Accelerated Bone Induction of Adult Rat Compact Bone Plate Scratched by Ultrasonic Scaler Using Acidic Electrolyzed Water
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials Used and Division of Groups
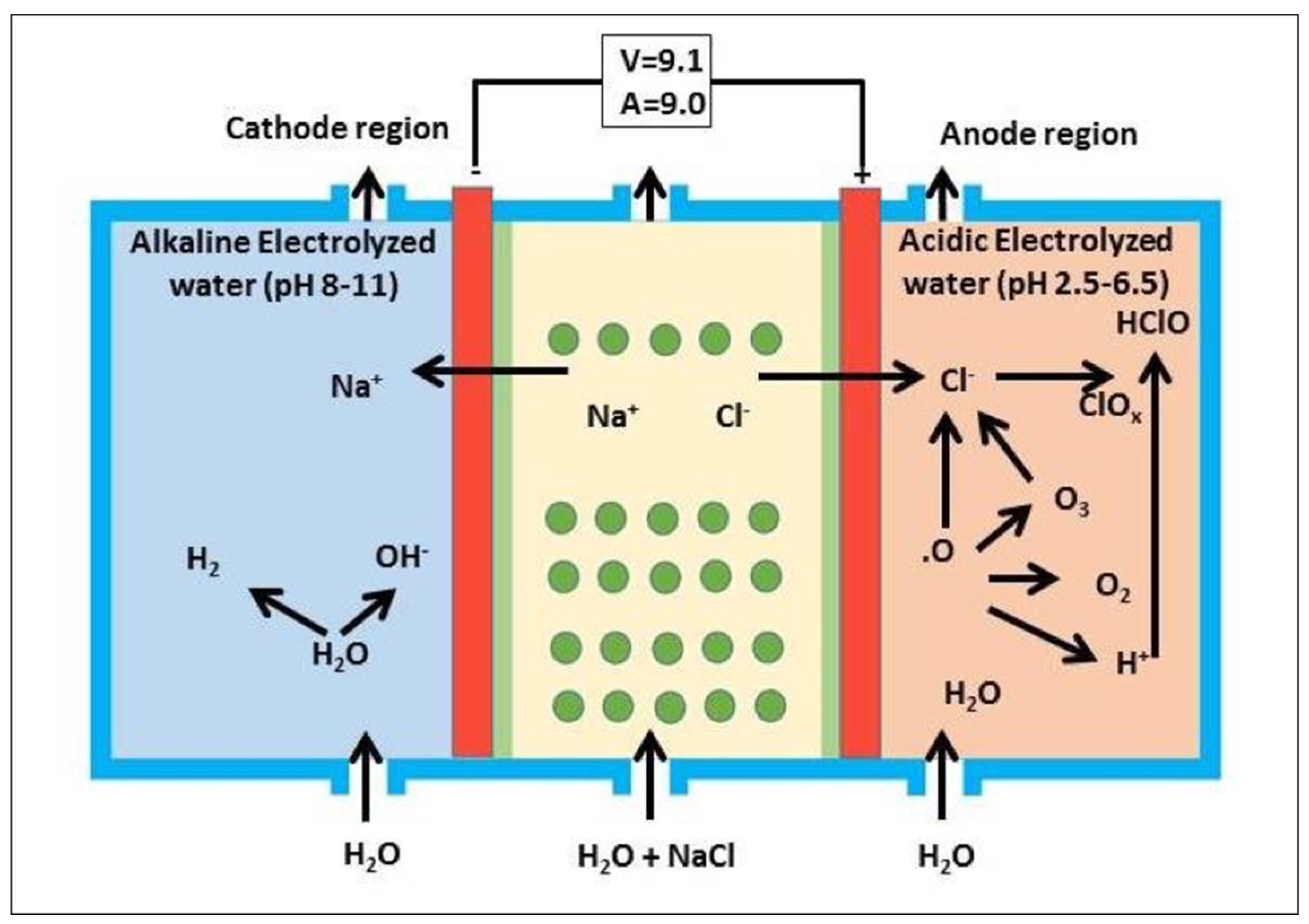
2.1.1. Acidic Electrolyzed Water (AEW)
2.1.2. Distilled Water (DW)
2.1.3. Ultrasonic Device
2.1.4. Animal Model
2.1.5. Division of Groups
2.2. Animal Experiment
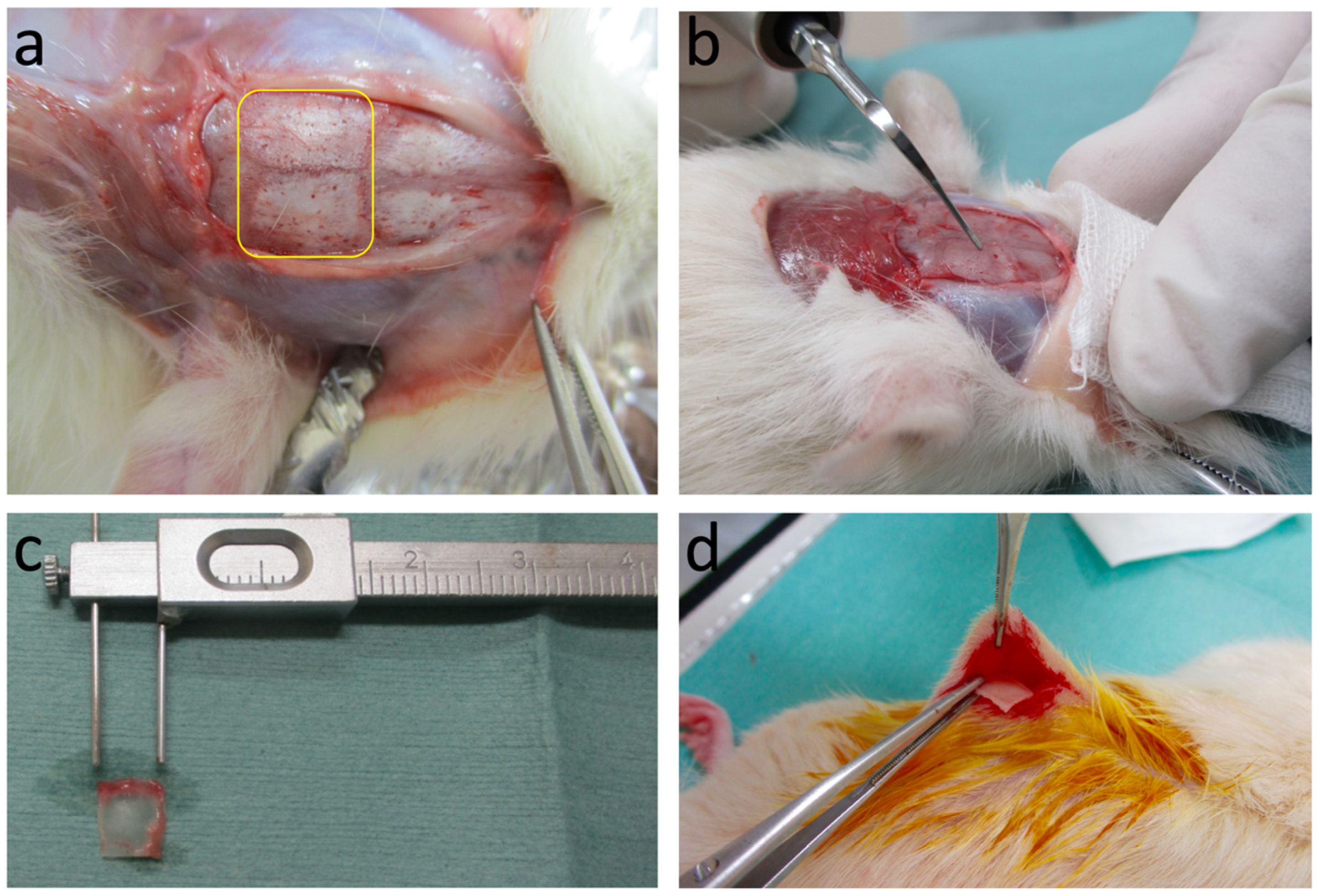
2.2.1. Preparation of Graft Samples
2.2.2. Topographical Analysis
2.2.3. Subcutaneous Graft in Host Rats
2.2.4. Histological Examination
2.2.5. Histomorphometric Measurements
3. Results
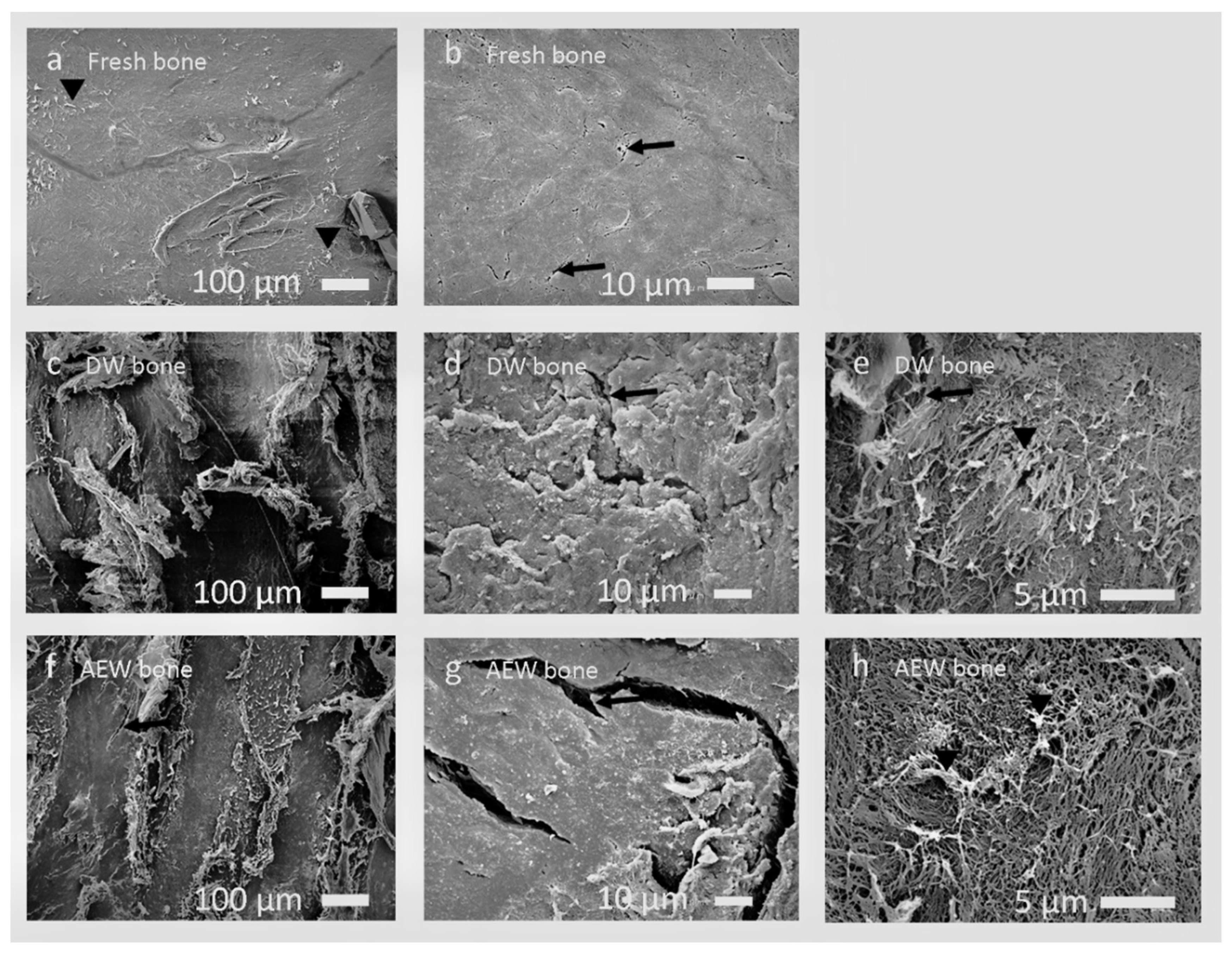
3.1. SEM Observation and EDS Analysis
3.1.1. Fresh Bone Group
3.1.2. DW Bone Group
3.1.3. AEW Bone Group
3.1.4. EDS Analysis
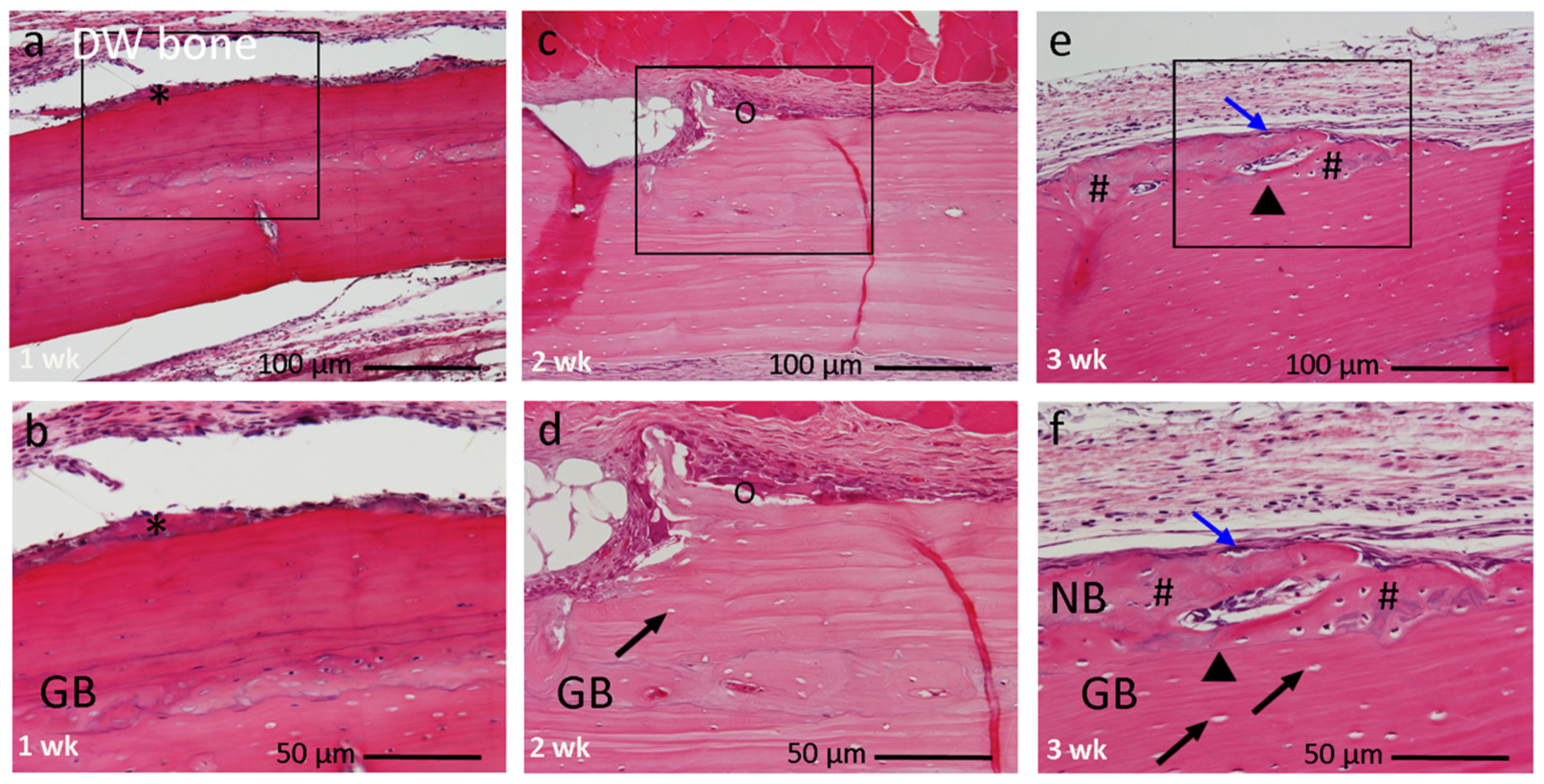
3.2. Histological Findings
3.2.1. Fresh Bone Group
3.2.2. DW Bone Group
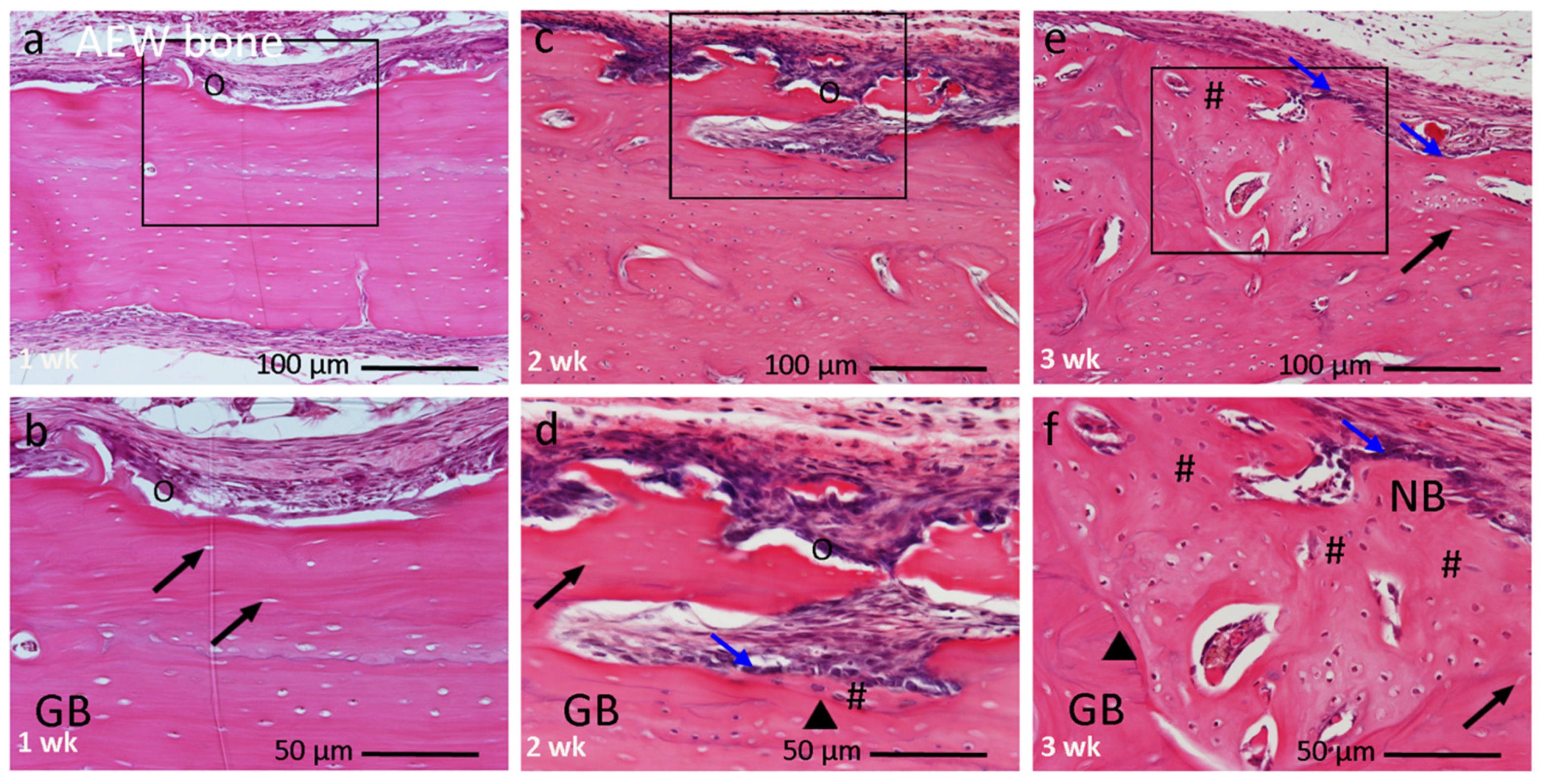
3.2.3. AEW Bone Group
3.3. Histomorphometric Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Florencio-Silva, R.; Sasso, G.R.d.S.; Sasso-Cerri, E.; Simoes, M.J.; Cerri, P.S. Biology of bone tissue: Structure, function, and factors that influence bone cells. BioMed Res. Int. 2015, 2015, 17. [Google Scholar] [CrossRef] [PubMed]
