Synthesis and Physicochemical Evaluation of Bees’ Chitosan-Based Hydrogels Modified with Yellow Tea Extract
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
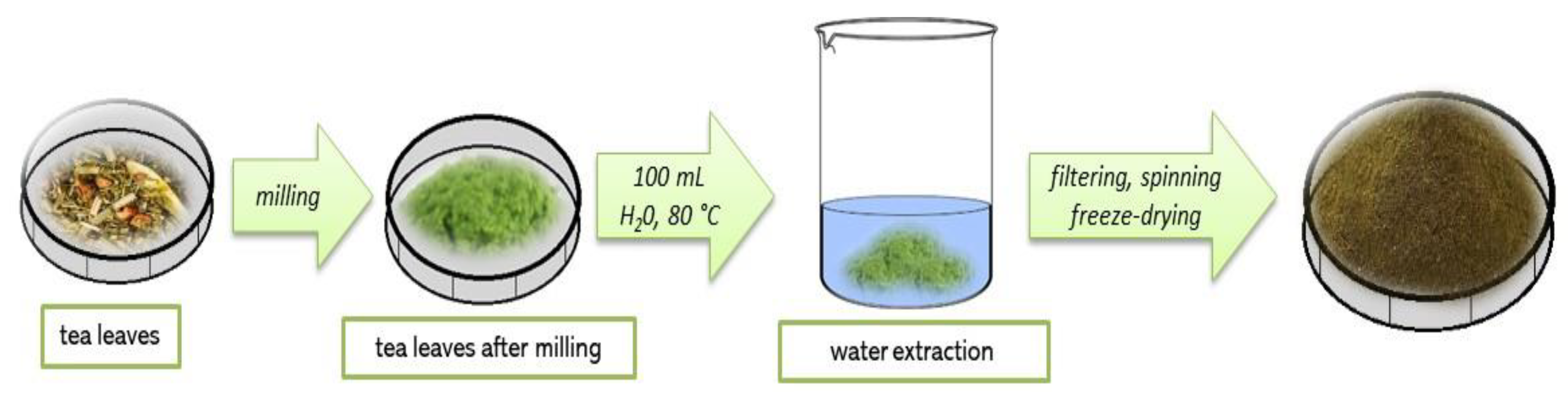
2.2. Preparation of Kekecha Yellow Tea Extract
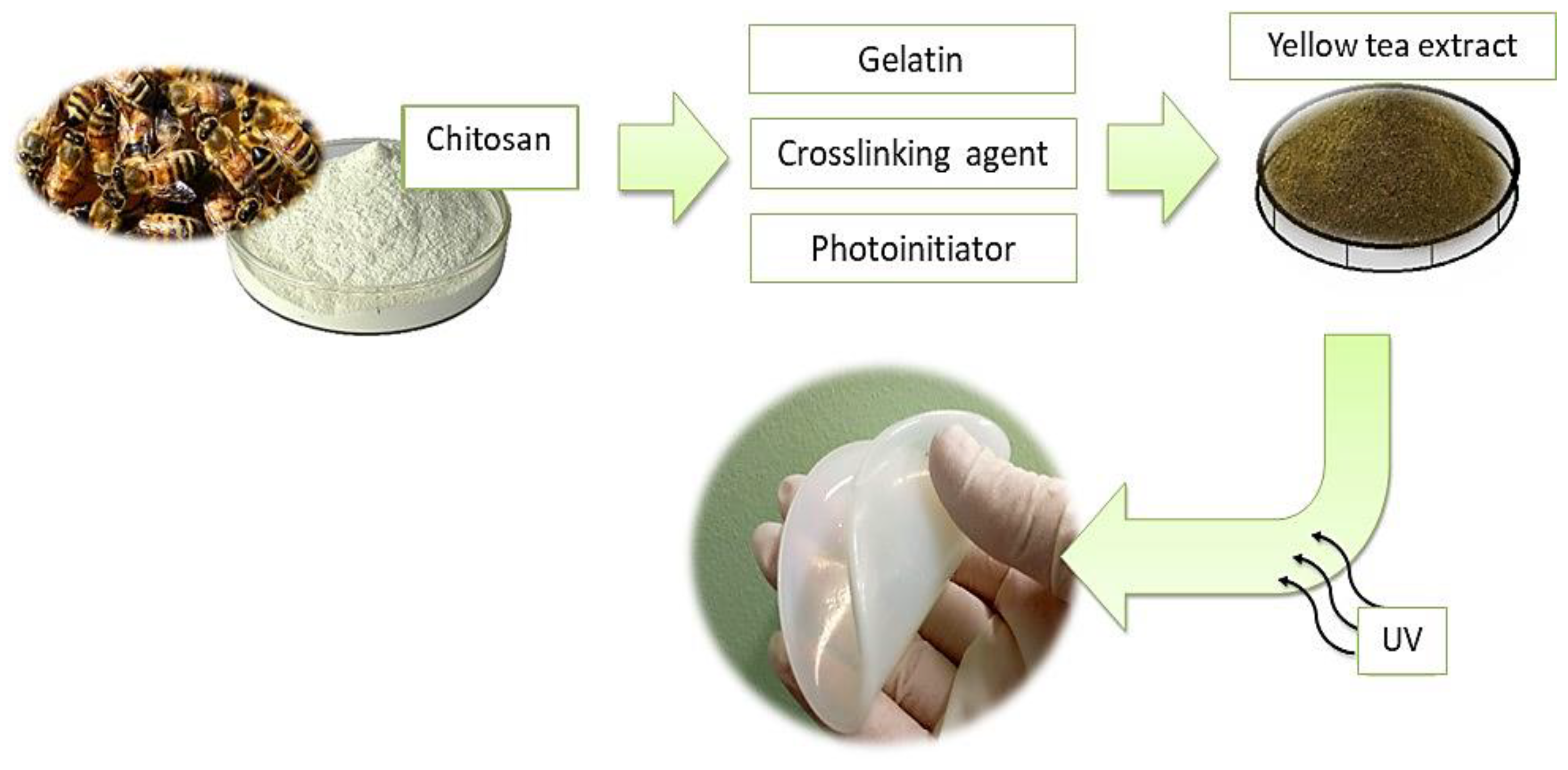
2.3. Synthesis of Hydrogels Modified with Yellow Tea
2.4. Methodology of Measurements
2.4.1. Characterization of the Sorption Properties of Hydrogels
2.4.2. Evaluation of the Mutual Interactions Between Hydrogels and Simulated PhysioLogical Liquids
2.4.3. Analysis of the Chemical Structure of Hydrogels via FT-IR Spectroscopy
2.4.4. Measurements of the Surface Contact Angles of Hydrogels
