Materials for High Temperature Liquid Lead Storage for Concentrated Solar Power (CSP) Air Tower Systems
Abstract
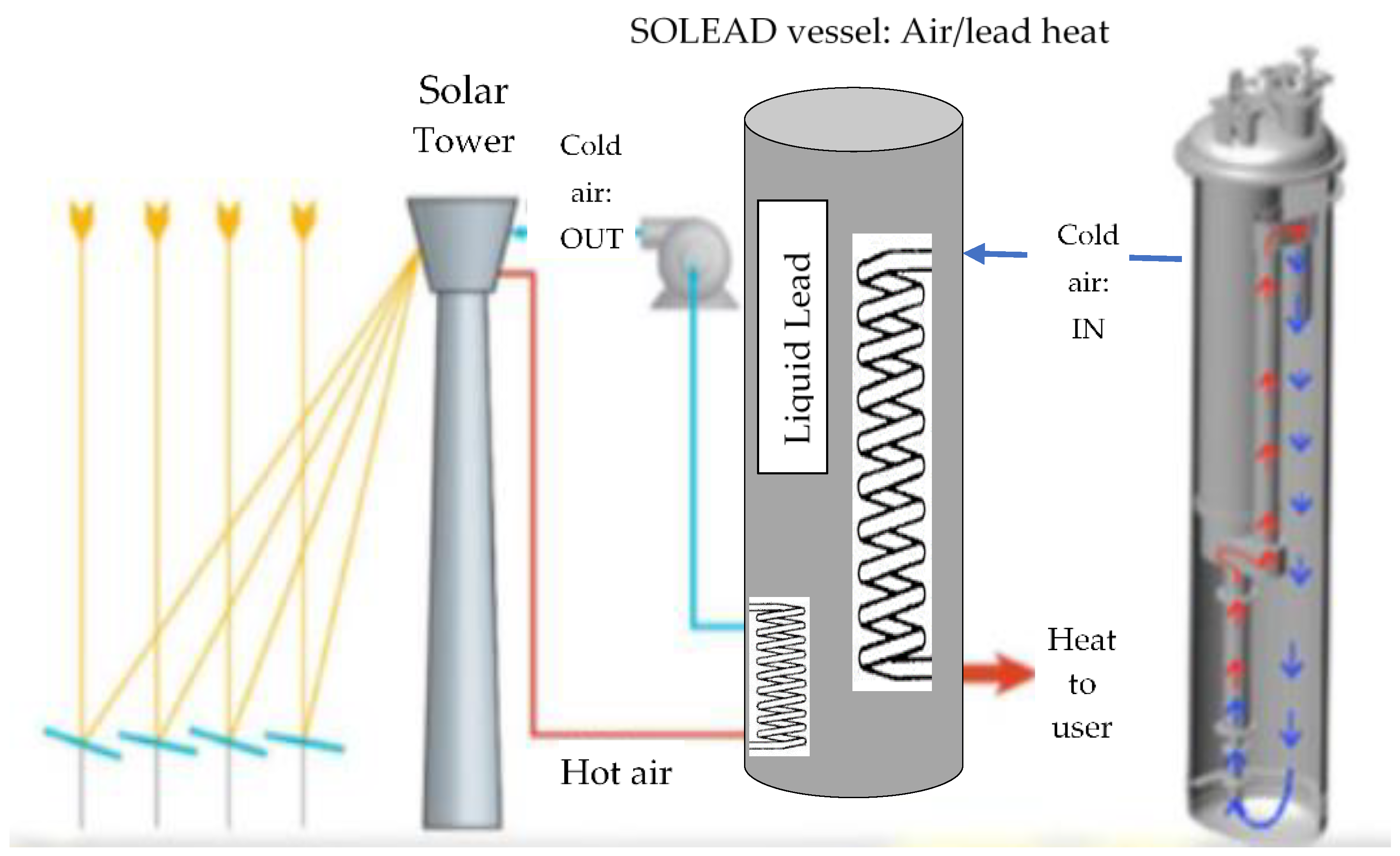
:1. Introduction