- Hauschka, P.V.; Mavrakos, A.E.; Iafrati, M.D.; Doleman, S.E.; Klagsbrun, M. Growth factors in bone matrix. Isolation of multiple types by affinity chromatography on heparin-Sepharose. J. Biol. Chem. 1986, 261, 12665–12674. [Google Scholar] [CrossRef]
- Bauer, T.W.; Muschler, G.F. Bone graft materials. An overview of the basic science. Clin. Orthop. Relat. Res. 2000, 371, 10–27. [Google Scholar] [CrossRef]
- Kikuchi, M.; Itoh, S.; Ichinose, S.; Shinomiya, K.; Tanaka, J. Self-organization mechanism in a bone-like hydroxyapatite/collagen nanocomposite synthesized in vitro and its biological reaction in vivo. Biomaterials 2001, 22, 1705–1711. [Google Scholar] [CrossRef]
- Urist, M.R. Bone: Formation by autoinduction. Science 1965, 150, 893. [Google Scholar] [CrossRef] [PubMed]
- Asahina, I. Bone morphogenetic proteins: Their history and characteristics. J. Hard Tissue Biol. 2014, 23, 283–286. [Google Scholar] [CrossRef][Green Version]
- Wang, W.; Yeung, K.W.K. Bone grafts and biomaterials substitutes for bone defect repair: A review. Bioact. Mater. 2017, 2, 224–247. [Google Scholar] [CrossRef]
- Solheim, E. Growth factors in bone. Int. Orthop. 1998, 22, 410–416. [Google Scholar] [CrossRef]
- Figueiredo, M.; Cunha, S.; Martins, G.; Freitas, J.; Judas, F.; Figueiredo, H. Influence of hydrochloric acid concentration on the demineralization of cortical bone. Chem. Eng. Res. Des. 2011, 89, 116–124. [Google Scholar] [CrossRef]