2.4.5. SEM Analysis of Hydrogel Surfaces
2.4.6. Analysis of the Tensile Strength of Hydrogels
3. Results and Discussion
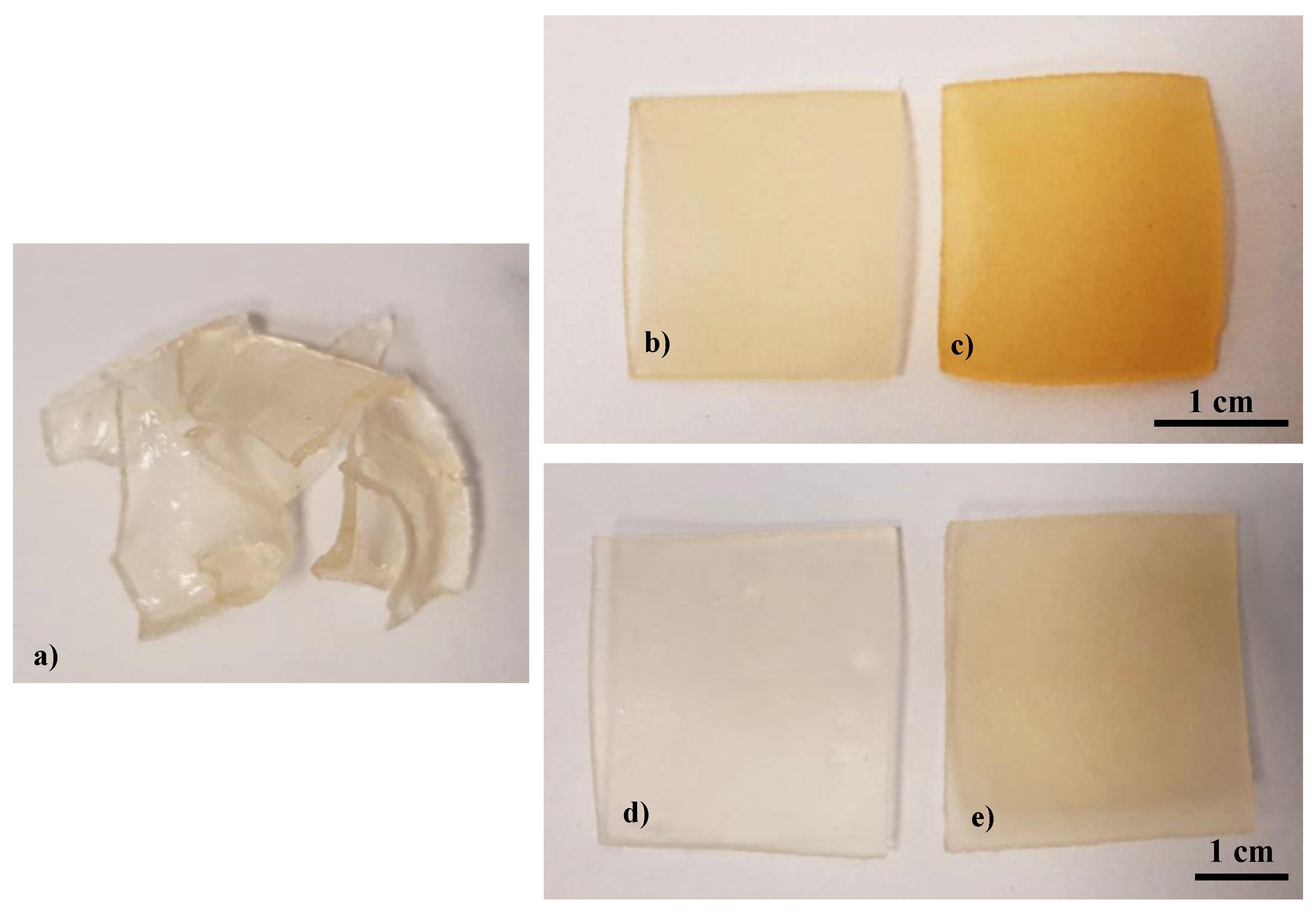
3.1. Synthesis of Hydrogels
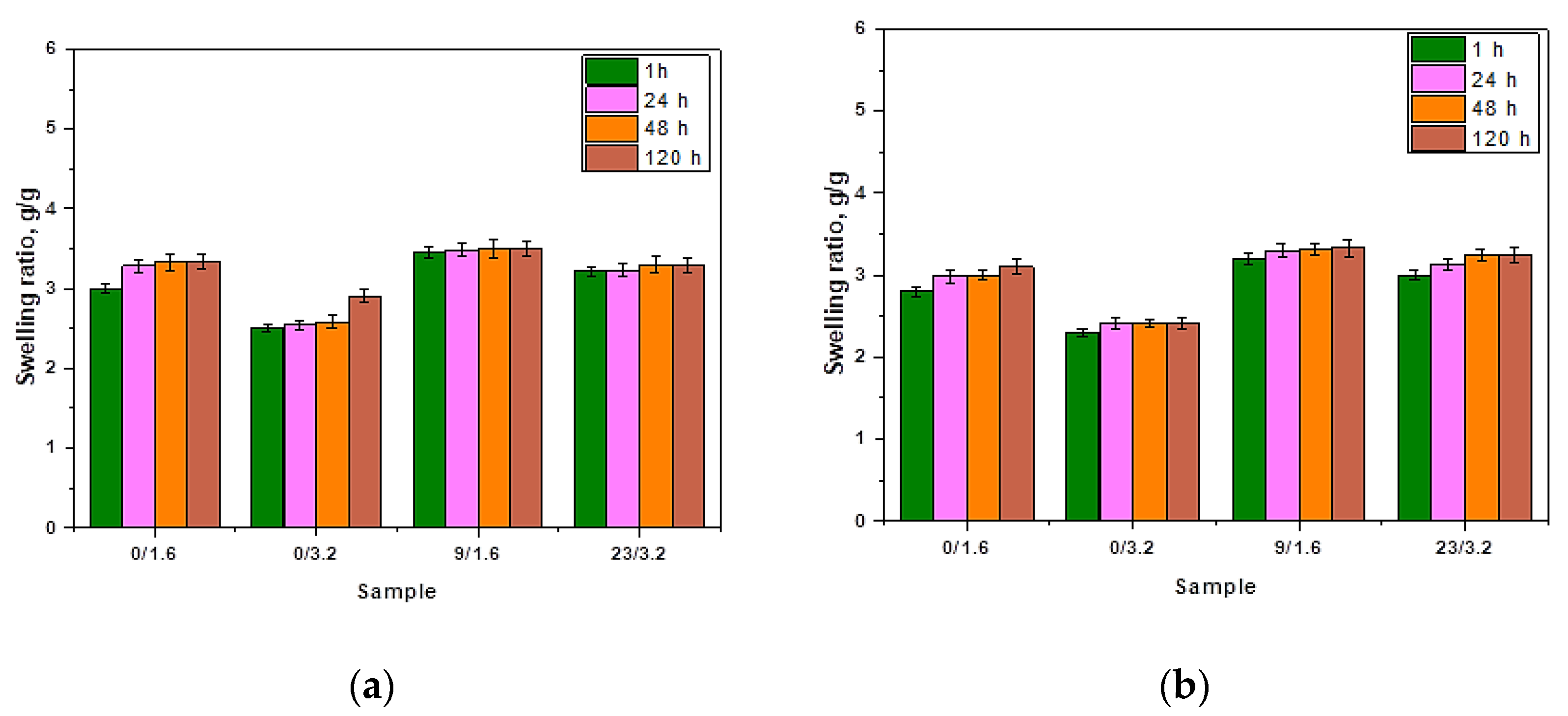
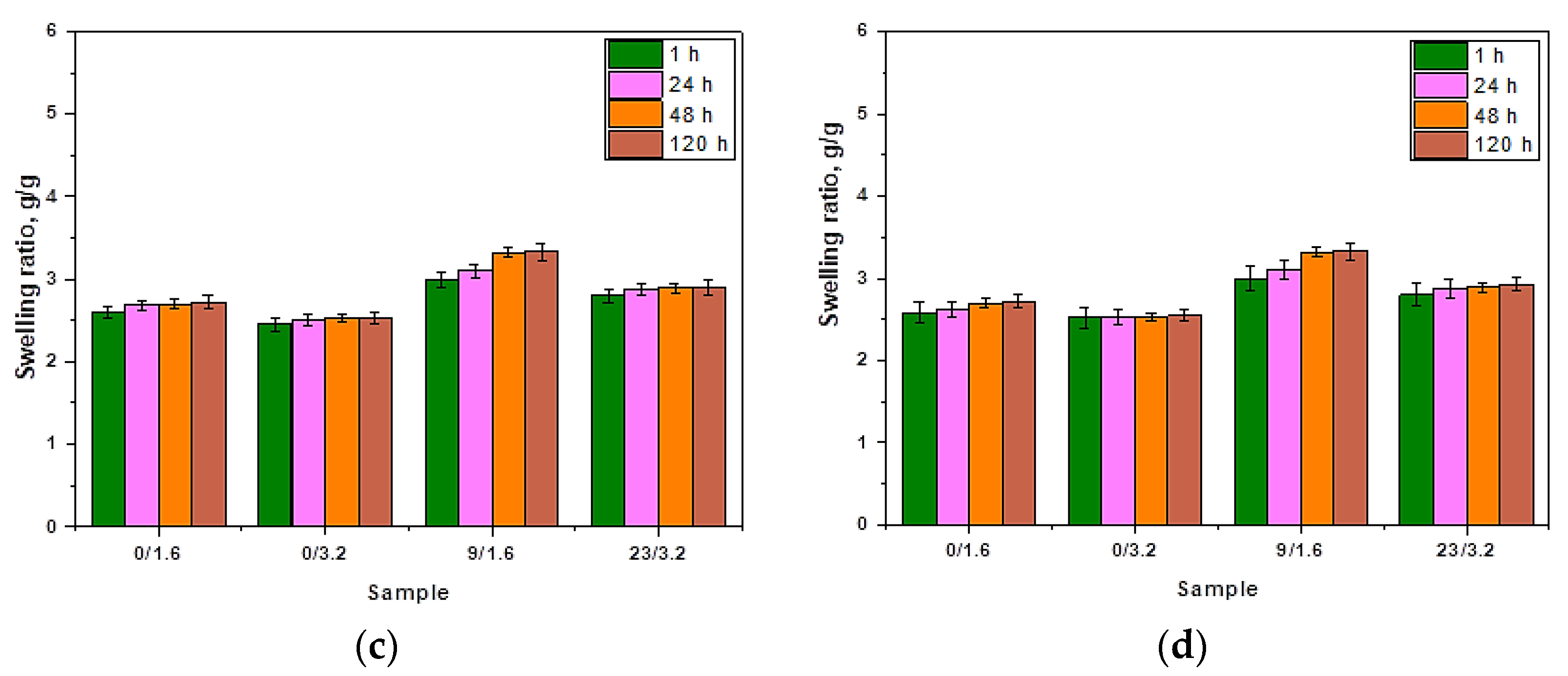
3.2. Results of Investigations on the Sorption Properties of Hydrogels
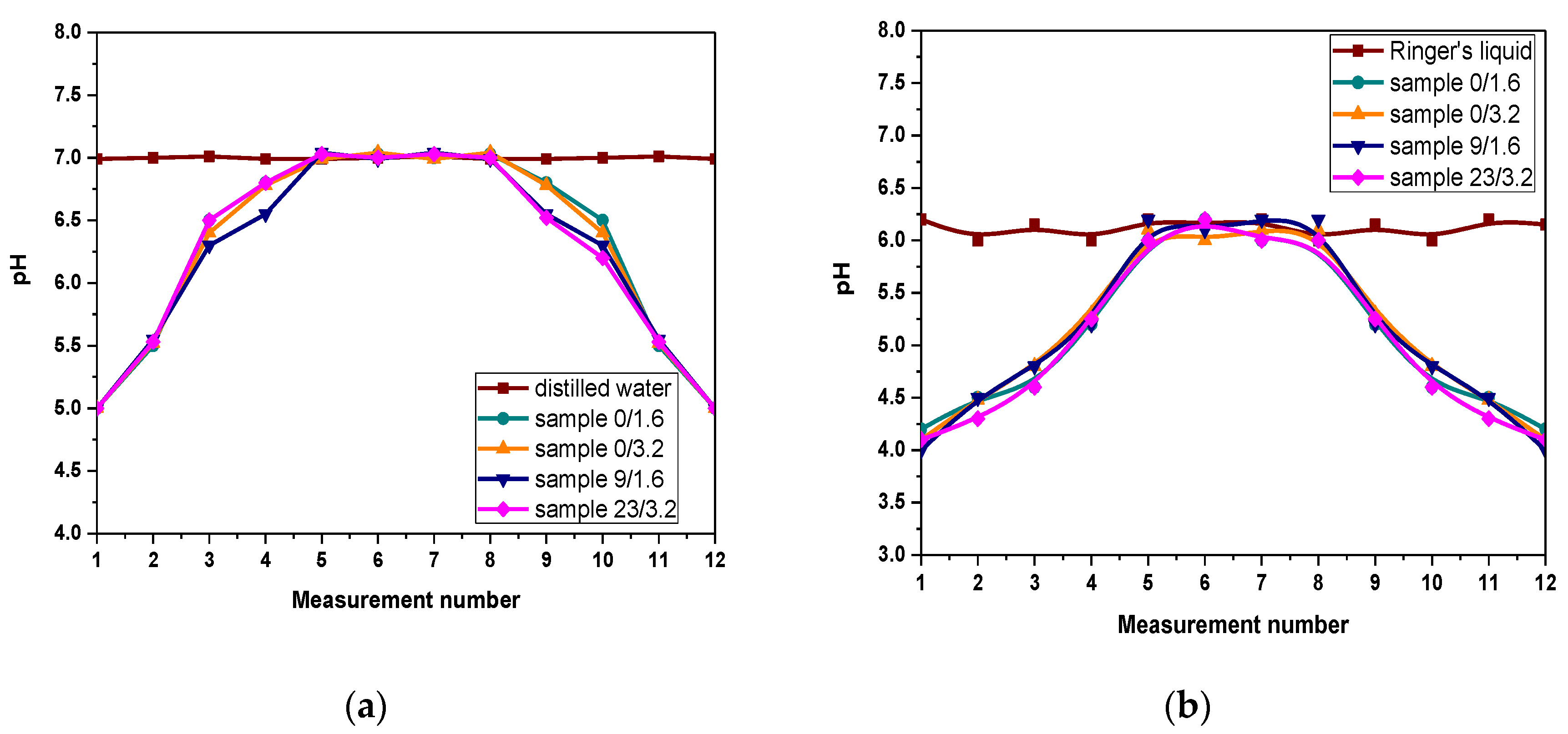
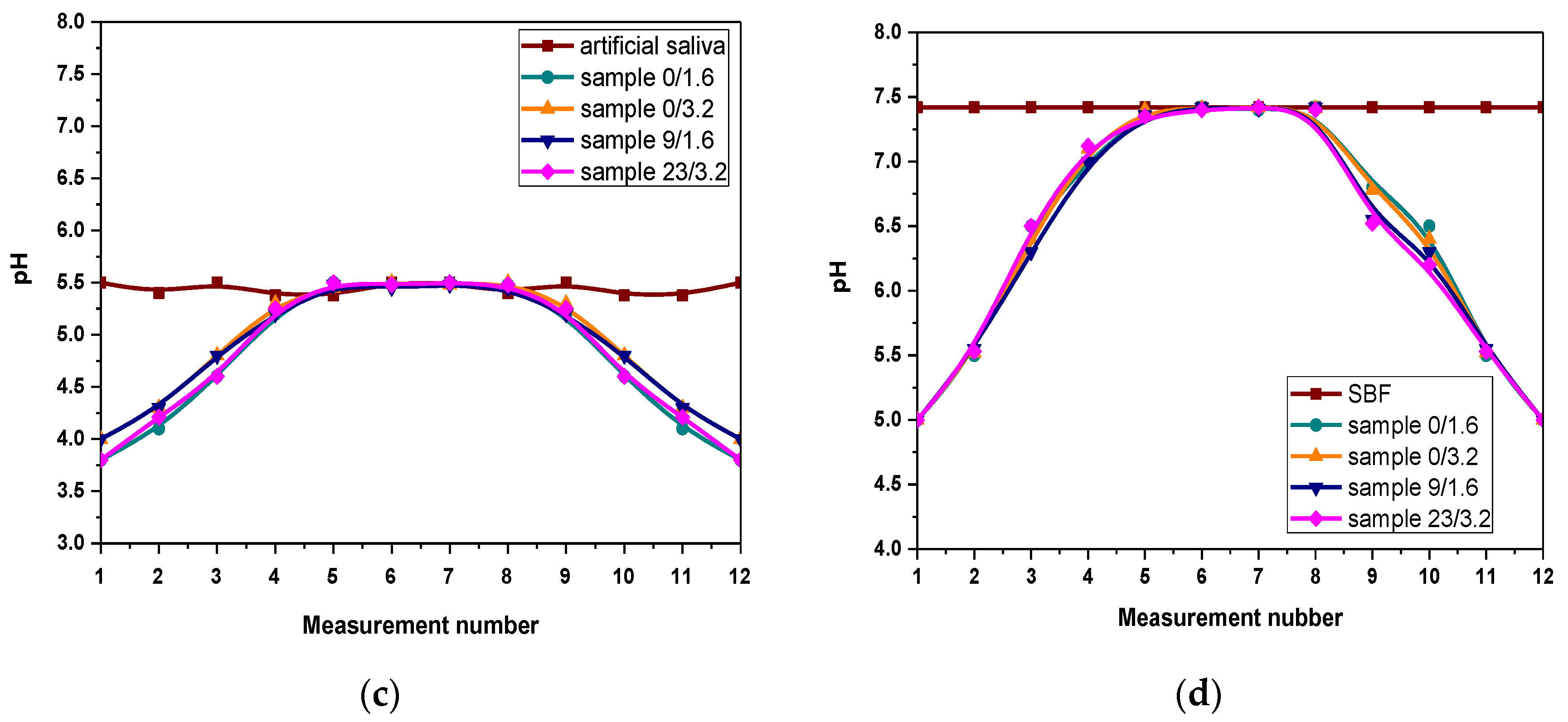
3.3. Results of Evaluation of the Mutual Interactions between Hydrogels and Simulated Physiological Liquids during Incubation Studies
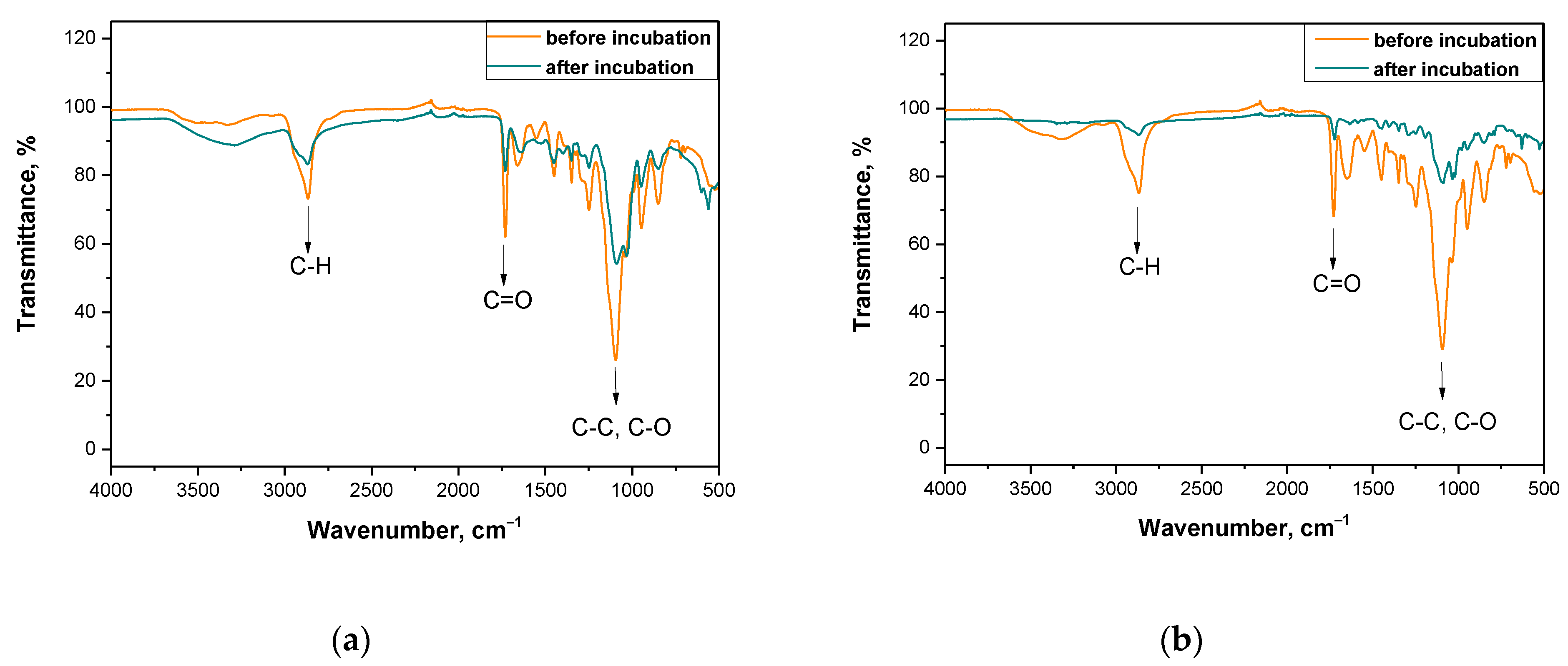
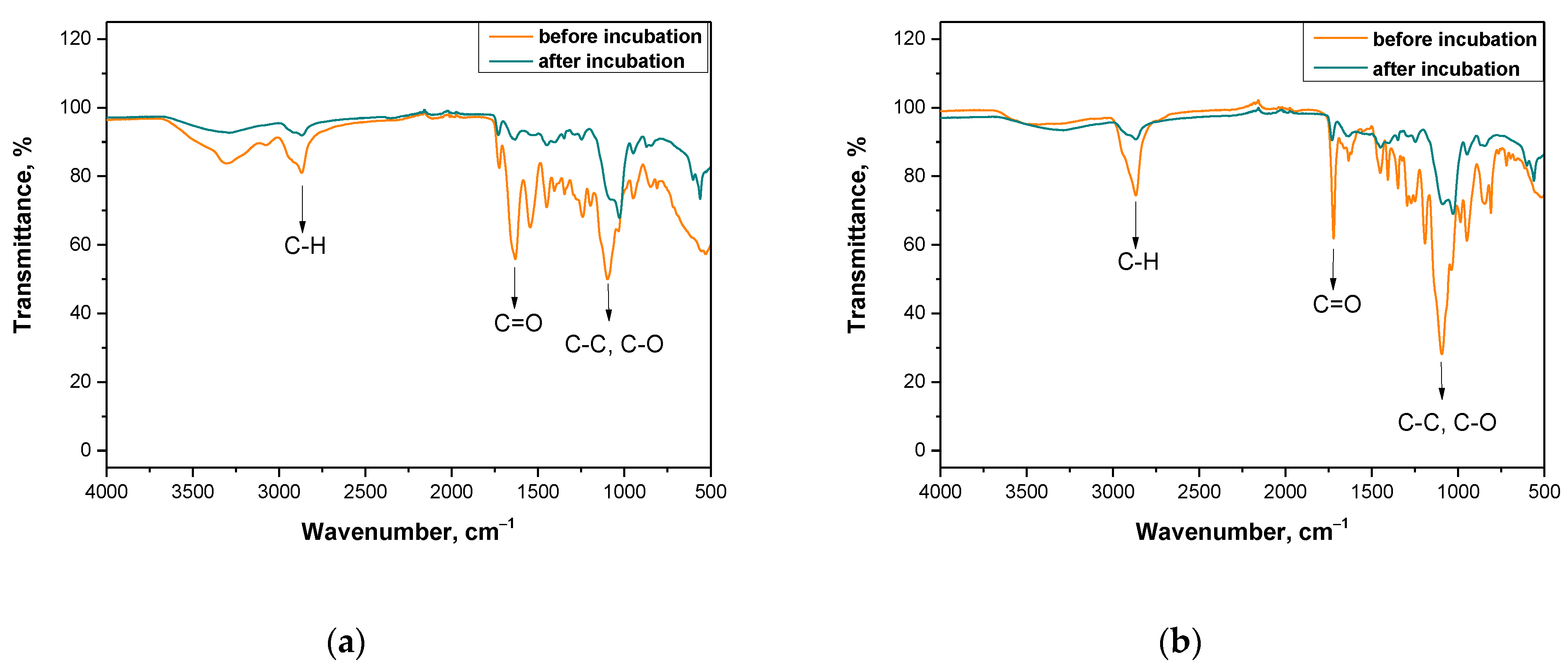
