Comparative Investigation of Collagen-Based Hybrid 3D Structures for Potential Biomedical Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
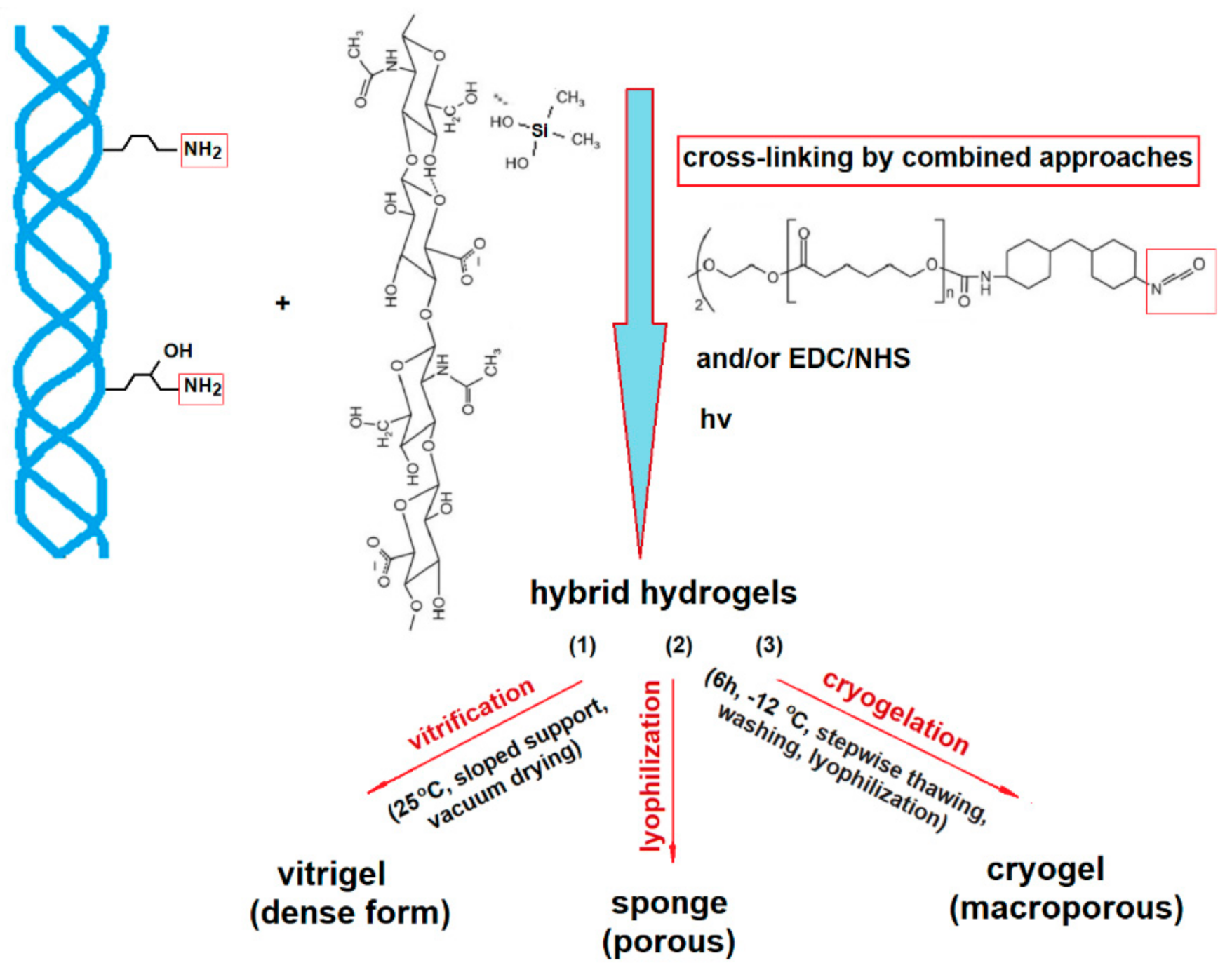
2.2. Hybrid 3D Structures Preparation
2.3. Methods
2.4. Drug Loading and Release
3. Results and Discussion
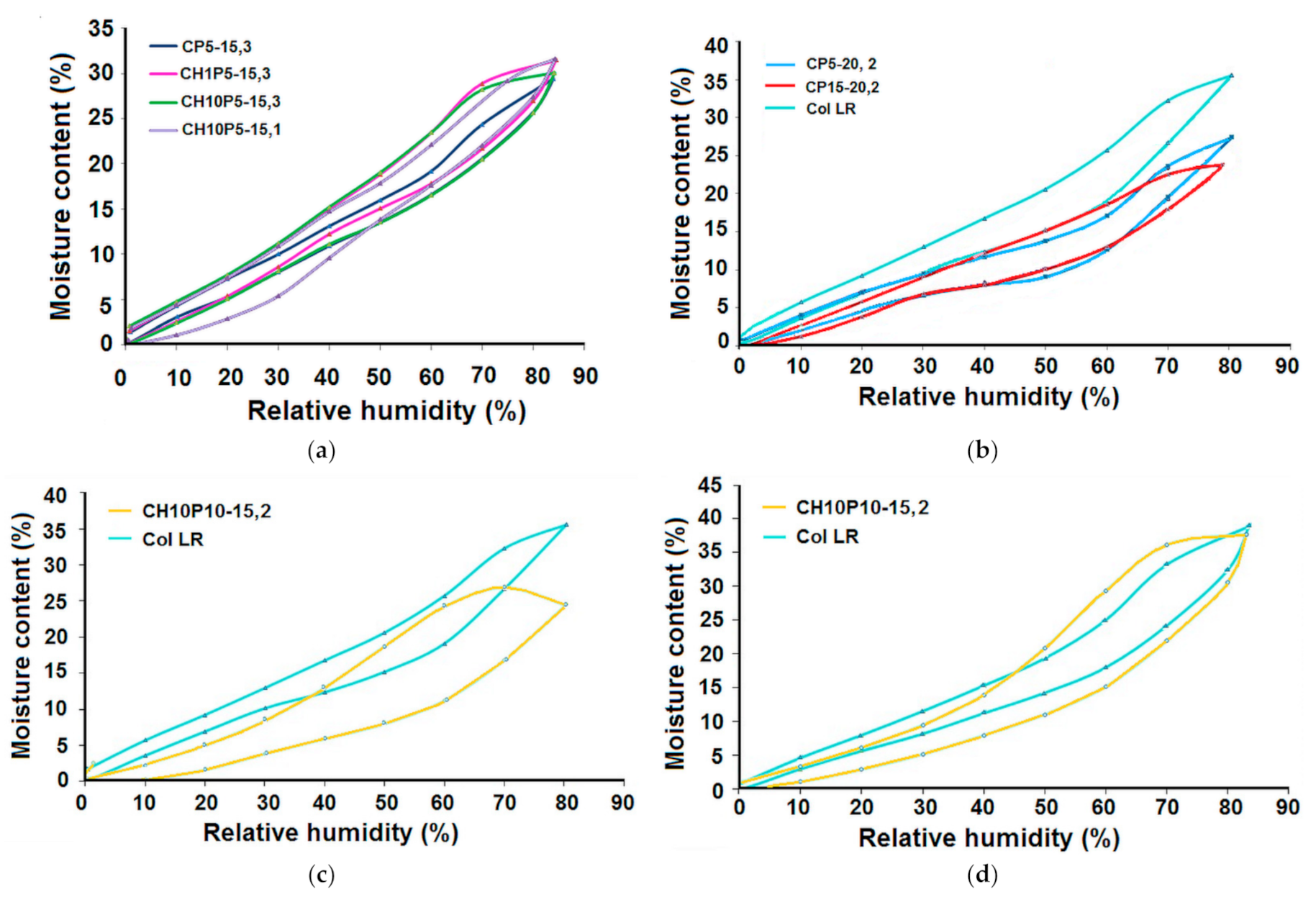
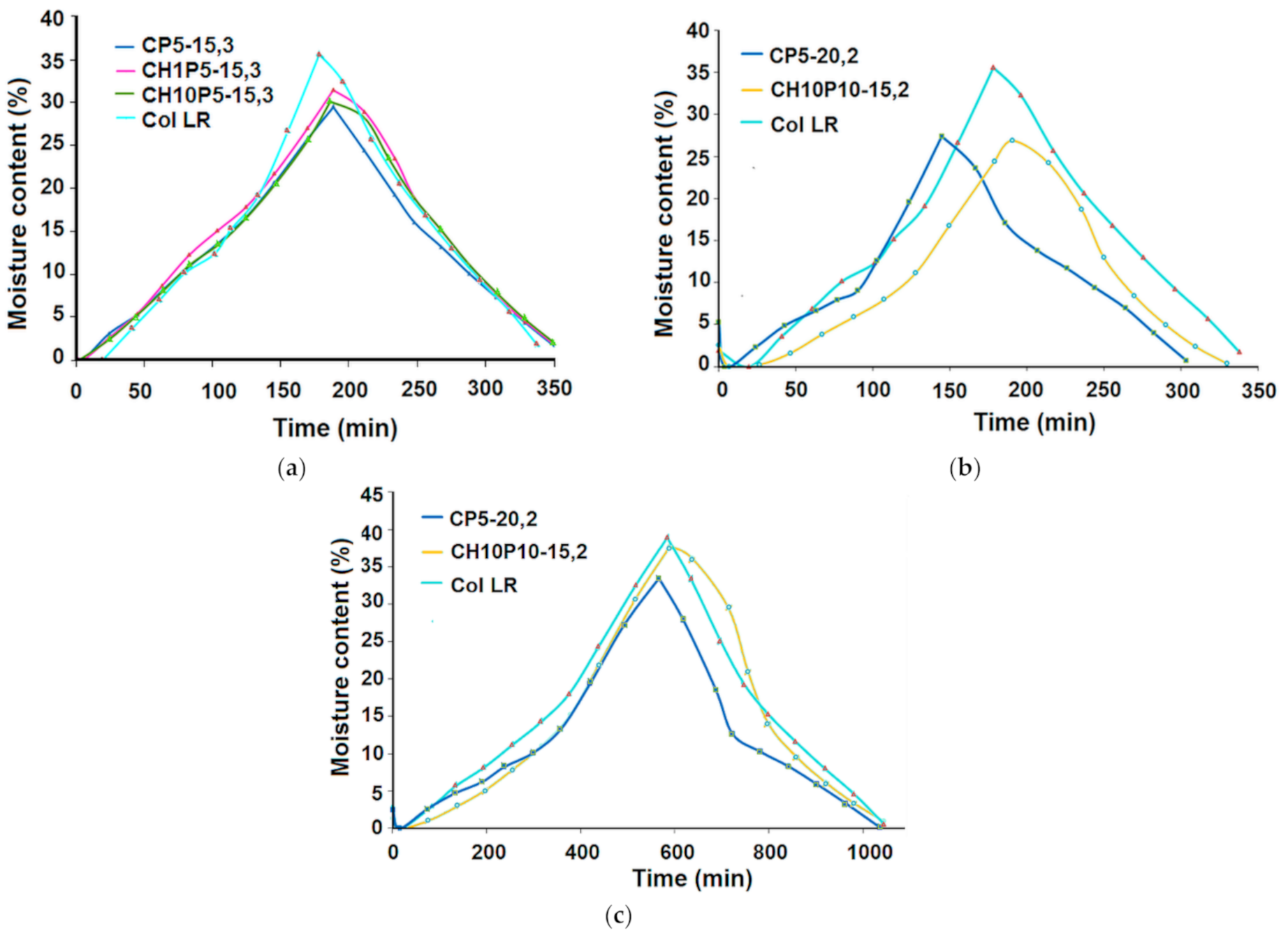
3.1. Water Uptake Ability and Water Vapour Sorption Behaviour
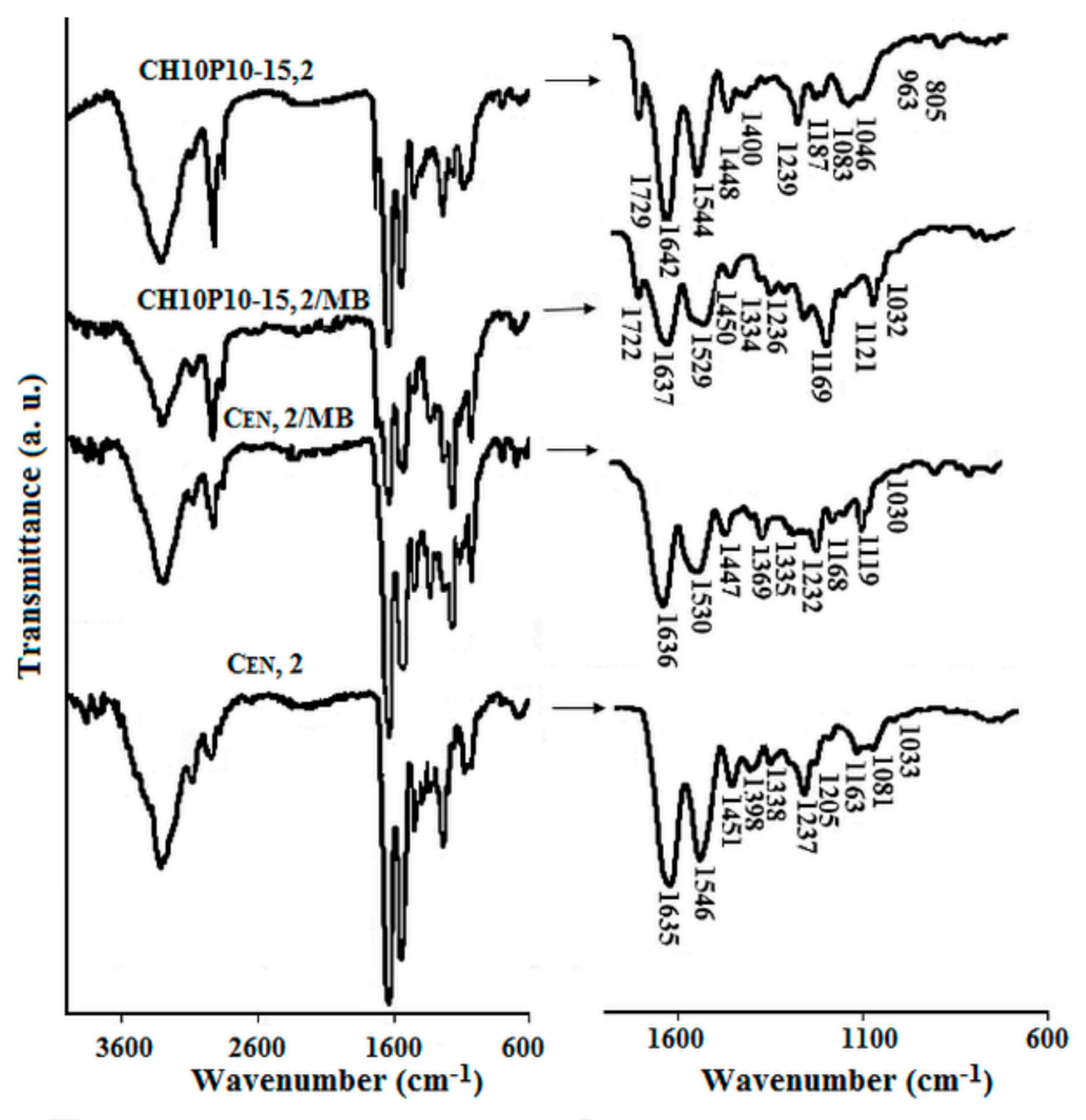
3.2. Characterization of Drug Loaded Collagen-Based Matrices
3.2.1. Loading with Drug Models
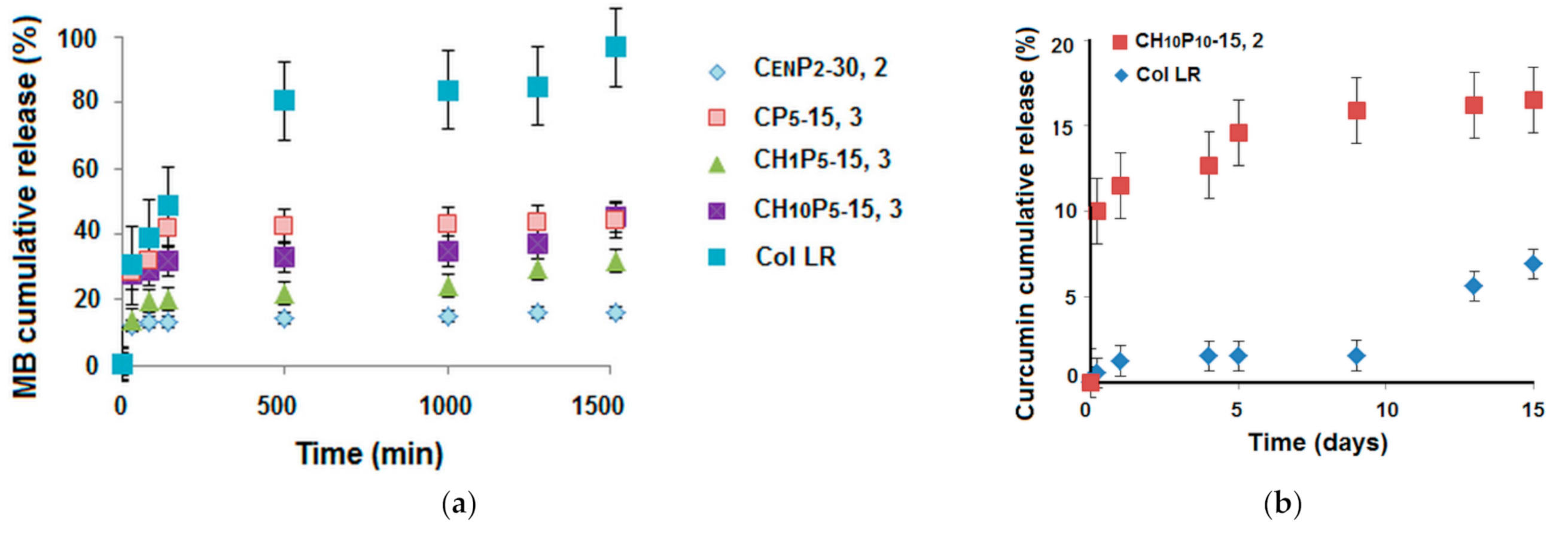
3.2.2. Drug Delivery
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- David, G. Collagen–based 3D structures–versatile, efficient materials for biomedical