- Pietrzak, W.S.; Ali, S.N.; Chitturi, D.; Jacob, M.; Woodell-May, J.E. BMP depletion occurs during prolonged acid demineralization of bone: Characterization and implications for graft preparation. Cell Tissue Bank 2011, 12, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Bonde, M.R.; Nester, S.E.; Khayat, A.; Smilanick, J.L.; Frederick, R.D.; Schaad, N.W. Comparison of effects of acidic electrolyzed water and NaOCl on Tilletia indica teliospore germination. Plant Dis. 1999, 83, 627–632. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.-R.; Hung, Y.-C.; Hsu, S.-Y.; Huang, Y.-W.; Hwang, D.-F. Application of electrolyzed water in the food industry. Food Control 2008, 19, 329–345. [Google Scholar] [CrossRef]
- Nakano, Y.; Akamatsu, N.; Mori, T.; Sano, K.; Satoh, K.; Nagayasu, T.; Miyoshi, Y.; Sugio, T.; Sakai, H.; Sakae, E.; et al. Sequential washing with electrolyzed alkaline and acidic water effectively removes pathogens from metal surfaces. PLoS ONE 2016, 11, e0156058. [Google Scholar] [CrossRef]
- Akazawa, T.; Murata, M.; Tabata, Y.; Ito, M. Microstructure and biocompatibility of hydroxyapatite porous ceramics designed by a partial dissolution-precipitation technique with supersonic treatment for bone regeneration. In Bone Regeneration; Tal, H., Ed.; InTech: Rijeka, Croatia, 2012; pp. 283–300. [Google Scholar]