3.4. Analysis of the Impact of Incubation in Simulated Body Liquids on the Chemical Structure of Hydrogels via FT-IR Technique
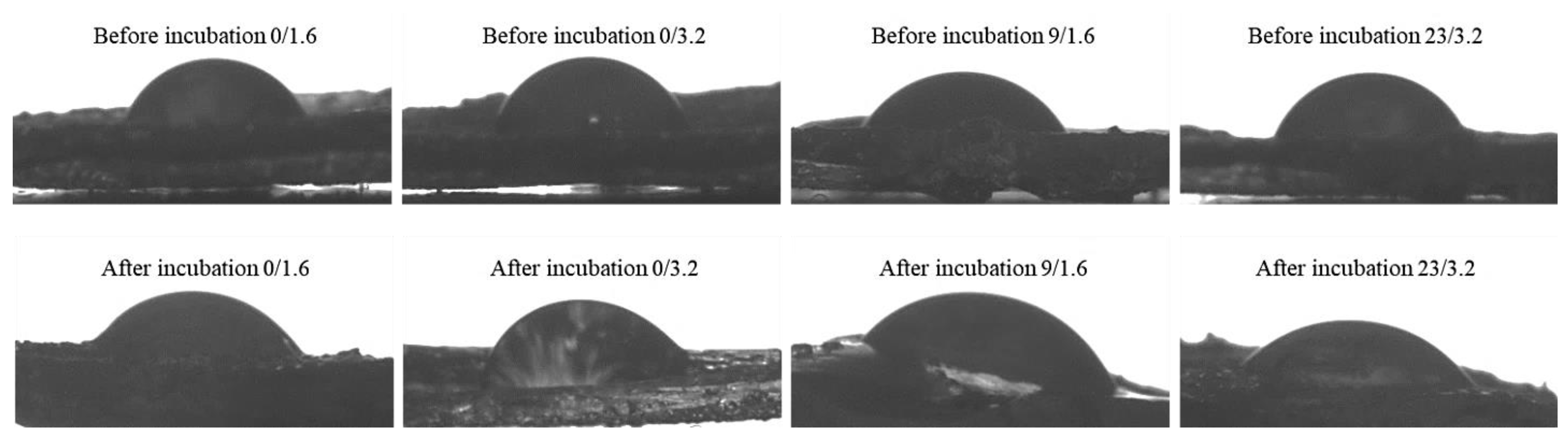
3.5. Results of Studies on the Wetting Properties of Hydrogels
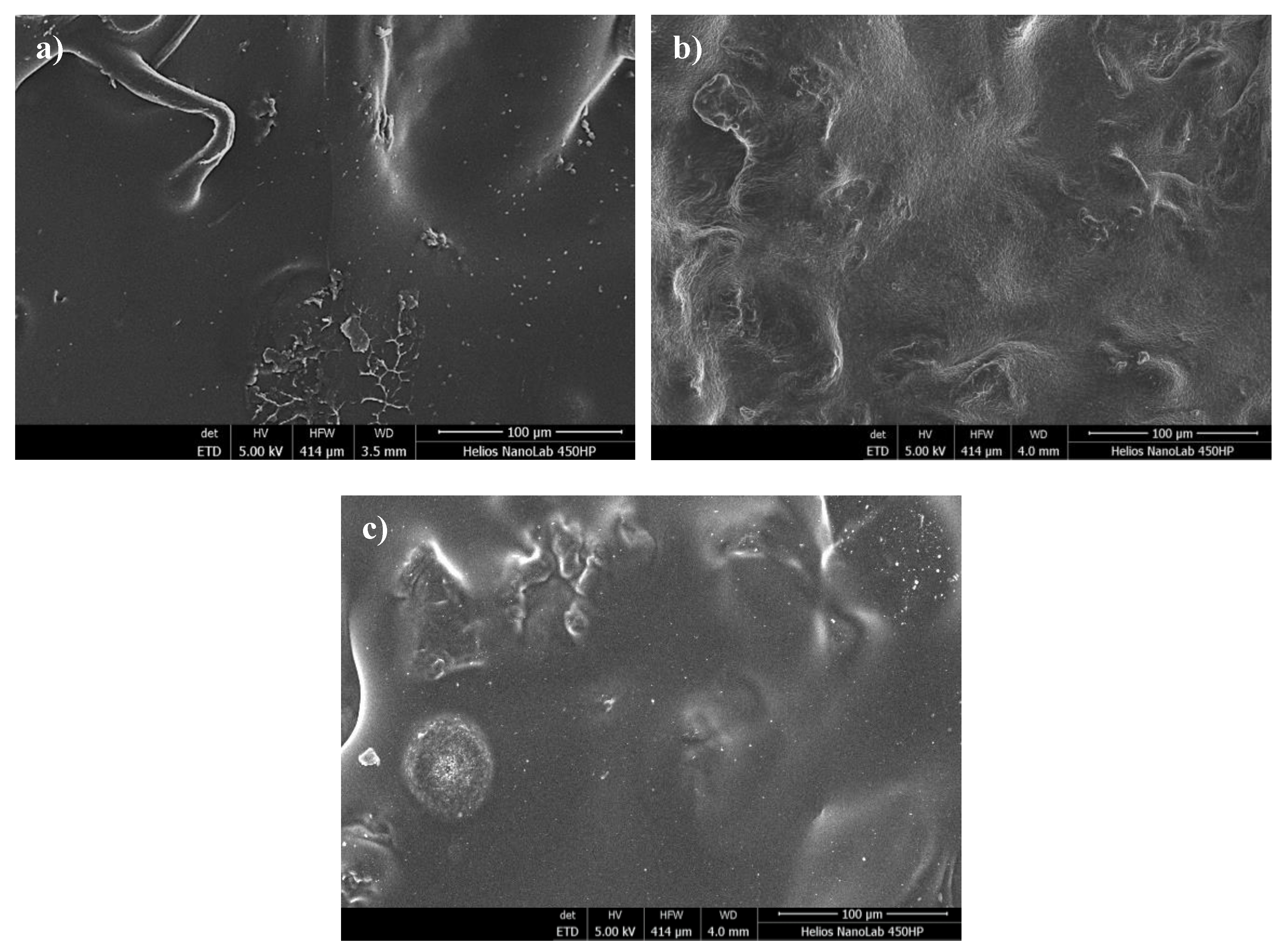
3.6. SEM Analysis of Hydrogel Surfaces
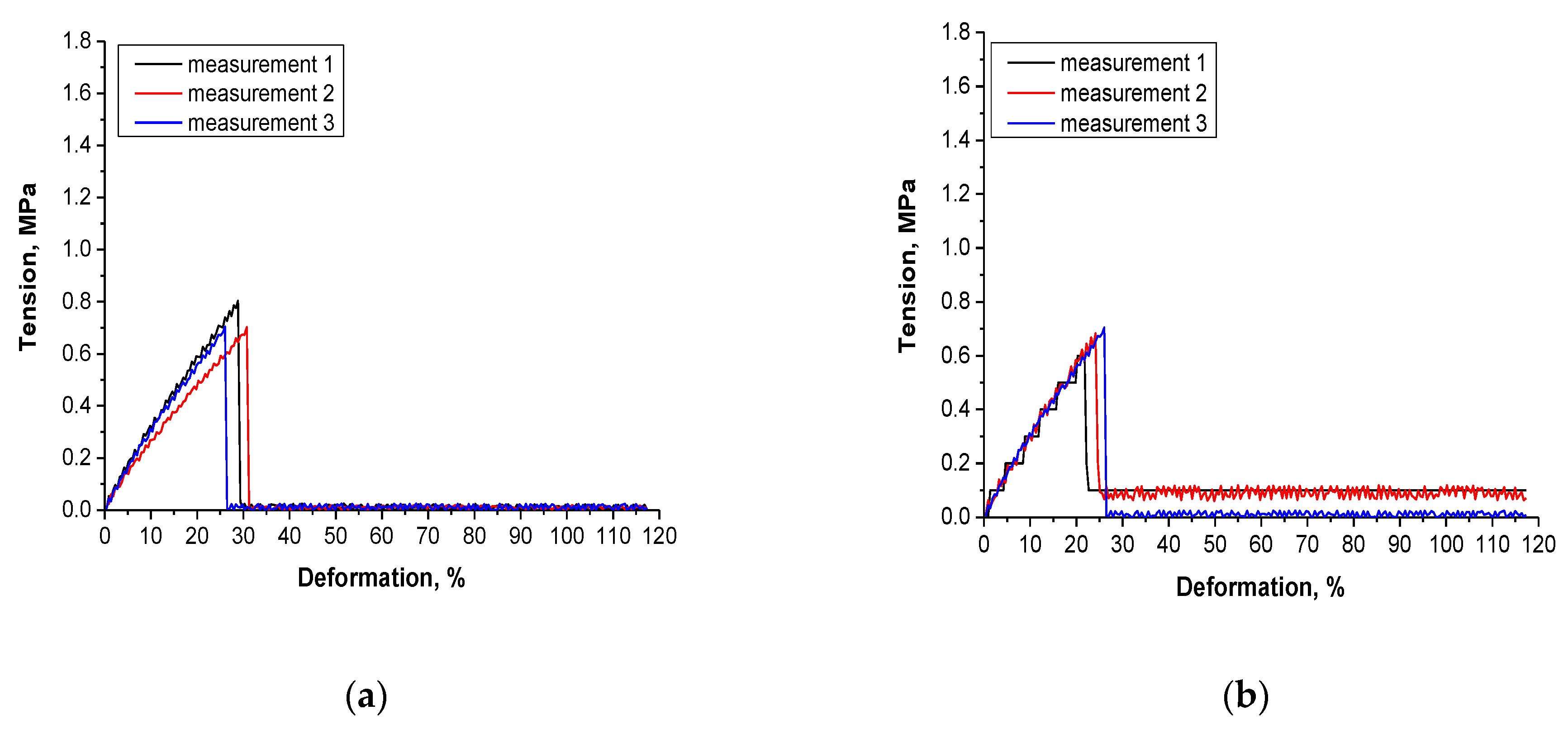
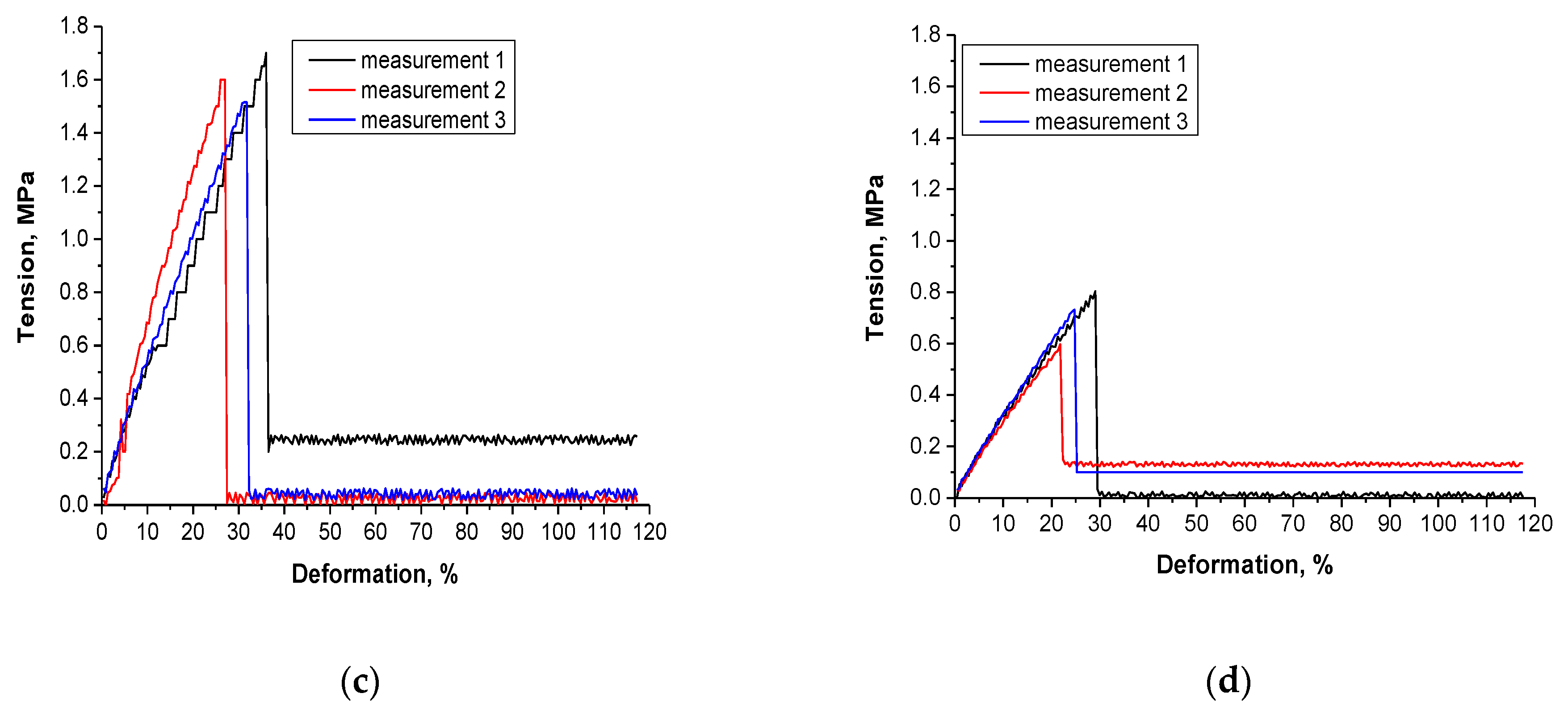
3.7. Results of the Investigations on the Tensile Strength of Hydrogels
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Asadi, N.; Pazoki-Toroudi, H.; Bakhshayesh, A.R.D.; Akbarzadeh, A.; Davaran, S.; Annabi, N. Multifunctional hydrogels for wound healing: Special focus on biomacromolecular based hydrogels. Int. J. Biol. Macromol. 