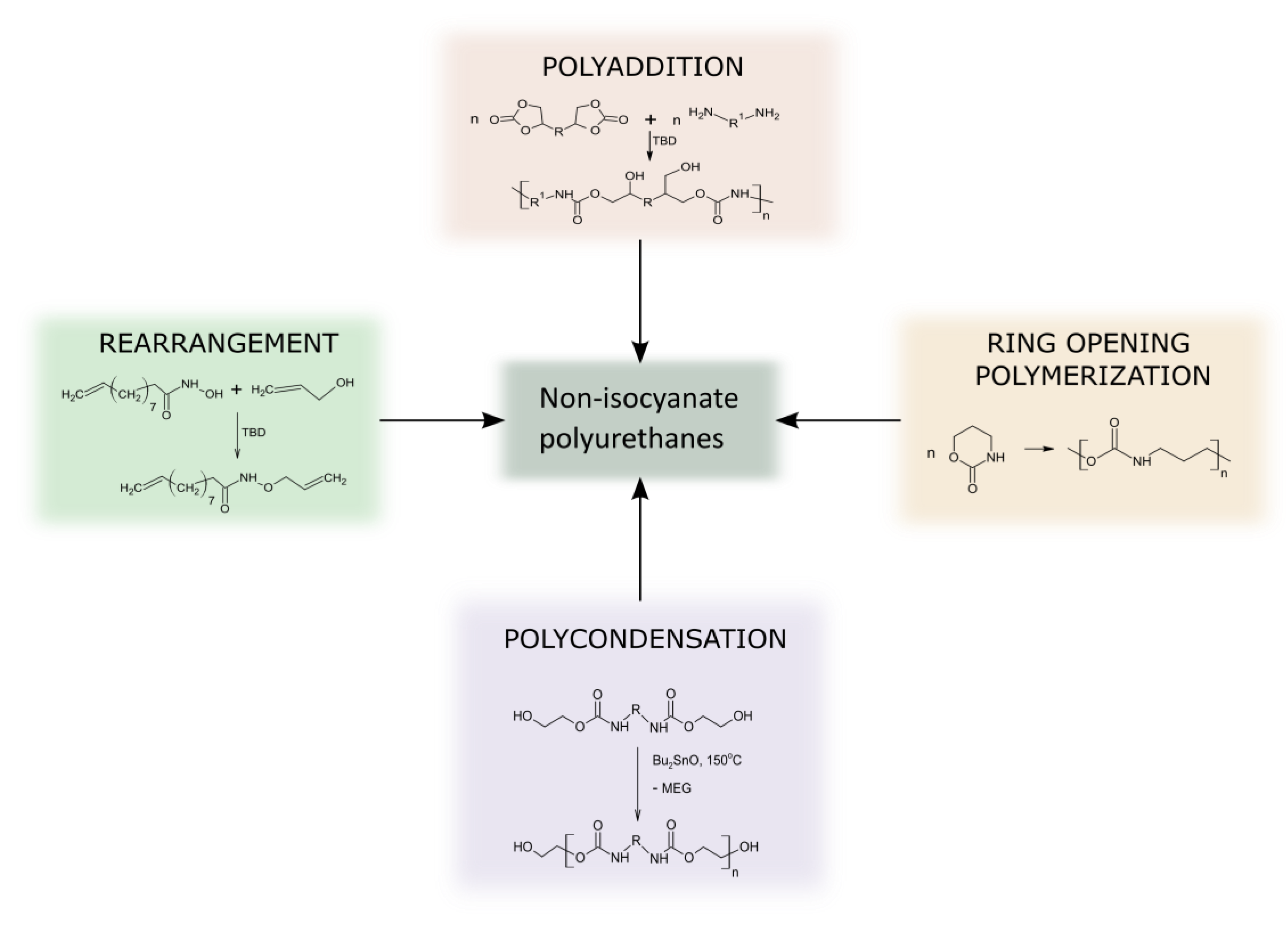
As conventional PUs are broadly modified using different types of reactive and non-reactive additives, this approach can also improve NIPU properties. NIPUs bearing primary and secondary hydroxyl side groups can undergo chemical modification, including grafting reactions and cross-linking with chemical agents able to react with OH- moiety.