- Akazawa, T.; Murata, M.; Minamida, Y.; Tingting, W.; Kabir, A.; Hino, J.; Tazaki, J.; Manabu, I.; Kimura, I. Bioactive surface structure and bio-absorption of human dentin granules designed by the supersonic demineralization and biomimetic coating technique. J. Hard Tissue Biol. 2012, 21, 351–358. [Google Scholar] [CrossRef][Green Version]
- Walmsley, A.D.; Laird, W.R.E.; Williams, A.R. Gas bubble fragmentation in an ultrasonic field. Ultrasonics 1985, 23, 170–172. [Google Scholar] [CrossRef]
- Walmsley, A.D.; Laird, W.R.E.; Williams, A.R. Dental plaque removal by cavitational activity during ultrasonic scaling. J. Clin. Periodontol. 1988, 15, 539–543. [Google Scholar] [CrossRef] [PubMed]
- Roberts, T.T.; Rosenbaum, A.J. Bone grafts, bone substitutes and orthobiologics: The bridge between basic science and clinical advancements in fracture healing. Organogenesis 2012, 8, 114–124. [Google Scholar] [CrossRef]
- Bae, D.S.; Waters, P.M.; Gebhardt, M.C. Results of free vascularized fibula grafting for allograft nonunion after limb salvage surgery for malignant bone tumors. J. Pediatr. Orthop. 2006, 26, 809–814. [Google Scholar] [CrossRef] [PubMed]
- Weibel, E.R. Measuring through the microscope: Development and evolution of stereological methods. J. Microsc. 1989, 155, 393–403. [Google Scholar] [CrossRef]
- Amler, M.H. Age factor in human alveolar bone repair. J. Oral. Implantol. 1993, 19, 138–142. [Google Scholar]