2. Materials and Methods
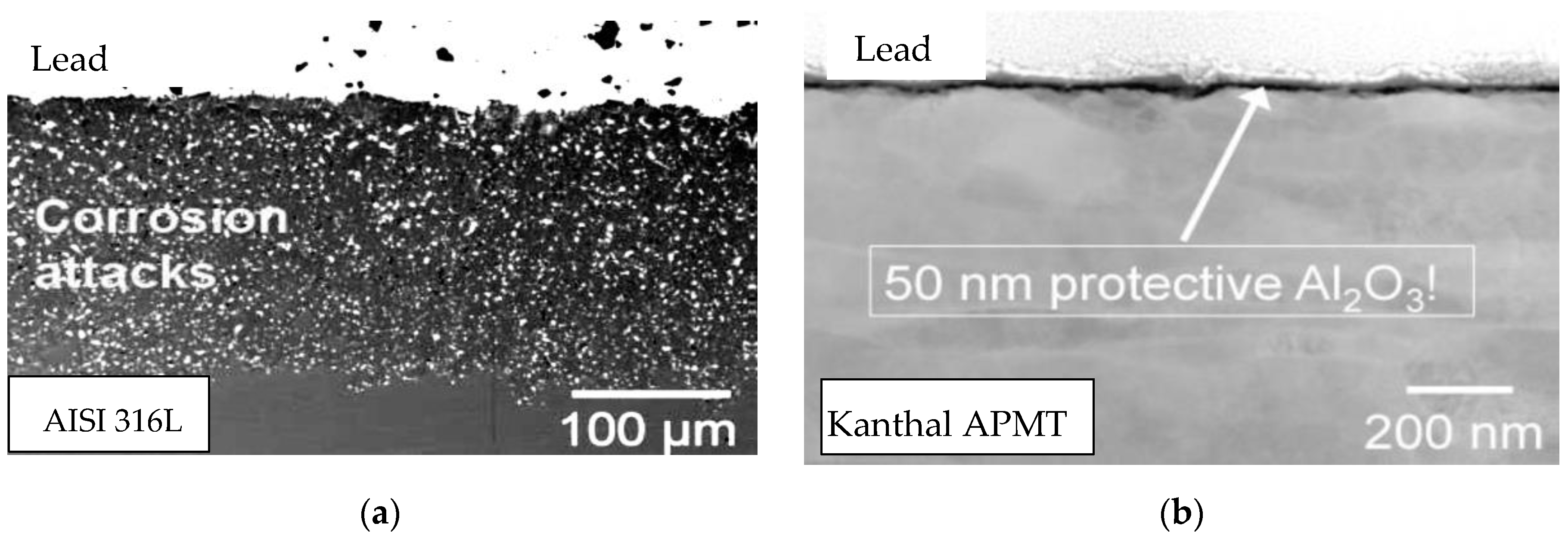
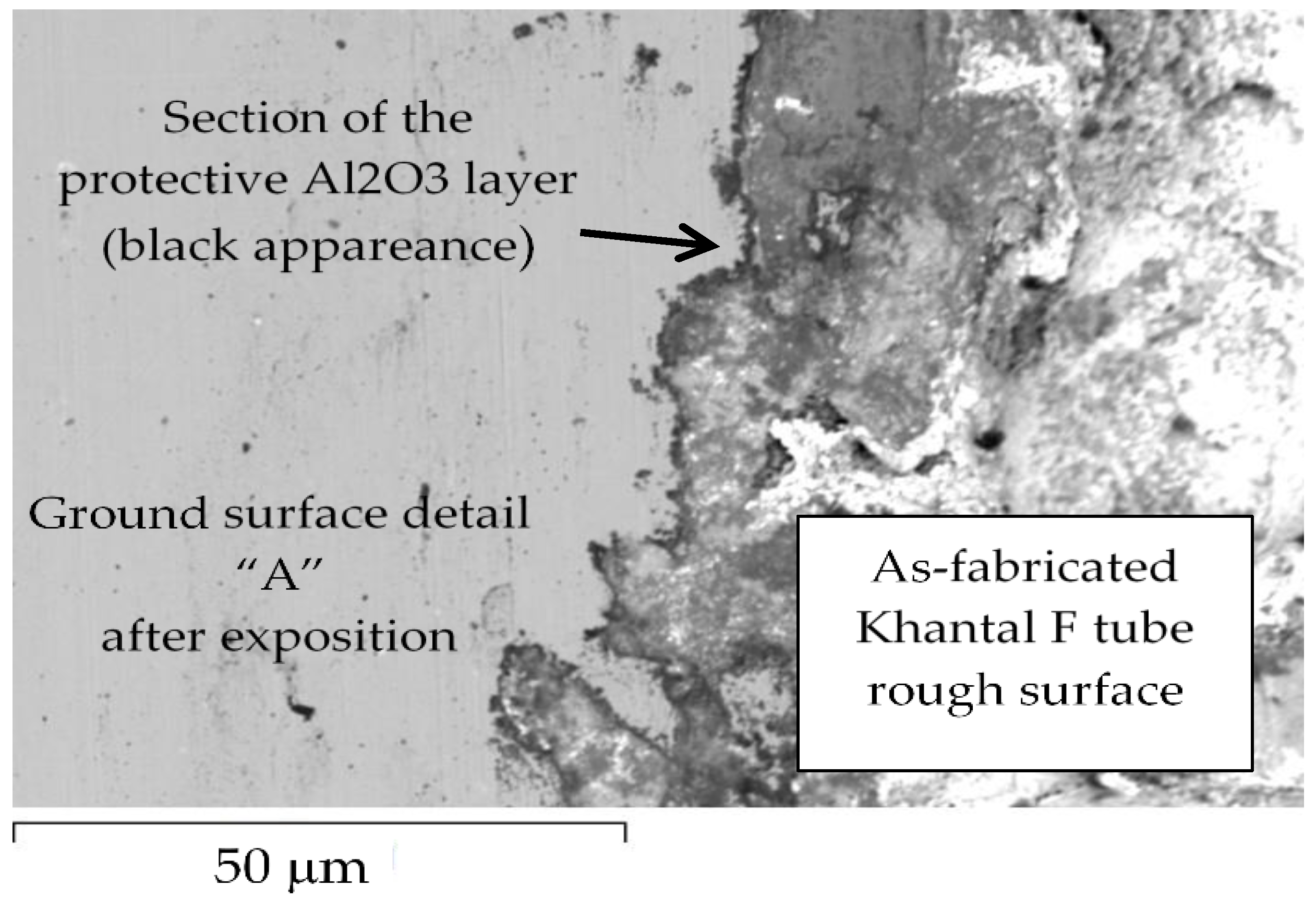
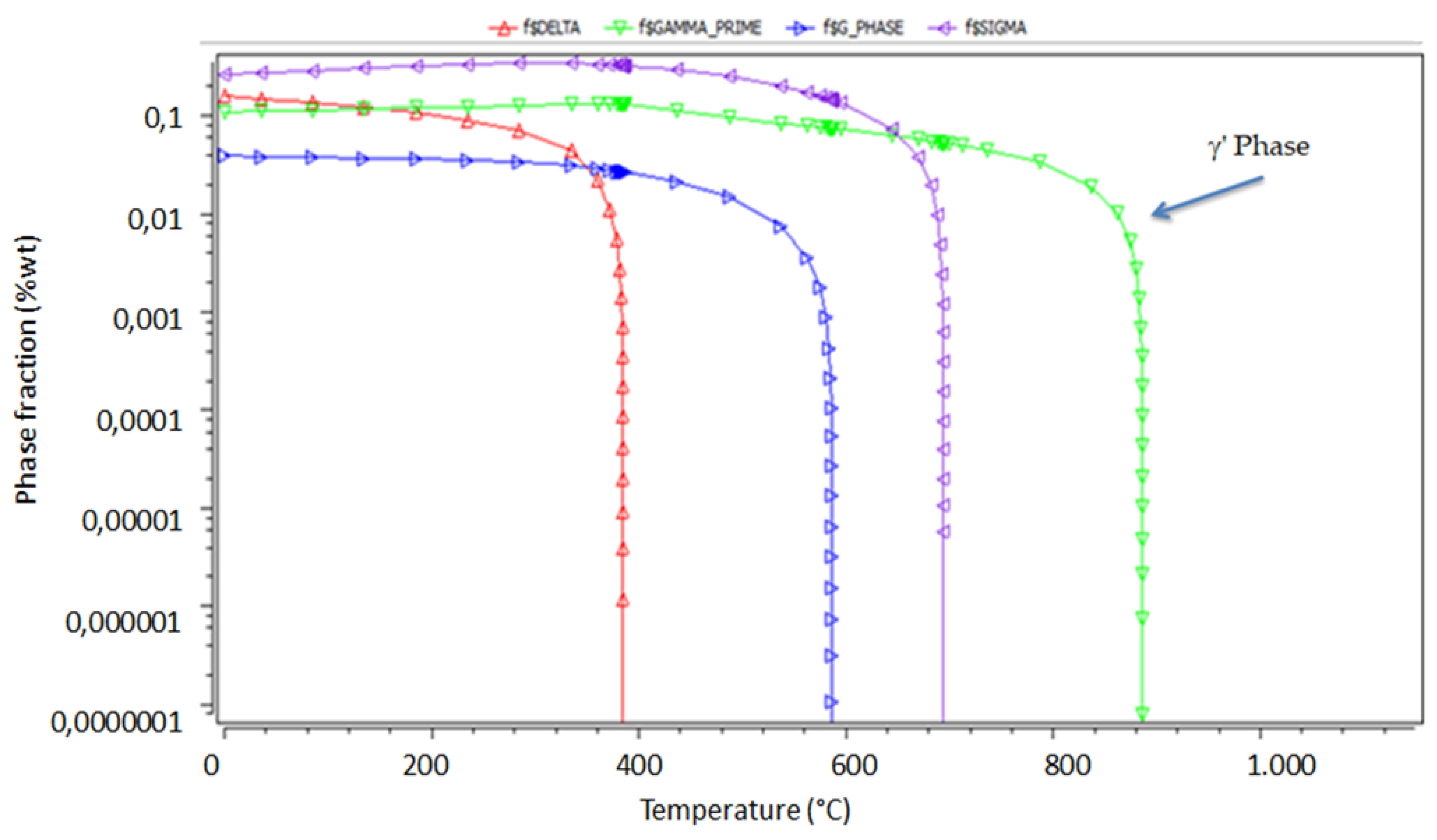
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Concentrating Solar Power Gen3 Demonstration Roadmap. Technical Report NREL/TP-5500-67464; January 2017. Available online: www.nrel.gov/publications (accessed on 15 April 2021).