In most cases, the synthesis process and the properties of obtained NIPU and NIPU composites are evaluated in the same way as conventional PU or any other polymeric materials. Characterization of substrates or products and monitoring of the process of synthesis are generally done by utilizing Fourier transform infrared spectroscopy (FTIR), nuclear magnetic resonance spectroscopy (NMR), X-Ray diffraction spectroscopy (XRD), scanning electron microscopy (SEM), and size exclusion chromatography (SEC). The methods used for mechanical property appraisal are tensile strength tests (measuring tensile strength itself, Young’s modulus, and elongation at break), impact strength tests, and various hardness tests (pencil hardness or Shore hardness). Thermal properties are usually tested using thermogravimetric analysis (TGA) and differential scanning calorimetry (DSC). Dynamic mechanical analysis (DMA) is performed regularly to determine thermo-mechanical properties. Chemical property tests include, but are not limited to, resistance for acid, alkali, various solvents, hydrolytic stability, water uptake, and anti-corrosive properties.
3.1. NIPU as a Polymer Matrix for Nanocomposites
Research interest has been focused recently on NIPU nanocomposites. They show improved properties with an addition of the nanofiller in the small amounts of 1–5%, which is much less than in the case of traditional micro-structured composites that often require much higher filler loads.
Along this line of interest, Dolui et al., devised a set of (NIPU)-blend-epoxy hybrid materials (HNIPU) based on sunflower oil that was reinforced by amine-functionalized graphene oxide (AF-GO). Usage of sunflower oil, a bio-basted raw material, benefits an already environmentally-friendly concept of non-isocyanate polyurethanes. Carbonate compound for HNIPU was obtained in the process of combining epoxidized sunflower oil (ESFO) and carbon dioxide (CO
2) in pressurized conditions 50 bar and the temperature of 120 °C. Carbonated sunflower oil (CSFO) bearing cyclo-carbonate moieties was obtained and later mixed with commercially available epoxy resin (up to 30 wt% in relation to CSFO). Isophorone diamine was used as a curing agent in solvent reaction at 70 °C in THF, followed by curing and post-curing at 90 °C and 130 °C for 48 h and 1 h, respectively. The HNIPU with 30 wt% epoxy was chosen to be modified with amine-functionalized graphene oxide, as it showed the best mechanical properties. The overall route of the synthesis, modification, and plausible interactions between polymer matrix and nanofiller are shown in
Scheme 13 [
33].
All the obtained nanocomposites (0.3, 0.6, and 1.0 wt% of AF-GO) showed improvement in mechanical properties in comparison to the unmodified polymer. Mechanical and other properties, such as thermal stability and flame retardancy, have improved with the addition of AF-GO. Superior properties of the composite materials with nanofiller may be attributed to hydrogen bonding and covalent interconnection of amine-modified graphene oxide with the polymer matrix. Enhancement of the properties of nanocomposites increases with AF-GO content. The same research group developed other types of NIPU composites using amine-functionalized multi-walled carbon nanotubes (AF-CNT) in the place of graphene oxide. As previously stated, carbonated sunflower oil (CSFO) and isophorone diamine (IPDA) were used [
33].
In the preparation protocol, multi-walled carbon nanotubes were first treated with concentrated nitric acid and ultra-sonicated. After additional processing (filtration, purification, and drying), functionalized carbon nanotubes (f-CNTs) were aminated by the addition of 3-amino propyl trimethoxy silane (APTMS). The obtained amine-functionalized multi-walled carbon nanotubes (AF-CNT) were then used as reactive filler in wt% of: 0.50, 1, 1.5, and 2 in relation to the weight of CSFO—
Scheme 14 [
34].
Properties of the obtained nanocomposites improved compared to reference NIPUs, which is explained by the presence of strong hydrogen interactions and covalent interlinkage of AF-CNTs with CSFO/IPDA polymer networks. The performance of obtained materials was enhanced with the loading of AF-CNTs up to 1.5 wt%. The thermal and mechanical features improved significantly, and the limiting oxygen index reached 30%, which suggests the synthesized compositions show self-extinguishing traits. The initial decomposition temperature was determined to be 274 °C, while the temperature of 50% weight loss was measured to be 390 °C for composition without any addition of CNTs. It reached 310 °C for the initial decomposition step, as well as 430 °C for 50% weight loss for composition with 1.5 wt% of CNTs [
34]. The value for non-filled material is higher than conventional PU materials based on sunflower oil (initial decomposition temperature ca. 250 °C, 50% weight loss at ca. 380 °C) [
35].