applications. In Biopolymer-Based Formulations: Biomedical and Food Applications; Pal, K., Banerjee, I., Sarkar, P., Kim, D., Deng, W.-P., Dubey, N.K., Majumder, K., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 881–906. [Google Scholar]
- Chattopadhyay, S.; Raines, R.T. Collagen–based biomaterials for wound healing. Biopolymers 2014, 101, 821–833. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.; Lu, H.; Kawazoe, N.; Chen, G. Pore size effect of collagen scaffolds on cartilage regeneration. Acta Biomater. 2014, 10, 2005–2013. [Google Scholar] [CrossRef] [PubMed]
- Higuchi, A.; Ling, Q.-D.; Hsu, S.-T.; Umezawa, A. Biomimetic cell culture proteins as extracellular matrices for stem cell differentiation. Chem. Rev. 2012, 112, 4507–4540. [Google Scholar] [CrossRef] [PubMed]
- Gordon, P.R.; Bailey, A.J. Chemical stabilisation of collagen as a biomimetic. Sci. World J. 2003, 3, 138–155. [Google Scholar]
- Yu, X.; Tang, C.; Xiong, S.; Yuan, Q.; Gu, Z.; Li, Z.; Hu, Y. Modification of Collagen for Biomedical Applications: A Review of Physical and Chemical Methods. Curr. Org. Chem. 2016, 20, 1797–1812. [Google Scholar] [CrossRef]
- Pan, Z.; Ye, H.; Wu, D. Recent advances on polymeric hydrogels as wound dressings. APL Bioeng. 2021, 5, 011504. [Google Scholar] [CrossRef]
- Stoica, A.E.; Chircov, C.; Grumezescu, A.M. Hydrogel Dressings for the Treatment of Burn Wounds: An Up-To-Date Overview. Materials 2020, 13, 2853. [Google Scholar] [CrossRef]