2021, 170, 728–750. [Google Scholar] [CrossRef]
- Ng, J.Y.; Obuobi, S.; Chua, M.L.; Zhang, C.; Hong, S.; Kumar, Y.; Gokhale, R.; Ee, P.L.R. Biomimicry of microbial polysaccharide hydrogels for tissue engineering and regenerative medicine—A review. Carbohydr. Polym. 2020, 241, 116345. [Google Scholar] [CrossRef] [PubMed]
- Alpaslan, D.; Dudu, T.E.; Aktas, N. Synthesis and charcterization of novel organo-hydrogel based agar, glycerol and peppermint oil as a natural drug carrier/release material. Mater. Sci. Eng. C 2021, 118, 111534. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J. Bioactive modification of poly(ethylene glycol) hydrogels for tissue engineering. Biomaterials 2010, 31, 4639–4656. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Yu, F.; Zheng, L.; Wang, R.; Yan, W.; Wang, Z.; Xu, J.; Wu, J.; Shi, D.; Zhu, L.; et al. Natural hydrogels for cartilage regeneration: Modification, preparation and application. J. Orthop. Translat. 2019, 17, 26–41. [Google Scholar] [CrossRef] [PubMed]
- Qu, B.; Luo, Y. Chitosan-based hydrogel beads: Preparations, modifications and applications in food and agriculture sectors—A review. Int. J. Biol. Macromol. 2020, 152, 437–448. [Google Scholar] [CrossRef]
- Borzacchiello, A.; Ambrosio, L. Structure-Property Relationships in Hydrogels. In Hydrogels. Biological Properties and Applications; Barbucci, R., Ed.; Springer: Milan, Italy, 2009; pp. 9–20. [Google Scholar]
- Fujiyabu, T.; Yoshikawa, Y.; Chung, U.; Sakai, T. Structure-property relationship of a model network containing solvent. Sci. Technol. Adv. Mater. 2019, 20, 608–621. [Google Scholar] [CrossRef] [Green Version]
- Ahearne, M.; Yang, Y.; Liu, K.K. Mechanical Characterization of Hydrogels for Tissue Engineering Applications. Top. Tissue Eng. 2008, 4, 1–16. Available online: https://www.oulu.fi/spareparts/ebook_topics_in_t_e_vol4/ (accessed on 10 May 2021).