As physical-chemical properties improved, the electromagnetic interference shielding effects of the sample with 1.5 wt% AF-CNTs were tested. The assessed sample exhibited microwave absorption of 217 dB at 10.86 GHz in the 8.2–12.4 GHz (X-band) frequency range while being 3 mm thick, suggesting NIPU/CNT nanocomposites show promise for screening utilization and shielding against electromagnetic radiation [
34].
Liu et al., obtained a batch of non-isocyanate polyurethane films in the process of reacting gallic-acid-based cyclic carbonate with difunctional amines. Further modification with polyhedral oligomeric silsesquioxane (POSS) particles bearing oxirane moieties yielded NIPU/POSS materials, with POSS covalently incorporated in the polymeric matrix. The gallic-acid-derived hybrid samples exhibited extraordinary mechanical and thermal properties (impact strength, adhesion, flexibility, pencil hardness), yet water resistance was not satisfying. The incorporation of POSS into polyurethane matrices improved pencil hardness, water resistance, and thermal performance of the obtained hybrid films, although their adhesion decreased slightly. As the thermal decomposition of unmodified NIPUs occurred at similar temperatures (around 360 °C) as for conventional PU, compositions with POSS particles were more thermally stable (decomposition temperature of ca. 380 °C) [
35,
36].
Kathalewar et al., prepared NIPU composites with an addition of surface-treated ZnO particles. The modification of ZnO was conducted via an aqueous precipitation method with cyclic carbonate functional alkoxy silane. This approach allowed surface-treated ZnO (TZnO) particles to bear pendant cyclic carbonate moieties and, in turn, function as a cross -linking agent for the matrix. NIPU matrix was obtained using cyclic carbonate derivative of epoxy resin (C-GY) and 4,9 dioxadodecane-1,12-diamine as a hardener, and dimethyl formamide was used as a solvent. Treated and untreated ZnO particles were added to the matrix in a
w/
w ratio of 1%, 2%, and 3%. The formulations were dissolved in a mixture of xylene and dimethyl carbonate, applied on mild steel and aluminum plates, and underwent a curing process at 70 °C and 135 °C for a total of 1.5 h. A conventional polyurethane formulation (CPU) based on acrylic polyol and N-3390 cross-linking agent was also prepared to be compared to ZnO–NIPU composites and NIPU coating without the addition of any particles (B-NIPU) [
37].
All obtained coatings were tested for mechanical and chemical properties. Overall properties of NIPU composited were expected to be influenced by the cross-linking of TZnO particles. Pencil hardness, cross hatch adhesion, and flexibility turned out to be similar in the case of all formulations. Interestingly, CPU flexibility was lower than NIPU coating without the addition of ZnO particles. It was attributed to the chemical structure of both formulations as CPU had a more cross-linked structure due to the usage of trifunctional (HDI-based) N-3390 cross-linking agent. The falling ball impact method was used to evaluate the impact resistance of obtained coatings. All NIPU coatings showed impact resistance of 70.8 lbs, while CPU achieved impact resistances of 53.1 lbs and 47.2 lbs in intrusion and extrusion testing methods, respectively. The scratch hardness of the CPU coating was similar to 3% TZnO coating. However, as the loading of the ZnO filler in NIPU compositions increased from 1% to 3%, their scratch hardness also improved. In the case of treated ZnO particles, it was attributed to higher cross-linking of the polymer network, as cyclic carbonate functional groups in the TZnO reacted with amine compounds. In contrast, untreated ZnO was present in the matrix only in the form of suspended particles [
38,
39]. This trend was also observed for abrasion resistance. Among obtained coatings, the highest abrasion resistance was observed for composites containing 3% TZnO NIPU formulation (3.3 mg weight loss). The abrasion resistance of untreated NIPU (5.1 mg weight loss) was superior to CPU (7.8 mg weight loss). The results of the dynamic mechanical analysis also corresponded with other testing methods. As the loading with ZnO nanofiller increased, the storage modulus of all compositions also improved. Yet again, surface-treated ZnO particles performed better than untreated ones. Glass transition temperature (
Tg) increased alongside the loading of treated and untreated ZnO particles, which was attributed to the restricted mobility of the matrix chains caused by interactions between the filler and NIPU [
37,
40].