- Glatzmaier, G.C. Summary Report for Concentrating Solar Power Thermal Storage Workshop: New Concepts and Materials for Thermal Energy Storage and Heat-Transfer Fluids; National Renewable Energy Laboratory: Lakewood, CO, USA, 2011; p. 3. [Google Scholar]
- Puppe, M.; Giuliano, S.; Frantz, C.; Uhlig, R.; Flesch, R.; Schumacher, R.; Ibraheem, W.; Schmalz, S.; Waldmann, B.; Guder, C.; et al. Techno-economic optimization of molten salt solar tower plants. In AIP Conference Proceedings; AIP Publishing: Perth, Australia, 13 December 2018. [Google Scholar]
- Haneklaus, N.; Zheng, Y.; Allelein, H.-J. Stop Smoking—Tube-In-Tube Helical System for Flameless Calcination of Minerals. Processes 2017, 5, 67. [Google Scholar] [CrossRef] [Green Version]
- Allen, T.R.; Crawford, D.C. Lead-Cooled Fast Reactor Systems and the Fuels and Materials Challenges. Sci. Technol. Nucl. Install. 2007, 2007, 97486. [Google Scholar] [CrossRef]
- He, Y.-L.; Qiu, Y.; Wang, K.; Yuan, F.; Wang, W.-Q.; Li, M.-J.; Guo, J.-Q. Perspective of concentrating solar power. Energy 2020, 198, 117373. [Google Scholar] [CrossRef]
- Püttgen, W.; Pant, M.; Bleck, W.; Seidl, I.; Rabitsch, R.; Testani, C. Selection of Suitable Tool Materials and Development of Tool Concepts for the Thixoforging of Steels. Steel Res. Int. 2006, 77, 342–348. [Google Scholar] [CrossRef]
- Blum, R.; Chen, Q.; Coussement, C.; Testani, C.; Verelst, L. Operational tests of superheater materials at high steam temperatures in a hard coal-fired steam generator. VGB PowerTech 2001, 81, 86–91. [Google Scholar]
- Gabrel, J.; Coussement, C.; Verelst, L.; Blum, R.; Chen, Q.; Testani, C. Superheater materials testing for USC boilers: Steam side oxidation rate of 9 advanced materials in industrial conditions. Mater. Sci. Forum 2001, 369–372, 931–938. [Google Scholar] [CrossRef]
- Di Piazza, I.; Tincani, A.; Marinari, R.; Valdiserri, M.; Bassini, S.; Rinaldi, A.; Turchetti, L.; Serra, M.; Antonelli, A.; Delfino, D. Conceptual Design of the CSP Lead Demonstrator SOLEAD. Adv. Mater. Lett. 2020, 11, 20061531. [Google Scholar] [CrossRef]
- Kondo, M.; Takahashi, M.; Suzuki, T.; Ishikawa, K.; Hata, K.; Qiu, S.; Sekimoto, H. Metallurgical Study on Erosion and Corrosion Behaviors of Steels Exposed to Liquid Lead-Bismuth Flow. J. Nucl. Mater. 2005, 343, 349–359. [Google Scholar] [CrossRef]
- Li, N. Lead-alloy coolant technology and materials e technology readiness level evaluation. Prog. Nucl. Energy 2008, 50, 140–151. [Google Scholar] [CrossRef]
- Kondo, M.; Takahashi, M.; Sawada, N.; Hata, K. Corrosion of Steels in Lead–Bismuth Flow. J. Nucl. Sci. Technol. 2006, 43, 107–116. [Google Scholar] [CrossRef]
- Konys, J.; Krauss, W.; Schroer, C.; Steiner, H.; Voss, Z.; Wedemeyer, O. Corrosion of Structural Materials in Liquid Lead Alloys for Nuclear Applications. Corrosion 2007, 63, 1124–1137. [Google Scholar] [CrossRef]
- Mustari, A.P.A.; Takashi, M. Corrosion Properties of Welded Ferritic-Martensitic Steels in Liquid Lead-Bismuth at 600 °C. J. Power Energy Syst. 2011, 5, 69–76. [Google Scholar] [CrossRef] [Green Version]
- Locatelli, G.; Mancini, M.; Todeschini, N. Generation IV nuclear reactors: Current status and future prospects. Energy Policy 2013, 61, 1503–1520. [Google Scholar] [CrossRef]
- Martinelli, L.; Dufrenoy, T.; Jaakou, K.; Rusanov, A.; Balbaud-Célérier, F. High temperature oxidation of Fe–9Cr–1Mo steel in stagnant liquid lead–bismuth at several temperatures and for different lead contents in the liquid alloy. J. Nucl. Mater. 2008, 376, 282–288. [Google Scholar] [CrossRef]