- Zhang, K.; Feng, W.; Jin, C. Protocol efficiently measuring the swelling rate of hydrogels. MethodsX 2020, 7, 100779. [Google Scholar] [CrossRef]
- Sievers, J.; Sperlich, K.; Stahnke, T.; Kreiner, C.; Eickner, T.; Martin, H.; Guthoff, R.F.; Schunemann, M.; Bohn, S.; Stachs, O. Determination of hydrogel swelling factors by two established and a novel non-contact continuous method. J. Appl. Polym. Sci. 2020, 138, e50326. [Google Scholar] [CrossRef]
- Harhaun, R.; Kunik, O.; Saribekova, D.; Lazzara, G. Biologically active properties of plant extracts in cosmetic emulsions. Microchem. J. 2020, 154, 104543. [Google Scholar] [CrossRef] [Green Version]
- Nicolai, M.; Pereira, P.; Vitor, R.F.; Reis, C.P.; Roberto, A.; Rijo, P. Antioxidant activity and rosmarinic acid content of ultrasound-assisted ethanolic extracts of medicinal plants. Measurement 2016, 89, 328–332. [Google Scholar] [CrossRef]
- Sahalie, N.A.; Abrha, L.H.; Tolesa, L.D. Chemical composition and antimicrobial activity of leave extract of Ocimum lamiifolium (Damakese) as a treatment for urinary tract infection. Cogent Chem. 2018, 4, 1440894. [Google Scholar] [CrossRef]
- Elisha, I.L.; Botha, F.S.; McGaw, L.J.; Eloff, J.N. The antibacterial activity of extracts of nine plant species with good activity against Escherichia coli against five other bacteria and cytotoxicity of extracts. BMC Complem. Altern. Med. 2017, 17, 133–142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Atef, N.M.; Shanab, S.M.; Negm, S.I.; Abbas, Y.A. Evaluation of antimicrobial activity of some plant extracts against antibiotic susceptible and resistant bacterial strains causing wound infection. Doc. Bull. Natl. Res. Cent. 2019, 43, 144. [Google Scholar] [CrossRef] [Green Version]
- Manilal, A.; Sabu, K.R.; Shewangizaw, M.; Aklilu, A.; Seid, M.; Merdekios, B.; Tsegaye, B. In vitro antibacterial activity of medicinal plants against biofilm-forming methicillin-resistant Staphylococcus aureus: Efficacy of Moringa stenopetala and Rosmarinus officinalis extracts. Heliyon 2020, 6, e03303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sabo, V.A.; Knezevic, P. Antimicrobial activity of Eucalyptus camaldulensis Dehn. plant extracts and essential oils: A review. Ind. Crops Prod. 2019, 132, 413–429. [Google Scholar] [CrossRef]
- Furman, B.L.; Candasamy, M.; Bhattamisra, S.K.; Veetil, S.K. Reduction of blood glucose by plant extracts and their use in the treatment of diabetes mellitus; discrepancies in effectiveness between animal and human studies. J. Ethnopharmacol. 2020, 247, 112264. [Google Scholar] [CrossRef]
- Abdel-Lateff, A.; Alarif, W.M.; Algandaby, M.M.; Alburae, N.A.; Abdel-Naim, A.B. Euryops arabicus displays anti-inflammatory activities in experimental models. J. Ethnopharmacol. 2020, 247, 112278. [Google Scholar] [CrossRef]
- Guo, X.; Ho, C.T.; Schwab, W.; Song, C.; Wan, X. Aroma compositions of large-leaf yellow tea and potential effect of theanine on volatile formation in tea. Food Chem. 2019, 280, 73–82. [Google Scholar] [CrossRef]
- Xu, J.; Wang, M.; Zhao, J.; Wang, Y.H.; Tang, Q.; Khan, I.A. Yellow tea (Camellia sinensis L.), a promising Chinese tea: Processing, chemical constituents and health benefits. Food Res. Int. 2018, 107, 567–577. [Google Scholar] [CrossRef]
- Andlauer, W.; Heritier, J. Rapid electrochemical screening of antioxidant capacity (RESAC) of selected tea samples. Food Chem. 2011, 125, 1517–1520. [Google Scholar] [CrossRef]
- Wang, Q.; Zhao, X.; Qian, Y.; Wang, R. In vitro antioxidative activity of yellow tea and its in vivo preventive effect on gastric injury. Exp. Ther. Med. 2013, 6, 423–426. [Google Scholar] [CrossRef] [Green Version]
- An, R.; Sun, L.; Xiang, L.; Zhang, W.; Li, Q.; Lai, X.; Wen, S.; Huo, M.; Li, D.; Sun, S. Effect of yellowing time on bioactive compounds in yellow tea and their antiproliferative capacity in HepG2 cells. Food Sci. Nutr. 2019, 7, 1838–1847. [Google Scholar] [CrossRef] [PubMed]
- Kujawska, M.; Ewertowska, M.; Adamska, T.; Ignatowicz, E.; Gramza-Michałowska, A.; Jodynis-Liebert, J. Protective effect of yellow tea extract on N-nitrosodiethylamine—Induced liver carcinogenesis. Pharm. Biol. 2016, 54, 1891–9000. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, N.; Chu, J.; Wang, M.; Chen, L.; Zhang, L.; Xie, Z.; Zhang, J.; Ho, C.T.; Li, D.; Wan, X. Large Yellow Tea Attenuates Macrophage-Related Chronic Inflammation and Metabolic Syndrome in High-Fat Diet Treated Mice. J. Agric. Food Chem. 2018, 66, 3823–3832. [Google Scholar] [CrossRef]
- Drabczyk, A.; Kudłacik-Kramarczyk, S.; Tyliszczak, B.; Rudnicka, K.; Urbaniak, M.; Michlewska, S.; Królczyk, J.B.; Gajda, P.; Pielichowski, K. Measurement methodology toward determination of structure-property relationship in acrylic hydrogels with starch and nanogold designed for biomedical applications. Measurement 2020, 156, 107608. [Google Scholar] [CrossRef]
- Tyliszczak, B.; Drabczyk, A.; Kudłacik-Kramarczyk, S.; Bialik-Wąs, K.; Kijkowska, R.; Sobczak-Kupiec, A. Preparation and cytotoxicity of chitosan based hydrogels modified with silver nanoparticles. Colloids Surf. B Biointerfaces 2017, 160, 325–330. [Google Scholar] [CrossRef]
- Tyliszczak, B.; Drabczyk, A.; Kudłacik-Kramarczyk, S.; Rudnicka, K.; Gatkowska, J.; Sobczak-Kupiec, A.; Jampilek, J. In vitro biosafety of pro-ecological chitosan-based hydrogels modified with natural substances. J. Biomed. Mater. Res. A 2019, 107, 2501–2511. [Google Scholar] [CrossRef] [PubMed]
- Elegbede, R.D.; Ilomuanya, M.O.; Sowemimo, A.A.; Nneji, A.; Joubert, E.; de Beer, D.; Koekemoer, T.; van de Venter, M. Effect of fermented and green Aspalathus linearis extract loaded hydrogel on surgical wound healing in Sprague Dawley rats. Wound Med. 2020, 29, 100186. [Google Scholar] [CrossRef]
- Ma, Z.; Garrido-Maestu, A.; Jeong, K.C. Application, mode of action, and in vivo activity of chitosan and its micro-and nanoparticles as antimicrobial agents: A review. Carbohydr. Polym. 2017, 176, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhuang, S. Antibacterial activity of chitosan and its derivatives and their interaction mechanism with bacteria: Current state and perspectives. Eur. Polym. J. 2020, 138, 109984. [Google Scholar] [CrossRef]
- Rodriguez-Rodriguez, R.; Espinosa-Andrews, H.; Velasquillo-Martinez, C.; Garcia-Carvajal, Z.Y. Composite hydrogels based on gelatin, chitosan and polyvinyl alcohol to biomedical applications: A review. Int. J. Polym. Mater. 2020, 69, 1–20. [Google Scholar] [CrossRef]
- Wang, W.; Xue, C.; Mao, X. Chitosan: Structural modification, biological activity and application. Int. J. Biol. Macromol. 2020, 164, 4532–4546. [Google Scholar] [CrossRef]
- Rashki, S.; Asgarpour, K.; Tarrahimofrad, H.; Hashemipour, M.; Ebrahimi, M.S.; Fathizadeh, H.; Khorshidi, A.; Khan, H.; Marzhoseyni, Z.; Salavati-Niasari, M.; et al. Chitosan-based nanoparticles against bacterial infections. Carbohydr. Polym. 2021, 251, 117108. [Google Scholar] [CrossRef] [PubMed]