Obtained coatings were also tested for their chemical resistance by various testing methods. All of the formulations showed superb resistance to both acid and alkali, evaluated by the immersion method. It was believed that hydrogen bonding had a shielding effect on hydroxyl groups present in the NIPU matrix, and thus, its acid resistance was not diminished. Excellent results were also obtained for solvent resistance in MEK and xylene. The hydrolytic stability was tested by submersion of obtained samples in boiling water for 8 h. Due to the presence of secondary hydroxyl groups in nano composites with ZnO and TZnO, their hydrolytic stability was superior to the conventional polyurethane. Moreover, loading of the NIPU matrix with ZnO fillers did not cause any unwanted porosity. The salt spray method was used to evaluate corrosion resistance. NIPU composite coatings did not show any damage after 500 h of salt spray test at 35 °C, while B-NIPU showed only minor corrosion of metal surface under the cross mark made on the coating. The results were comparable to those of conventional polyurethane [
37].
Fleischer et al., prepared composites filled with cellulose carbonate and matrices obtained from carbonate compound derivatives of glycerol (GGC), trimethylolpropane (TMC) and pentaerythritol (PEC) glycidyl ethers, and citric acid amino amides (CAA), based on triethyl citrate hexamethylene diamine (HMDA). The fact that most of the listed materials are bio-based only benefits the idea of NIPUs being environmentally friendly materials. Respective cyclic carbonates were mixed with HMDA or CAA and, subsequently, cured at 70 °C for 8 h. For the preparation of composites, cellulose filler was mixed with carbonate and amine compounds and cured under the same conditions. Such a synthetic route is a convenient method to obtain non-isocyanate polyurethanes and their composites according to green chemistry regulation. Thermal and mechanical properties were evaluated for obtained NIPU and their respective composites with cellulose. Glass transition temperatures were 20 °C for GGC–HMDA, 47 °C for TMC–HMDA, and 51 °C for PEC–HMDA. When NIPUs were obtained with citric acid amino amides, their
Tg increased by 16 °C for GGC–CAA and 9 °C for TMC–CAA.
Tg of PEC–CAA remained unchanged. Young’s modulus of both GGC-based NIPUs was uniform at 7 MPa. On the contrary, it increased from 590 MPa for TMC–HMDA to 1230 MPa for TMC–CAA and 660 MPa for PEC–HMDA to 1740 MPa for PEC–CAA. There is an observable trend for all of the mentioned values: the usage of citric acid amino amides as curing agents rather than hexamethylene diamine results in increasing in glass transition temperature and Young’s modulus. Tensile strength shows the same trend while elongation at break lowers. All those interactions are believed to be due to the tri-functionality of CAA and di-functionality of HMDA. In terms of carbonate compound structures, both GGC-based (difunctional)NIPUs are flexible, as their structure is much less cross-linked than TMC– and PEC–NIPUs (both are tri-functional). However, when GGC is blended with other carbonates, interesting synergies are observed. PEC–GGC 3:1
w/
w mixture of carbonates reacted with HMDA gives NIPUs with tripled stiffness and tensile strength compared to the values of the single carbonate compositions. For blends cured with CAA, the results are alike. An extensive explanation of the genesis of this blend synergism may be done as more complex research is to be conducted. The carbonate blend of TMPC–GGC 1:2
w/
w was chosen to be modified with cellulose and cellulose carbonate. In both formulations, the polymer matrix was loaded with 5 wt% of filler. Cellulose carbonate–NIPU composite showed Young’s modulus of 2600 MPa in comparison to 2100 MPa for non-filled NIPU. Tensile strength and elongation at break were almost unchanged. Better compatibility of carbonated cellulose with NIPU matrix is reflected in the improvement of Young’s compared to unmodified cellulose filler, as cellulose carbonate can react with NIPU matrix via urethane linkages. Other carbohydrates, e.g., nanocellulose can also be added to NIPU matrices via this method [
41].