- Zhang, D.; Wu, X.; Chen, J.; Lin, K. The development of collagen based composite scaffolds for bone regeneration. Bioact. Mater. 2018, 3, 129–138. [Google Scholar] [CrossRef]
- Madaghiele, M.; Demitri, C.; Sannino, A.; Ambrosio, L. Polymeric hydrogels for burn wound care: Advanced skin wound dressings and regenerative templates. Burns Trauma 2014, 2, 153–161. [Google Scholar] [CrossRef] [Green Version]
- Dodwad, V.; Vaish, S.; Mahajan, A.; Chhokra, M. Local drug delivery in periodontics: A strategic intervention. Int. J. Pharm. Pharm. Sci. 2012, 4, 30–34. [Google Scholar]
- Yannas, I.V.; Tzeranis, D.S.; Harley, B.A.; So, P.T.C. Biologically active collagen-based scaffolds: Advances in processing and characterization. Phil. Trans. R. Soc. A 2010, 368, 2123–2139. [Google Scholar] [CrossRef] [Green Version]
- Niiyama, H.; Kuroyanagi, Y. Development of novel wound dressing composed of hyaluronic acid and collagen sponge containing epidermal growth factor and vitamin C derivative. J. Artif. Organs 2014, 17, 81–87. [Google Scholar] [CrossRef]
- Ying, H.; Zhou, J.; Wang, M.; Su, D.; Ma, Q.; Lv, G.; Chen, J. In situ formed collagen-hyaluronic acid hydrogel as biomimetic dressing for promoting spontaneous wound healing. J. Mater. Sci. Eng. C 2019, 101, 487–498. [Google Scholar] [CrossRef]
- Tavakoli, S.; Klar, A.S. Advanced hydrogels as wound dressings. Biomolecules 2020, 10, 1169. [Google Scholar] [CrossRef] [PubMed]
- Saghazadeh, S.; Rinoldi, C.; Schot, M.; Kashaf, S.S.; Sharifi, F.; Jalilian, E.; Nuutila, K.; Giatsidis, G.; Mostafalu, P.; Derakhshandeh, H.; et al. Drug delivery systems and materials for wound healing applications. Adv. Drug Deliv. Rev. 2018, 127, 138–166. [Google Scholar] [CrossRef] [PubMed]
- Litwiniuk, M.; Krejner, A.; Speyrer, M.S.; Gauto, A.R.; Grzela, T. Hyaluronic Acid in Inflammation and Tissue Regeneration. Wounds 2016, 28, 78–88. [Google Scholar] [PubMed]
- Diaconescu, R.; Simionescu, B.C.; David, G. Control and prediction of degradation of biopolymer based hydrogels with poly(ε-caprolactone) subunits. Int. J. Biol. Macromol. 2014, 71, 147–154. [Google Scholar] [CrossRef]
- Huang, G.; Huang, H. Application of hyaluronic acid as carriers in drug delivery. Drug Deliv. 2018, 25, 766–772. [Google Scholar] [CrossRef]
- Available online: www.biosiltech.com/ingredients/dsh-cn (accessed on 10 June 2021).
