact and Prospects of Green Chemistry for Textile Technology; The Textile Institute Book Series; Shahid-ul-Islam, B.S., Ed.; Woodhead Publishing: Sawston, UK, 2019. [Google Scholar]
- Guibal, E. Interactions of metal ions with chitosan-based sorbents: A review. Sep. Purif. Technol. 2004, 38, 43–74. [Google Scholar] [CrossRef]
- Yusof, N.A.A.; Zain, N.M.; Pauzi, N. Synthesis of Chitosan/Zinc Oxide Nanoparticles Stabilized by Chitosan via Microwave Heating. Bull. Chem. React. Eng. Catal. 2019, 14, 450–458. [Google Scholar] [CrossRef]
- Krishnapriya, R.; Praneetha, S.; Murugan, A.V. Energy-efficient, microwave-assisted hydro/solvothermal synthesis of hierarchical flowers and rice grain-like ZnO nanocrystals as photoanodes for high performance dye-sensitized solar cells. CrystEngComm 2015, 17, 8353. [Google Scholar] [CrossRef]
- Nithya, A.; Jothivenkatachalam, K. Chitosan assisted synthesis of ZnO nanoparticles: An efficient solar light driven photocatalyst and evaluation of antibacterial activity. J. Mater. Sci. Mater. Electron. 2015, 26, 10207–10216. [Google Scholar] [CrossRef]
- Bhadra, P.; Mitra, M.K.; Das, G.C.; Dey, R.; Mukherjee, S. Interaction of chitosan capped ZnO nanorods with Escherichia coli. Mater. Sci. Eng. C 2011, 31, 929–937. [Google Scholar] [CrossRef]
- AbdElhady, M.M. Preparation and Characterization of Chitosan/Zinc Oxide Nanoparticles for Imparting Antimicrobial and UV Protection to Cotton Fabric. Int. J. Carbohydr. Chem. 2012, 840591. [Google Scholar] [CrossRef]
- Rajkumar, C.; Srivastava, R.K. UV—Visible photoresponse properties of self-seeded and polymer mediated ZnO flower-like and biconical nanostructures. Results Phys. 2019, 15, 102647. [Google Scholar] [CrossRef]
- Hu, H.; Xin, J.H.; Hu, H.; Chan, A.; He, L. Glutaraldehyde–chitosan and poly (vinyl alcohol) blends, and fluorescence of their nano-silica composite films. Carbohydr. Polym. 2013, 91, 305–313. [Google Scholar] [CrossRef]
- Samanta, P.K.; Bandyopadhyay, A.K. Chemical growth of hexagonal zinc oxide nanorods and their optical properties. Appl. Nanosci. 2012, 2, 111–117. [Google Scholar] [CrossRef]
- Choi, H.M.; Cloud, R.M. Natural sorbents in oil spill cleanup. Environ. Sci. Technol. 1992, 26, 772–776. [Google Scholar] [CrossRef]
- Zafar, M.N.; Dar, Q.; Nawaz, F.; Zafar, M.N.; Iqbal, M.; Nazar, M.F. Effective adsorptive removal of azo dyes over spherical ZnO nanoparticles. J. Mater. Res. Technol. 2019, 8, 713–725. [Google Scholar] [CrossRef]
- Schuster, E.; Eckardt, J.; Hermansson, A.-M.; Larsson, A.; Loren, N.; Altskar, A.; Strom, A. Microstructural, mechanical and mass transport properties of isotropic and capillary alginate gels. Soft. Matter. 2014, 10, 357–366. [Google Scholar] [CrossRef] [PubMed]










| Sample | Density, g/cm3 (15 °C) | Viscosity, mm2/s (40 °C) |
|---|---|---|
| oil | 0.830 | 3.3 |
| diesel fuel | 0.827 | 2.9 |
| motor oil 15W/40 | 0.940 | 106.0 |
| Component Mass% | Mg | Al | Si | P | S | Cl | K | Ca | Ti | Fe | Zn | Matrix |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ch | 0.01 | 0.01 | 0.00 | 0.00 | 0.03 | 0.06 | 0.03 | 0.03 | 0.30 | 0.02 | 0.00 | 99.50 |
| ChZn | 0.67 | 0.25 | 0.27 | 0.08 | 0.18 | 0.33 | 0.54 | 1.39 | 1.46 | 0.14 | 10.25 | 84.44 |
| ZnChZn | 0.58 | 0.17 | 0.33 | 0.06 | 0.18 | 0.32 | 0.72 | 1.33 | 1.97 | 0.15 | 12.19 | 82.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toteva, V.; Staneva, D.; Grabchev, I. Pollutants Sorbent Made of Cotton Fabric Modified with Chitosan-Glutaraldehyde and Zinc Oxide Particles. Materials 2021, 14, 3242. https://doi.org/10.3390/ma14123242
Toteva V, Staneva D, Grabchev I. Pollutants Sorbent Made of Cotton Fabric Modified with Chitosan-Glutaraldehyde and Zinc Oxide Particles. Materials. 2021; 14(12):3242. https://doi.org/10.3390/ma14123242
Chicago/Turabian StyleToteva, Vesislava, Desislava Staneva, and Ivo Grabchev. 2021. "Pollutants Sorbent Made of Cotton Fabric Modified with Chitosan-Glutaraldehyde and Zinc Oxide Particles" Materials 14, no. 12: 3242. https://doi.org/10.3390/ma14123242
APA StyleToteva, V., Staneva, D., & Grabchev, I. (2021). Pollutants Sorbent Made of Cotton Fabric Modified with Chitosan-Glutaraldehyde and Zinc Oxide Particles. Materials, 14(12), 3242. https://doi.org/10.3390/ma14123242