Yang et al., prepared and studied NIPU composites filled with corundum (Al
2O
3) and silicon carbide (SiC). Ceramic fillers were also covered with various carbon sources (namely dopamine, glucose, and graphene oxide) and, subsequently, thermally treated to produce hybrid materials bearing a graphene outer layer with ceramic material inside. NIPU matrix was obtained from trimethylolpropane glycidyl ether carbonate (TMPGC) and hexamethylene diamine (HMDA) or diethylenetriamine (DETA) as curing agents. NIPU composites were prepared by mixing graphenatedated ceramic fillers with carbonate substrate and later addition of either HMDA or DETA. The curing process was conducted for 14 h at 80 °C, and later on, the post-curing process was applied for 4 h at 100 °C (
Scheme 15) [
42].
Composites with 10 or 30 wt% of selected fillers were evaluated for their thermal and mechanical properties. The reference DETA-cured NIPU had Young’s modulus of 3900 MPa. A Young’s modulus of 7800 MPa was achieved for its composite filled with 30 wt% of corundum filler untreated with graphene. However, tensile strength and elongation at break were observed to be lowered (from circa 100 MPa to 90 MPa and from 3% to 1.2%, respectively) due to the stiffening effect of the filler. NIPU-bearing hydroxyl and amine groups were believed to interact with Al cations present in corundum. When filler particles were graphenated, reduced interfacial adhesion was thought to be the cause of worsening stiffness and tensile strength of obtained materials. In the case of SiC filler, significant enhancement of Young’s modulus (to 5100 MPa from 2400 MPa) was observed for graphenated-particle-based dopamine, with TMPGC–HMDA matrix at 10 wt% content of the filler. In terms of thermal properties, utilizing differential scanning calorimetry, differences in
Tg between reference NIPUs and their composite materials were not significantly high (up to ±5 °C). Two-point electrical conductivity measurements were undertaken to assess the electrical properties of obtained composites. Only samples obtained with diethylenetriamine showed electrical conductivity when filled with 30 wt% graphenated fillers. Thus, such low electrical conductivity may be the base for obtained materials to be eligible for electromagnetic shielding applications [
42].
Pössel et al., prepared NIPU composites containing γ-Al(OH)
3 (O-gibbsite) nanoplatelets, which were subsequently modified by surface treatment with lysine to obtain ly-gibbsite nanofiller. Utilizing the freeze-drying method, a gibbsite was obtained in the form of a powder. To facilitate better compatibility with the non-isocyanate matrix, gibbsite was treated with L-lysine, which was done before the freeze-drying process. Two carbonate compounds: trimethylolpropane glycidylethercarbonate (TMPGC) and pentaerythritol glycidylethercarbonate (PGC), as well as two amine compounds: hexamethylenediamine (HMDA) and diethylene triamine (DETA), were used for the synthesis of the matrices. Due to the volatile nature of amine compounds, the first step of composite preparation was to disperse gibbsite fillers in the cyclic carbonate substrate by a high shear mixer. After the addition of respective amine, NIPU materials were cured at 80 °C overnight and underwent a post-curing process for 4 h at 100 °C [
43].
Thermal and mechanical attributes were determined for obtained samples. Effects were observed to be similar to other NIPU composites [
42]: filling the polymer matrix with O-gibbsite (untreated with L-lysine) caused Young’s modulus to increase by 180% for TMPGC–HMDA, 140% for TMPGC–DETA, and 150% for PGC–DETA, respective to their non-filled counterparts. At the same time, tensile strength and elongation at break were observed to diminish. Modification with lysine proved to improve the performance of gibbsite fillers. For example, loading NIPU composites with up to 40 wt% of ly-gibbsite caused Young’s modulus to improve from 2800 MPa for TMPGC–HMDA to 6000 MPa for its composite. Improvement of similar magnitude was also observed for TMPGC–DETA- and PGC–DETA-based materials. Thin coatings (100 μm) were prepared with obtained NIPU composite formulations. Samples with untreated O-gibbsite happened to be opaque due to the formation of large O-gibbsite agglomerates. On the contrary, ly-gibbsite–NIPU composite remained translucent, even when the content of filler was 40 wt%. Ly-gibbsite was also used as a means to improve flame retardancy for NIPU. While halogen-containing compounds are known to be used for such applications, avoiding them comes in with accordance to green chemistry postulates. Using a gas burner to set NIPU composite samples aflame was conducted as a means to assess their properties. A flammable cotton piece was set below the burning samples to check for fire spread possibilities. Without any surprise, reference NIPUs turned out to be highly flammable, with burning droplets dripping down on the cotton and setting it aflame. Ly-gibbsite added in the amount of 30 wt% to TMPGC–HDMA and PGC–DETA matrices significantly enhanced their flame retardancy [
43].