- Wainwright, M.; Crossley, K.B. Methylene Blue–a Therapeutic Dye for All Seasons? J. Chemother. 2002, 14, 431–443. [Google Scholar] [CrossRef]
- Hussain, Z.; Thu, H.E.; Amjad, M.W.; Hussain, F.; Ahmed, T.A.; Khan, S. Exploring recent developments to improve antioxidant, anti-inflammatory and antimicrobial efficacy of curcumin: A review of new trends and future perspectives. Mater. Sci. Eng. 2017, C77, 1316–1326. [Google Scholar] [CrossRef]
- Anand, P.; Kunnumakkara, A.B.; Newman, R.A.; Aggarwal, B.B. Bioavailability of curcumin: Problems and promises. Mol. Pharm. 2007, 4, 807–818. [Google Scholar] [CrossRef]
- Jankun, J.; Wyganowska-Świątkowska, M.; Dettlaff, K.; Jelińska, A.; Surdacka, A.; Wątróbska-Świetlikowska, D.; Skrzypczak-Jankun, E. Determining whether curcumin degradation/condensation is actually bioactivation (Review). Int. J. Mol. Med. 2016, 37, 1151–1158. [Google Scholar] [CrossRef] [Green Version]
- Maier, S.; Maier, V.; Buciscanu, I. Novel procedure for large-scale purification of atelocollagen, by selective precipitation. J. Am. Leather Chem. Assoc. 2010, 1051–1058. [Google Scholar]
- David, G.; Simionescu, B.C.; Maier, S.; Balhui, C. Micro-/nanostructured polymeric materials: Poly(e-caprolactone) crosslinked collagen sponges. Dig. J. Nanomater. Biostruct. 2011, 6, 1575–1585. [Google Scholar]
- David, G.; Cristea, M.; Balhui, C.; Timpu, D.; Doroftei, F.; Simionescu, B.C. Effect of cross-linking methods on structure and properties of poly(e-caprolactone) stabilized hydrogels containing biopolymers. Biomacromolecules 2012, 13, 2263–2272. [Google Scholar] [CrossRef]
- Simionescu, B.C.; Neamtu, A.; Balhui, C.; Danciu, M.; Ivanov, D.; David, G. Macroporous structures based on biodegradable polymers-candidates for biomedical application. J. Biomed. Mater. Res. A 2013, 101A, 2689–2698. [Google Scholar] [CrossRef]
- Loh, Q.L.; Choong, C. Three-dimensional scaffolds for tissue engineering applications: Role of porosity and pore size. Tissue Eng. Part B Rev. 2013, 19, 485–502. [Google Scholar] [CrossRef] [Green Version]
- Brown, D.F.; Kothari, D. Comparison of antibiotic discs from different sources. J. Clin. Pathol. 1975, 28779–28783. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scott, J.E. Proteoglycan-fibrillar collagen interactions. Biochem. J. 1988, 252, 313–323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Movasaghi, Z.; Rehman, S.; Rehman, I.U. Fourier Transform Infrared (FTIR) Spectroscopy of Biological Tissues. App. Spectrosc. Rev. 2008, 43, 134–179. [Google Scholar] [CrossRef]
- Ovchinnikov, O.V.; Evtukhova, A.V.; Kondratenko, T.S.; Smirnov, M.S.; Khokhlov, V.Y.; Erina, O.V. Manifestation of intermolecular interactions in FTIR spectra of methylene blue molecules. Vib. Spectrosc. 2016, 86, 181–189. [Google Scholar] [CrossRef]
- Kolev, T.M.; Velcheva, E.A.; Stamboliyska, B.A.; Spiteller, M. DFT and Experimental Studies of the Structure and Vibrational Spectra of Curcumin. Int. J. Quantum Chem. 2005, 102, 1069–1079. [Google Scholar] [CrossRef]