- Wetzel, T.; Pacio, J.; Marocco, L.; Weisenburger, A.; Heinzel, A.; Hering, W.; Schroer, C.; Muller, G.; Konys, J.; Stieglitz, R.; et al. Liquid metal technology for concentrated solar power systems: Contributions by the German research program. AIMS Energy 2014, 2, 89–98. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, M.; Uchida, S.; Hata, K.; Matsuzawa, T.; Osada, H.; Kasahara, Y.; Sawa, N.; Okubo, Y.; Obara, T.; Yusibani, E. PbBi-cooled direct contact boiling water small reactor. Prog. Nucl. Energy 2005, 47, 190–201. [Google Scholar] [CrossRef]
- Takahashi, M.; Uchida, S.; Kasahara, Y. Design study on reactor structure of Pb–Bi-cooled direct contact boiling water fast reactor (PBWFR). Prog. Nucl. Energy 2008, 50, 197–205. [Google Scholar] [CrossRef]
- Kondo, M.; Takahashi, M. Corrosion resistance of Si- and Al-rich steels in flowing lead–bismuth. J. Nucl. Mater. 2006, 356, 203–212. [Google Scholar] [CrossRef]
- Heinzel, A.; Kondo, M.; Takahashi, M. Corrosion of steels with surface treatment and Al-alloying by GESA exposed in lead–bismuth. J. Nucl. Mater. 2006, 350, 264–270. [Google Scholar] [CrossRef]
- Rivai, A.K.; Takahashi, M. Compatibility of surface-coated steels, refractory metals and ceramics to high temperature lead–bismuth eutectic. Prog. Nucl. Energy 2008, 50, 560–566. [Google Scholar] [CrossRef]
- Rivai, A.K.; Takahashi, M. Corrosion Investigations of Al-Fe-coated Steels, High Cr Steels, Refractory Metals and Ceramics in Lead Alloys at 700 °C. J. Nucl. Mater. 2010, 398, 146–152. [Google Scholar] [CrossRef]
- Short, M.P.; Ballinger, R.G.; Hänninen, H.E. Corrosion resistance of alloys F91 and Fe–12Cr–2Si in lead–bismuth eutectic up to 715 °C. J. Nucl. Mater. 2013, 434, 259–281. [Google Scholar] [CrossRef]
- Li, N. Active control of oxygen in molten lead–bismuth eutectic systems to prevent steel corrosion and coolant contamination. J. Nucl. Mater. 2002, 300, 73–81. [Google Scholar] [CrossRef]
- Takaya, S.; Furukawa, T.; Inoue, M.; Fujisawa, T.; Okuda, T.; Abe, F.; Ohnuki, S.; Kimura, A. Corrosion resistance of Al-alloying high Cr–ODS steels in stagnant lead–bismuth. J. Nucl. Mater. 2010, 398, 132–138. [Google Scholar] [CrossRef]
- Kondo, M.; Takahashi, M. Metallurgical Analysis of a Tube Ruptured in the Lead Bismuth Corrosion Test Facility. J. Nuclear Sci. Technol. 2006, 43, 174–178. [Google Scholar] [CrossRef]
- Weisenburger, A.; Müller, G.; Heinzel, A.; Jianu, A.; Muscher, H.; Kieser, M. Corrosion: Al containing corrosion barriers and mechanical properties of steels foreseen as structural materials in liquid lead alloy cooled nuclear systems. Nucl. Eng. Des. 2011, 241, 1329–1334. [Google Scholar] [CrossRef]
- Takaya, S.; Furukawa, T.; Müller, G.; Heinzel, A.; Jianu, A.; Weisenburger, A.; Aoto, K.; Inoue, M.; Okuda, T.; Abe, F.; et al. Al-containing ODS steels with improved corrosion resistance to liquid lead–bismuth. J. Nucl. Mater. 2012, 428, 125–130. [Google Scholar] [CrossRef]
- Yamaki, E.; Takahashi, M. Corrosion Resistance of Fe-Al Alloy-Coated Ferritic/martensitic Steel under Bending Stress in High Temperature Lead-Bismuth Eutectic. J. Nucl. Sci. Technol. 2011, 48, 797–804. [Google Scholar] [CrossRef]
- Rinaldi, A.; Barbieri, G.; Testani, C. NEXTOWER Horizon 2020 Research and Innovation Programme under Grant Agreement No 721045, Work Package 5—ENEA, Final Technical Report. under final review.