3.2. NIPU as an Additive
Apart from being polymeric matrices, NIPUs may act as additives, modifiers, or fillers incorporated into different polymers. In such a way, new material properties can be achieved by utilizing NIPUs’ inherent properties and the formation of favorable interactions with the polymer matrix.
For instance, non-isocyanate polyurethane (NIPU) was applied to solve the brittleness issue of poly(propylene carbonate) (PPC), which shows poor elongation at break (below 10% at 20 °C) that critically restricts its applications. Thus, separate NIPUs were obtained and introduced to PCC to enhance its properties. NIPU was chosen due to its non-toxic synthesis, low glass transition temperature, and capability to create hydrogen bonding between carbonyl or oxygen units in poly(propylene carbonate). After the synthesis, NIPU and PCC were melt-blended in a mixer in a variety of different weight ratios (NIPU/PPC = 1/99, 2.5/97.5, 5/95, 8/92,10/90, 13/87, or 15/85). The abovementioned ratios of NIPU/PCC had a significant impact on the miscibility and the morphology of obtained materials, which was connected with the equilibrium between inter and intermolecular hydrogen linkages formed between poly(propylene carbonate) and polyurethane. As the amount of NIPU in the composition grew, the fraction of intermolecular hydrogen linkages between two copolymers also increased. Below the amount of 10 wt%, NIPU dispersed equally in poly(propylene carbonate), and the shift from brittle to durable occurred when L/d reached a critical value of 1.74, where L and d were center-to-center distance and the diameter of the particles, respectively. The unnotched impact strength grew three times (to above 20 KJ/m
2) compared with neat PPC (slightly above 8 20 KJ/m
2) when the amount of introduced NIPU reached 10 wt%. Flocculation of NIPU was observed when the NIPU loading reached 13 wt%, which lead to a decline in toughness. Elongation at break for PCC was measured to be around 10%, but while modified with NIPU, it increased up to 30% when filler loading was over 8 wt%. It increased tensile strength (from around 53 MPa for PPC) by 15 MPa for formulations filled with 2.5 wt% of NIPU. On the contrary, elongation at break and the tensile strength diminished with the addition of 13 wt% of NIPU. Such a phenomenon was noticed to occur in PLA/hyperbranched polyamide blend [
44] and PPC/low-molecular-weight urethane [
45].
Mechanical properties, chemical structure, and morphology of an epoxy resin modified by combining nanoclay nanoparticles NIPU were studied by Białkowska and co-workers. The use of nanoclays as strengthening fillers for epoxy resins has already been widely studied. However, not much research has been done to reinforce epoxy resins by incorporating non-isocyanate polyurethanes. Based on preliminary studies, epoxy-nanoclay nanocomposite containing 1 wt% of bentonite was chosen for further modification with different amounts of NIPU, yielding hybrid epoxy composites. As for the NIPU, an MNF 30 oligomer (i.e., oligomer based on urea and formaldehyde, containing 30% mol of 2-hydroxy-6-naphthalenesulfonic acid [HNSA], with an equimolar proportion of hard and soft segments) was used due to its outstanding tensile strength (reaching 12.7 MPa) and a sufficient elongation at break, reaching 110%, amongst compositions tested before [
46].