- Nagai, N.; Qin, C.-L.; Nagatsuka, H.; Inoue, M.; Ishiwari, Y. Age effects on ectopic bone formation induced by purified bone morphogenetic protein. Int. J. Oral Maxillofac. Surg. 1999, 28, 143–150. [Google Scholar] [CrossRef]
- Jergesen, H.E.; Chua, J.; Kao, R.T.; Kaban, L.B. Age Effects on Bone Induction by Demineralized Bone Powder. Clin. Orthop. Relat. Res. 1991, 268, 253–259. [Google Scholar]
- Qin, C.-L.; Murata, M.; Nagatsuka, H.; Inoue, M.; Nagai, N. Aging and ectopic bone formation induced by partially purified bone morphogenetic protein. Jpn. J. Oral Biol. 1997, 39, 572–582. [Google Scholar] [CrossRef]
- Sengupta, P. The laboratory rat: Relating its age with human’s. Int. J. Prev. Med. 2013, 4, 624–630. [Google Scholar]
- Lin, F.H.; Chen, T.M.; Lin, C.P.; Lee, C.J. The merit of sintered PDLLA/TCP composites in management of bone fracture internal fixation. Artif. Organs 1999, 23, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Zadpoor, A.A. Bone tissue regeneration: The role of scaffold geometry. Biomater. Sci. 2015, 3, 231–245. [Google Scholar] [CrossRef]
- Zerbo, I.R.; Bronckers, A.L.J.J.; De Lange, G.L.; Burger, E.H.; Van Beek, G.J. Histology of human alveolar bone regeneration with a porous tricalcium phosphate. Clin. Oral Implants Res. 2001, 12, 379–384. [Google Scholar] [CrossRef]