- Rivai, A.K.; Takahashi, M. Corrosion characteristics of materials in Pb–Bi under transient temperature conditions. J. Nucl. Mater. 2010, 398, 139–145. [Google Scholar] [CrossRef]
- Ejenstam, J.; Shen, R.; Rinaldi, A.; Ivermark, M.; Olovsjö, J.N.; Chandrasekaran, D. Alumina Forming Alloys for Higher Efficiency in Thermal Energy Storage Systems. In Proceedings of the High-Temperature Corrosion and Protection of Materials (HTCPM) 2021 International Congress, Toulouse, France, 29 March–2 April 2021. [Google Scholar]
- Sandvik 253MA Data Sheet. Available online: https://www.materials.sandvik/en/materials-center/material-datasheets/tube-and-pipe-seamless/sandvik-253-ma/ (accessed on 15 April 2021).
- Sandvik Sanicro 25 Data Sheet. Available online: https://www.materials.sandvik/en/materials-center/material-datasheets/tube-and-pipe-seamless/sanicro-25/ (accessed on 15 April 2021).
- Sandvik Sanicro 31HT Data Sheet. Available online: https://www.materials.sandvik/en/materials-center/material-datasheets/tube-and-pipe-seamless/sanicro-31ht/ (accessed on 15 April 2021).
- Specification Sheet: Alloy 800H/800HT. Available online: https://www.specialmetals.com/assets/smc/documents/alloys/incoloy/incoloy-alloys-800h-800ht.pdf (accessed on 15 April 2021).