Nanoclay was mixed in, becoming a 15% dispersion in acetone. Ultrasonic homogenization was applied to ensure the uniformity of the mixture. Oligomeric urethane was added in up to 15 wt% in its liquid state. Finally, a stoichiometric amount of curing agent was added and mixed, and afterward, the compositions were put inside aluminum molds, ensuring shapes preferred for later mechanical testing. The curing process was conducted at room temperature, followed by post-curing at 60 °C. Tolerance to slow (the critical stress intensity factor
Kc) and fast crack propagations (the impact strength-IS) and the flexural strength in three-point bending were determined for samples with different amounts of NIPU. The epoxy formulations exhibited higher ductility, superior flexural strain at break, better flexural energy to break, together with brittle fracture energy. Compositions loaded with 10% NIPU and 1% clay exhibited the highest impact strength and
Kc among other samples (including reference). A negligible reduction in the flexural strength in comparison to the pure epoxy sample can be seen. However, a very significant increase in
εb is observed upon the incorporation of NIPU. The explanation for the improvement in the resistance to slow and fast crack propagations may be found by analyzing SEM micrographs (
Figure 4). Hybrid epoxy composites displayed rugged and stratified fracture surfaces with notable plastic yielding, while the pure epoxy counterpart exhibited a smooth surface, ordinary for fragile polymers, without plastic yielding or an elongated structure [
47].
3.3. NIPU as a Building Block in Copolymer Synthesis
Monomers such as esters, amides, and epoxy compounds are utilized to synthesize NIPU copolymer hybrid materials. Most attention is paid to epoxy compounds as they are often intermediates in obtaining cyclic carbonates from different kinds of raw materials, including those from renewable sources [
6].
Hence, Haniffa et al., obtained non-isocyanate polyurethane by using 1,3-diaminopropane (DM) and isophorone diamine (IPDA) utilized in the role of curing agents. The raw material was Jatropha curcas oil (JCO). Substantial unsaturated fatty acid content and satisfactory oxidation stability of Jatropha curcas oil make it possible for this oil to be used for numerous NIPU commercial applications. Additionally, it is a renewable resource, which only adds to the concept of NIPUs being environmentally friendly materials. Carbonated JCO (designated CJCO) was synthesized through a two-step process. First, JCO was epoxidized with hydrogen peroxide, with formic acid being present in the reaction environment. Then, CJCO was subjected to carbon dioxide fixation with tetrabutylammonium bromide (TBAB) as a catalyst. Alkyd resin of JCO was also treated in the same way, leading to carbonated alkyd resin (CC-AR). CJCO was mixed with various ratios of CC-AR (
w/
w) to boost its characteristics and reacted with 1,3-diaminopropane (DM) and isophorone diamine (IPDA), as shown in
Scheme 16 [
48].
Thermomechanical properties of the obtained NIPUs turned out to be effectively altered alongside the CC-AR weight ratio and amine amount. The CJCO cured with DM displayed outstanding elongation at break of 230%, yet low Young’s modulus. CJCO/CC-AR mixed in 1:3 ratio showed circa threefold improved Young’s modulus of 680 MPa and better
Tg value of 44 °C with IPDA in relation to DM. The reason for this may be that the cycloaliphatic structure of IPDA creates steric hindrance, or it is due to a highly cross-linked network structure. However, CJCO/CC-AR blend with CC-AR ratio of 1:4 exhibited a decrease in modulus and tensile strength in relation to NIPU-based composition with a lower CC-AR ratio. Thus, the further increase of CC-AR in the CJCO/CC-AR blend is believed to result in slipping amongst the chains of the saturated matrices alongside the further reduction in terms of thermomechanical properties [
48].