- Kuboki, Y.; Takita, H.; Kobayashi, D.; Tsuruga, E.; Inoue, M.; Murata, M.; Nagai, N.; Dohi, Y.; Ohgushi, H. BMP-Induced osteogenesis on the surface of hydroxyapatite with geometrically feasible and nonfeasible structures: Topology of osteogenesis. J. Biomed. Mater. Res. 1998, 39, 190–199. [Google Scholar] [CrossRef]
- Urist, M.R.; Strates, B.S. The classic: Bone morphogenetic protein. Clin. Orthop. Relat. Res. 2009, 467, 3051–3062. [Google Scholar] [CrossRef] [PubMed]
- Blum, B.; Moseley, J.; Miller, L.; Richelsoph, K.; Haggard, W. Measurement of Bone Morphogenetic Proteins and Other Growth Factors in Demineralized Bone Matrix. Orthopedics 2004, 27, S161–S165. [Google Scholar] [CrossRef]
- Kimura, I.; Wei, T.; Akazawa, T.; Murata, M. Characterization of demineralization behavior of bovine bone granules related to particulate properties. Adv. Powder Technol. 2017, 28, 740–746. [Google Scholar] [CrossRef]
- Mo, X.T.; Yang, Z.M.; Qin, T.W. Effects of 20% demineralization on surface physical properties of compact bone scaffold and bone remodeling response at interface after orthotopic implantation. Bone 2009, 45, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.K.; Wang, C.H.; Ting, C.C.; Wei, S.I.; Du, J.K. Effect of different acidities of electrolyzed water on dentin surface roughness, decalcification and microhardness -a preliminary study. Dent. Mater. J. 2016, 35, 803–809. [Google Scholar] [CrossRef] [PubMed]