- Peers, S.; Montembault, A.; Ladavière, C. Chitosan hydrogels for sustained drug delivery. J. Control. Release 2020, 326, 150–163. [Google Scholar] [CrossRef]
- Chen, P.; Liu, L.; Pan, J.; Mei, J.; Li, C.; Zheng, Y. Biomimetic composite scaffold of hydroxyapatite/gelatin-chitosan core-shell nanofibers for bone tissue engineering. Mater. Sci. Eng. C 2019, 97, 325–335. [Google Scholar] [CrossRef]
- Leonhardt, E.E.; Kang, N.; Hamad, M.A.; Wooley, K.L.; Elsabahy, M. Absorbable hemostatic hydrogels comprising composites of sacrificial templates and honeycomb-like nanofibrous mats of chitosan. Nat. Commun. 2019, 10, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.; Xing, X.; Tan, H.; Jia, Y.; Zhou, T.; Chen, Y.; Ling, Z.; Hu, X. Covalently antibacterial alginate-chitosan hydrogel dressing integrated gelatin microspheres containing tetracycline hydrochloride for wound healing. Mater. Sci. Eng. C 2017, 70, 287–295. [Google Scholar] [CrossRef]
- Ragab, T.I.M.; Nada, A.A.; Ali, E.A.; Shalaby, A.S.G.; Soliman, A.A.F.; Emam, M.; El Raey, M.A. Soft hydrogel based on modified chitosan containing P. granatum peel extract and its nanoforms: Multiparticulate study on chronic wounds treatment. Int. J. Biol. Macromol. 2019, 135, 407–421. [Google Scholar] [CrossRef]
- Pankongadisak, P.; Suwantong, O. Enhanced properties of injectable chitosan-based thermogelling hydrogels by silk fibroin and longan seed extract for bone tissue engineering. Int. J. Biol. Macromol. 2019, 138, 412–424. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, F.; Zhao, R.; Wang, C.; Hu, K.; Sun, Y.; Politis, C.; Shavandi, A.; Nie, L. Polyvinyl Alcohol/Sodium Alginate Hydrogels Incorporated with Silver Nanoclusters via Green Tea Extract for antibacterial Applications. Des. Monomers Polym. 2020, 23, 118–133. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.; Park, K.; Yoon, Y.; Kim, H.S.; Kim, H.J.; Choi, J.W.; Lee, D.Y.; Chun, H.J.; Yang, D.H. Visible Light-Cured Antibacterial Collagen Hydrogel Containing Water-Solubilized Triclosan for Improved Wound Healing. Materials 2021, 14, 2270. [Google Scholar] [CrossRef]
- Nurzynska, A.; Klimek, K.; Palka, K.; Szajnecki, Ł.; Ginalska, G. Curdlan-Based Hydrogels for Potential Application as Dressings for Promotion of Skin Wound Healing—Preliminary In Vitro Studies. Materials 2021, 14, 2344. [Google Scholar] [CrossRef] [PubMed]
- Tyliszczak, B.; Drabczyk, A.; Kudłacik, S.; Bialik-Wąs, K.; Sobczak-Kupiec, A. Beetosan/Chitosan from bees—Preparation and properties. Int. J. Adv. Sci. Eng. Technol. 2016, 4, 118–120. [Google Scholar]
- Liu, Y.; Weng, R.; Wang, W.; Wei, X.; Li, J.; Chen, X.; Liu, Y.; Lu, F.; Lu, Y. Tunable physical ad mechanical properties of gelatin hydrogel after transglutaminase crosslinking on two gelatin types. Int. J. Biol. Macromol. 2020, 162, 405–413. [Google Scholar] [CrossRef] [PubMed]
- Kokubo, T.; Takadama, H. How useful is SBF in predicting in vivo bone bioactivity? Biomaterials 2006, 27, 2907–2915. [Google Scholar] [CrossRef]
- Ebrahimi, A.; Esfahani, H.; Fattah-alhosseini, A.; Imantalab, O. In vitro electrochemical study of TiB/TiB2 composite coating on titanium in Ringer’s solution. J. Alloys Compd. 2018, 765, 826–834. [Google Scholar] [CrossRef]
- Shah, R.; Saha, N.; Saha, P. Influence of temperature, pH and simulated biological solutions on swelling and structural properties of biomineralized (CaCO3) PVP-CMC hydrogel. Prog. Biomater. 2015, 4, 123–136. [Google Scholar] [CrossRef] [Green Version]
- Kipcak, A.S.; Ismail, O.; Doymaz, I.; Piskin, S. Modelling and Investigation of the Swelling Kinetics of Acrylamide-Sodium Acrylate Hydrogel. J. Chem. 2014, 281063. [Google Scholar]
- Drozdov, A.D.; de Claville Christiansen, J. The effects of pH and ionic strength on equilibrium swelling of polyampholyte gels. Int. J. Solids Struct. 2017, 110, 192–208. [Google Scholar] [CrossRef]
- Feng, D.; Bai, B.; Wang, H.; Suo, Y. Enhanced mechanical stability and sensitive swelling performance of chitosan/yeast hybrid hydrogel beads. New J. Chem. 2016, 40, 3350–3362. [Google Scholar] [CrossRef]
- Holloway, J.L.; Spiller, K.L.; Lowman, A.M.; Palmese, G.R. Analysis of the in vitro swelling behavior of poly(vinyl alcohol) hydrogels in osmotic pressure solution for soft tissue replacement. Acta Biomater. 2011, 7, 2477–2482. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Lin, S.; Feng, Q.; Dong, C.; Yang, G.; Li, L.; Bian, L. Nanocomposite hydrogels stabilized by self-assembled multivalent bisphosphonate-magnesium nanoparticles mediate sustained release of magnesium ion and promote in-situ bone regeneration. Acta Biomater. 2017, 64, 389–400. [Google Scholar] [CrossRef]
- Thongchai, K.; Chuysinuan, P.; Thanyacharoen, T.; Techasakul, S.; Ummartyotin, S. Characterization, release, and antioxidant activity of caffeic acid-loaded collagen and chitosan hydrogel composites. J. Mater. Res. Technol. 2020, 9, 6512–6520. [Google Scholar] [CrossRef]
- Khorasani, M.T.; Joorabloo, A.; Moghaddam, A.; Shamsi, H.; Moghadam, Z.M. Incorporation of ZnO nanoparticles into heparinised polyvinyl alcohol/chitosan hydrogels for wound dressing application. Int. J. Biol. Macromol. 2018, 114, 1203–1215. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Yang, K.; Zhao, D.; Yang, H. Thermal- and salt-activated shape memory hydrogels based on a gelatin/polyacrylamide double network. RSC Adv. 2019, 9, 18619–18626. [Google Scholar] [CrossRef] [Green Version]

| No. | Base Solution, mL | Yellow Tea Extract, % (v/v) | Crosslinking Agent, mL | Photoinitiator, mL | Sample Notation |
|---|---|---|---|---|---|
| 1. | 10 | 0 | 1.6 | 0.1 | 0/1.6 |
| 2. | 0 | 3.2 | 0/3.2 | ||
| 3. | 9 | 1.6 | 9/1.6 | ||
| 4. | 23 | 3.2 | 23/3.2 | ||
| 5. | 33 | 3.2 | 33/3.2 |
| Sample | Surface Wetting Angle, ° | |
|---|---|---|
| Before Incubation | After Incubation | |
| 0/1.6 | 91.7 ± 2.8 | 85.5 ± 1.2 |
| 0/3.2 | 93.5 ± 1.3 | 87.7 ± 2.3 |
| 9/1.6 | 89.2 ± 1.7 | 70.2 ± 1.4 |
| 23/3.2 | 90.3 ± 0.9 | 55.0 ± 2.1 |
| Sample | Deformation under 0.6 MPa Tension, % | Maximum Deformation, % |
|---|---|---|
| 0/1.6 | 19 | 27 |
| 0/3.2 | 21 | 24 |
| 9/1.6 | 8 | 23 |
| 23/3.2 | 20 | 20 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kudłacik-Kramarczyk, S.; Drabczyk, A.; Głąb, M.; Gajda, P.; Jaromin, A.; Czopek, A.; Zagórska, A.; Tyliszczak, B. Synthesis and Physicochemical Evaluation of Bees’ Chitosan-Based Hydrogels Modified with Yellow Tea Extract. Materials 2021, 14, 3379. https://doi.org/10.3390/ma14123379
Kudłacik-Kramarczyk S, Drabczyk A, Głąb M, Gajda P, Jaromin A, Czopek A, Zagórska A, Tyliszczak B. Synthesis and Physicochemical Evaluation of Bees’ Chitosan-Based Hydrogels Modified with Yellow Tea Extract. Materials. 2021; 14(12):3379. https://doi.org/10.3390/ma14123379
Chicago/Turabian StyleKudłacik-Kramarczyk, Sonia, Anna Drabczyk, Magdalena Głąb, Paweł Gajda, Anna Jaromin, Anna Czopek, Agnieszka Zagórska, and Bożena Tyliszczak. 2021. "Synthesis and Physicochemical Evaluation of Bees’ Chitosan-Based Hydrogels Modified with Yellow Tea Extract" Materials 14, no. 12: 3379. https://doi.org/10.3390/ma14123379