- IN617—Special Metals Data Sheet. Available online: https://www.specialmetals.com/documents/technical-bulletins/inconel/inconel-alloy-617.pdf (accessed on 15 April 2021).

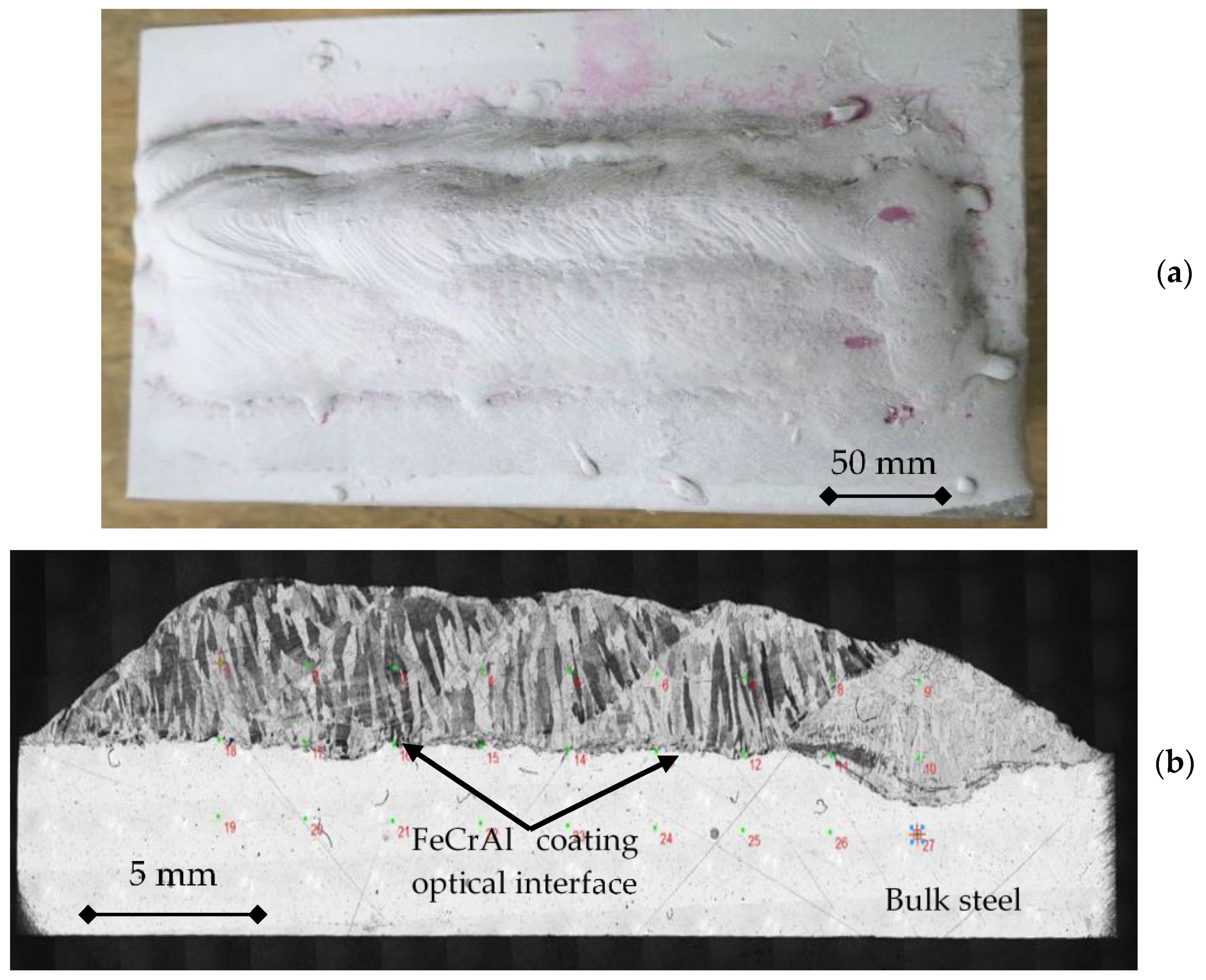
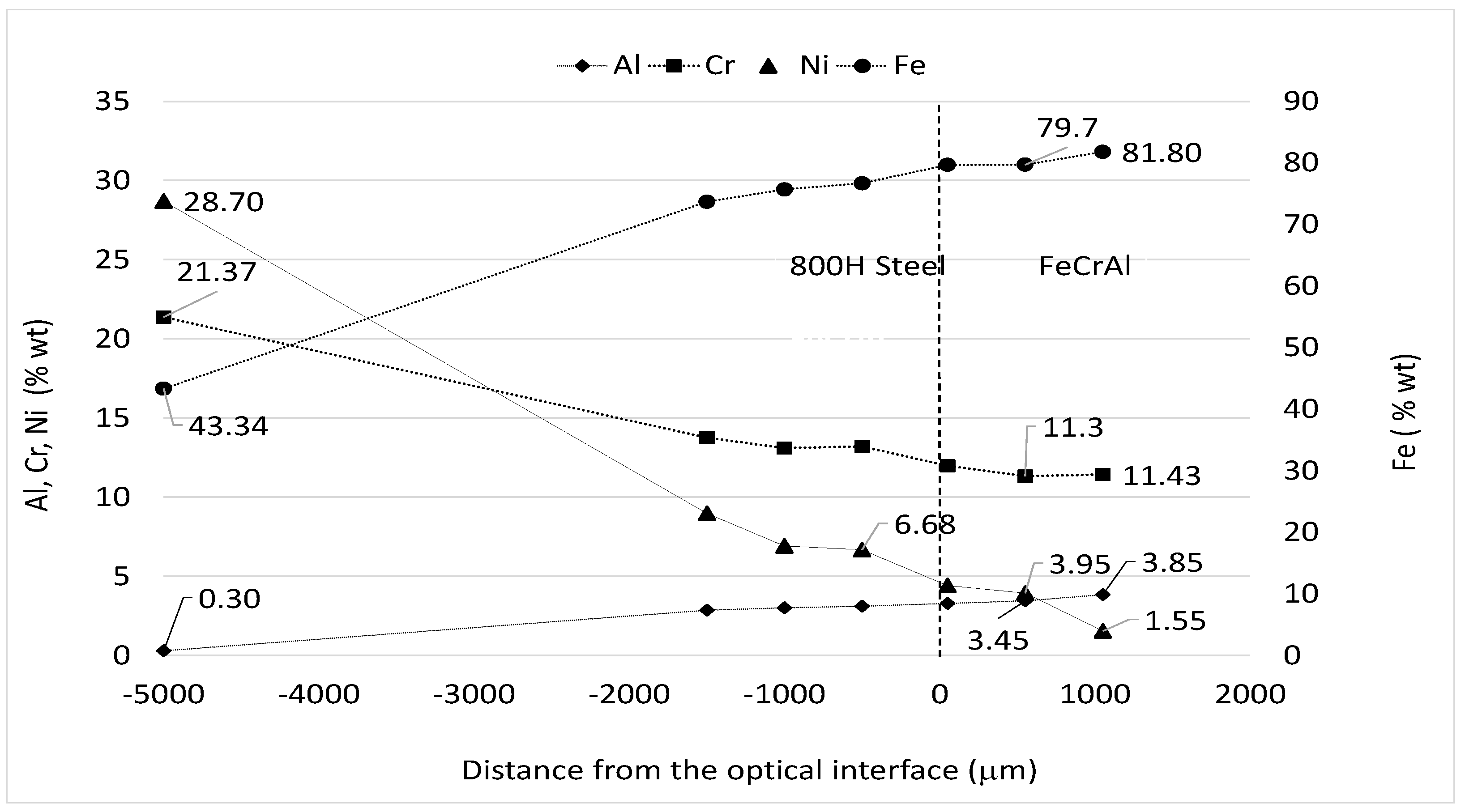
| Material (%wt) | Fe | Cr | Al | Si | Mn | C | Ni | RE |
|---|---|---|---|---|---|---|---|---|
| Nextower 1 | BAL | 9.5–13 | 3.8–4.2 | <0.5 | <0.7 | <0.08 | <0.5 | 0.33 |
| Nextower 2 | BAL | 11–14 | 3.2–4.2 | 1–2 | <0.7 | <0.08 | <0.5 | 0.59 |
| Material | C | Si | Cr | Ni | Al | Ti | W | Co | Cu | Nb | Mo | Fe |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 253MA | 0.08 | 1.6 | 21 | 11 | - | - | - | - | - | - | - | BAL |
| Sanicro 25 | <0.1 | 0.2 | 22.5 | 25 | - | - | 3.6 | 1.5 | 3.0 | 0.5 | - | BAL |
| Sanicro 31HT | 0.07 | 0.6 | 20.5 | 30.5 | 0.5 | 0.5 | - | - | - | - | - | BAL |
| Steel 800H | <0.1 | <0.6 | 21 | 31 | <0.6 | <0.6 | 0.5 | BAL | ||||
| IN617 | 0.1 | <1.0 | 22 | 44.5 | 1.3 | - | - | 10–15 | - | - | 9.0 | <3.0 |
| Material | Creep (10,000 h) | Creep (100,000 h) | Ref. |
|---|---|---|---|
| 253MA | T = 800 °C—σ = 28 MPa; | σ = 15 MPa | Sandvik 253MA data sheet [35] |
| Sanicro 25 | T = 800 °C—σ = 50 MPa | σ = 25 MPa | Sandvik Sanicro 25 data sheet [36] |