- Murata, M.; Inoue, M.; Arisue, M.; Kuboki, Y.; Nagal, N. Carrier-dependency of cellular differentiation induced by bone morphogenetic protein in ectopic sites. Int. J. Oral Maxillofac. Surg. 1998, 27, 391–396. [Google Scholar] [CrossRef]
- Sanchez, D.G.; Hiepen, C.; Knaus, P.; Ten, D.P. Bone morphogenetic protein singnaling in bone homeostasis. Bone 2015, 80, 43–59. [Google Scholar] [CrossRef]
- Seref-Ferlengez, Z.; Basta-Pljakic, J.; Kennedy, O.D.; Philemon, C.J.; Schaffler, M.B. Structural and mechanical repair of diffuse damage in cortical bone in vivo. J. Bone Miner. Res. 2014, 29, 2537–2544. [Google Scholar] [CrossRef]
- Prideaux, M.; Findlay, D.M.; Atkins, G.J. Osteocytes: The master cells in bone remodelling. Curr. Opin. Pharmacol. 2016, 28, 24–30. [Google Scholar] [CrossRef]
- Kobayashi, S.; Takahashi, H.E.; Ito, A.; Saito, N.; Nawata, M.; Horiuchi, H.; Ohta, H.; Ito, A.; Iorio, R.; Yamamoto, N.; et al. Trabecular minimodeling in human iliac bone. Bone 2003, 32, 163–169. [Google Scholar] [CrossRef]

| Element | Mean Peak Intensity % ± SD | ||
|---|---|---|---|
| NS | DW | AEW | |
| Carbon | 42.44 | 45.85 | 45.21 ± 1.95 |
| Nitrogen | 37.69 | 37.72 | 38.55 ± 0.53 |
| Oxygen | 17.61 | 14.25 | 15.66 ± 2.05 |
| Sodium | 0.14 | 0.20 | 0.12 ± 0.10 |
| Magnesium | 0.03 | 0.02 | 0.01 ± 0.00 |
| Phosphorus | 1.08 | 1.06 | 0.09 ± 0.01 |
| Chlorine | 0.10 | 0.00 | 0.32 ± 0.05 |
| Calcium | 0.90 | 0.86 | 0.03 ± 0.00 |
| Total | 99.99 | 99.96 | 99.99 |
| Bone Type | Explant Period | ||