- Dharunya, G.; Duraipandy, N.; Lakra, R.; Korapatti, P.S.; Jayavel, R.; Kiran, M.S. Curcumin cross–linked collagen aerogels with controlled anti–proteolytic and pro-angiogenic efficacy. Biomed. Mater. 2016, 11, 045011. [Google Scholar] [CrossRef] [PubMed]
- Misra, S.; Heldin, P.; Hascall, V.C.; Karamanos, N.K.; Skandalis, S.S.; Markwald, R.R.; Ghatak, S. Hyaluronan–CD44 interactions as potential targets for cancer therapy. FEBS J. 2011, 278, 1429–1443. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gopinath, D.; Ahmed, M.R.; Gomathi, K.; Chitra, K.; Sehgal, P.K.; Jayakumar, R. Dermal wound healing processes with curcumin incorporated collagen films. Biomaterials 2004, 25, 1911–1917. [Google Scholar] [CrossRef]





| Code | ESR (g × g−1) | Porosity (%) |
|---|---|---|
| Col LR | 27.9 ± 0.3 | 93.5 ± 0.5 |
| CH1P5-15,1 | 5.2 ± 0.2 | 67.0 ± 1.0 |
| CH10P5-15,1 | 3.7 ± 0.1 | 59.0 ±1.5 |
| CH10P30-15,1 | 2.8 ± 0.1 | 54.0 ± 0.5 |
| CP5-10,2 | 38.5 ± 0.4 | 94.0 ± 0.9 |
| CP5-20,2 | 30.1 ± 0.3 | 94.0 ± 0.6 |
| CP15-10,2 | 27.5 ± 0.2 | 93.9 ± 0.5 |
| CP15-20,2 | 26.0 ± 0.5 | 94.5 ± 0.6 |
| CP30-10,2 | 19.5 ± 0.5 | 91.2 ± 0.9 |
| CP30-20,2 | 17.5 ± 0.7 | 92.0 ± 1.1 |
| CEN,2 | 19.0 ± 0.8 | 95.6 ± 0.5 |
| CENP2,2 | 15.0 ± 0.3 | 92.8 ± 1.0 |
| CENP2-30,2 | 28.0 ± 0.5 | 94.9 ± 1.4 |
| CP5-15,3 | 27.0 ± 0.3 | 94.4 ± 0.5 |
| CH1P5-15,3 | 15.0 ± 0.2 | 94.0 ± 0.4 |
| CH10P5-15,3 | 11.0 ± 0.1 | 93.0 ± 0.3 |
| CH10P10-15,2 | 7.0 ± 0.1 | 70.0 ± 0.2 |
| CH10P50-15,3 | 6.0 ± 0.2 | 57.0 ± 0.8 |
| Code | Loading Capacity (mg MB/g Sample) |
|---|---|
| Col LR | 28.5 ± 1.0 |
| CH1P5-15, 1 | 23.8 ± 1.5 |
| CH10P5-15, 1 | 14.4 ± 1.8 |
| CP5-10, 2 | 25.4 ± 2.0 |
| CP5-20, 2 | 17.6 ± 1.6 |
| CP15-10,2 | 13.1 ± 1.2 |
| CP15-20,2 | 18.7 ± 1.0 |
| CP30-10,2 | 22.3 ± 1.7 |
| CP30-20,2 | 37.9 ± 1.0 |
| CEN,2 | 25.9 ± 0.8 |
| CENP2,2 | 32.2 ± 1.0 |
| CENP2-30,2 | 36.1 ± 0.5 |
| CH10P10-15,2 | 14.0 ± 0.5 |
| CP5-15, 3 | 27.9 ± 1.0 |
| CH1P5-15, 3 | 18.0 ± 0.7 |
| CH10P5-15, 3 | 14.9 ± 0.3 |
| CH10P50-15, 3 | 13.0 ± 0.6 |
| Code | Loading Capacity (%) |
|---|---|
| Col LR | 12.7 ± 0.4 |
| CENP2-30,2 | 14.7 ± 0.1 |
| CH10P5-15,3 | 15.0 ± 0.4 |
| CH10P10,2 | 15.4 ± 0.3 |
| CH10P30-15,1 | 16.5 ± 0.2 |
| CH10P50-15,3 | 17.2 ± 0.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
David, G.; Bargan, A.I.; Drobota, M.; Bele, A.; Rosca, I. Comparative Investigation of Collagen-Based Hybrid 3D Structures for Potential Biomedical Applications. Materials 2021, 14, 3313. https://doi.org/10.3390/ma14123313
David G, Bargan AI, Drobota M, Bele A, Rosca I. Comparative Investigation of Collagen-Based Hybrid 3D Structures for Potential Biomedical Applications. Materials. 2021; 14(12):3313. https://doi.org/10.3390/ma14123313
Chicago/Turabian StyleDavid, Geta, Alexandra I. Bargan, Mioara Drobota, Adrian Bele, and Irina Rosca. 2021. "Comparative Investigation of Collagen-Based Hybrid 3D Structures for Potential Biomedical Applications" Materials 14, no. 12: 3313. https://doi.org/10.3390/ma14123313