| Sanicro 31HT | T = 800 °C | σ = 27 MPa | Sandvik Sanicro 31HT data sheet [37] |
| Steel 800H | T = 815 °C—σ = 36 MPa | σ = 27 MPa | Specification Sheet: Alloy 800H/800HT, (UNS N08810, UNS N08811) [38] |
| IN617 | T = 815 °C—σ = 69MPa; T = 870 °C—σ = 40 MPa | - | IN617—Special Metals Data Sheet, [39] |
| Technique | Coating Speed (mm/s) | Diameter of Wire (mm) | Number of Passes (Nr) | Mean Total Thickness (mm) |
|---|---|---|---|---|
| Gas Tungsten Arc Welding | 20 | 1.3 | 4 | 5.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rinaldi, A.; Barbieri, G.; Kosykh, E.; Szakalos, P.; Testani, C. Materials for High Temperature Liquid Lead Storage for Concentrated Solar Power (CSP) Air Tower Systems. Materials 2021, 14, 3261. https://doi.org/10.3390/ma14123261
Rinaldi A, Barbieri G, Kosykh E, Szakalos P, Testani C. Materials for High Temperature Liquid Lead Storage for Concentrated Solar Power (CSP) Air Tower Systems. Materials. 2021; 14(12):3261. https://doi.org/10.3390/ma14123261
Chicago/Turabian StyleRinaldi, Antonio, Giuseppe Barbieri, Eduard Kosykh, Peter Szakalos, and Claudio Testani. 2021. "Materials for High Temperature Liquid Lead Storage for Concentrated Solar Power (CSP) Air Tower Systems" Materials 14, no. 12: 3261. https://doi.org/10.3390/ma14123261