|---|---|---|---|
| 1 wk | 2 wk | 3 wk | |
| Fresh bone (%) | 0 | 0 | 0 |
| DW bone (%) | 0 | 0 | 8.2 ± 1.0 ** |
| AEW bone (%) | 0 | 4.2 ± 0.4 ** | 13.4 ± 1.3 ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shakya, M.; Murata, M.; Yokozeki, K.; Akazawa, T.; Nagayasu, H.; Adhikari, B.R.; Upadhyaya, C. Accelerated Bone Induction of Adult Rat Compact Bone Plate Scratched by Ultrasonic Scaler Using Acidic Electrolyzed Water. Materials 2021, 14, 3347. https://doi.org/10.3390/ma14123347
Shakya M, Murata M, Yokozeki K, Akazawa T, Nagayasu H, Adhikari BR, Upadhyaya C. Accelerated Bone Induction of Adult Rat Compact Bone Plate Scratched by Ultrasonic Scaler Using Acidic Electrolyzed Water. Materials. 2021; 14(12):3347. https://doi.org/10.3390/ma14123347
Chicago/Turabian StyleShakya, Mamata, Masaru Murata, Kenji Yokozeki, Toshiyuki Akazawa, Hiroki Nagayasu, Bhoj Raj Adhikari, and Chandan Upadhyaya. 2021. "Accelerated Bone Induction of Adult Rat Compact Bone Plate Scratched by Ultrasonic Scaler Using Acidic Electrolyzed Water" Materials 14, no. 12: 3347. https://doi.org/10.3390/ma14123347
APA StyleShakya, M., Murata, M., Yokozeki, K., Akazawa, T., Nagayasu, H., Adhikari, B. R., & Upadhyaya, C. (2021). Accelerated Bone Induction of Adult Rat Compact Bone Plate Scratched by Ultrasonic Scaler Using Acidic Electrolyzed Water. Materials, 14(12), 3347. https://doi.org/10.3390/ma14123347