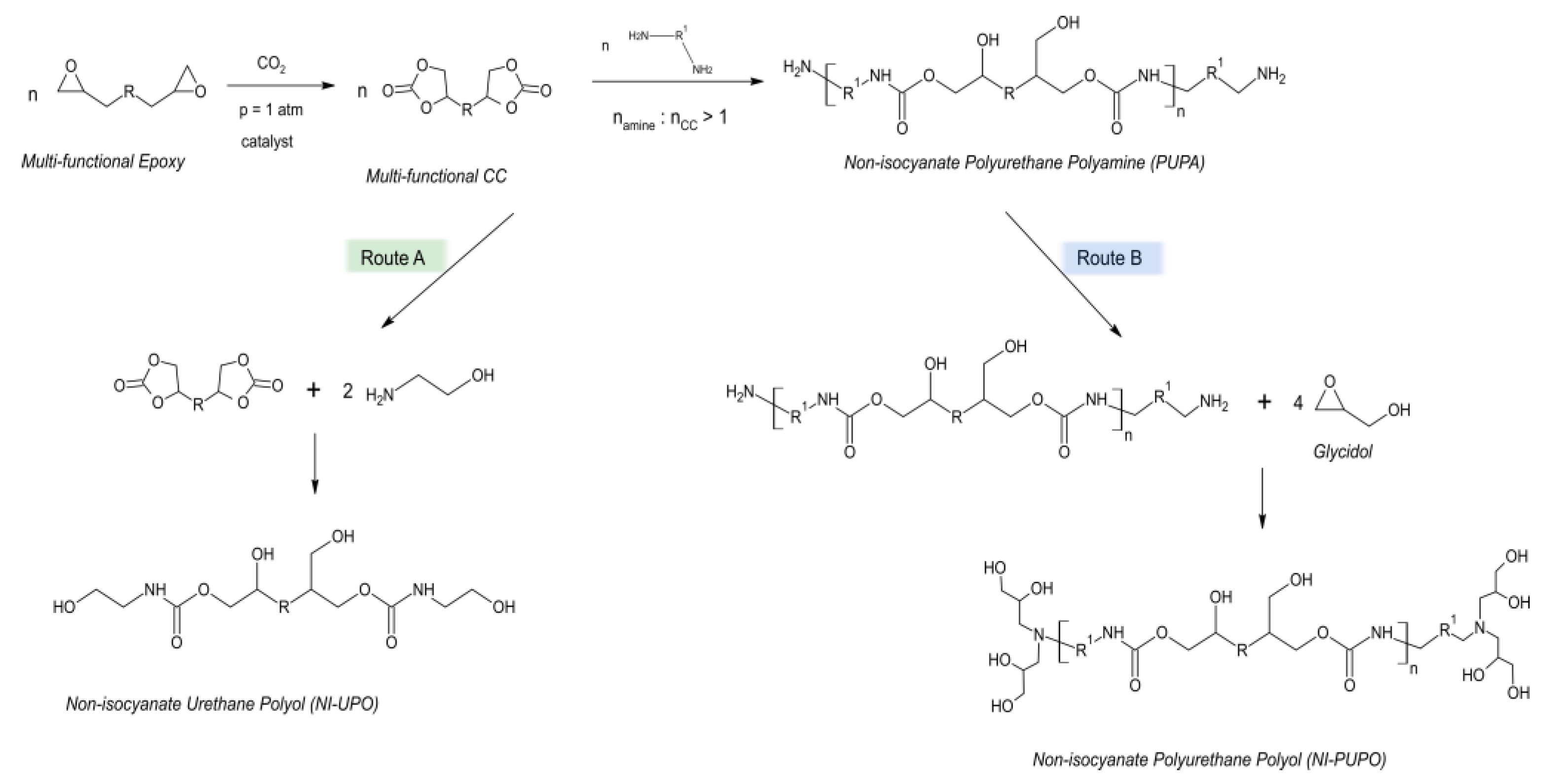
Asemani et al., obtained NIPU coatings by (1) synthesizing cyclic carbonates via catalytic fixation of CO
2 by epoxy compounds and then (2) by reacting them with an excess quantity of amine to, in turn, obtain polyurethane polyamines (PUPAs). Final products were prepared by mixing stoichiometric amounts of PUPAs and aliphatic epoxies as cross-linking compounds—
Scheme 17 [
49].
The obtained results revealed that well-designed NIPU oligomers led to materials showing extraordinary performance, being flexible in low temperatures and resistant to chemical agents. Such customization is possible by choosing proper cyclic carbonate and amine types and varying their relative molar ratios. Generally speaking, non-isocyanate polyurethane coatings with mechanical, physical, and chemical properties similar to traditional polyurethane coatings can be obtained by deliberate choice of NIPU building blocks. On the contrary, the thermal stability of NIPU coatings was reported to be remarkably lowered in relation to traditional polyurethane coatings [
49].
Pathak et al., synthesized a non-isocyanate polyurethane coating based on dehydrated castor oil fatty acid (DCOFA) and tris-2-hydroxyethyl isocyanurate (THEIC). Non-toxic THEIC provided excellent thermal and chemical resistance to the polymer due to its heterocyclic nature, enabling its usage in numerous practices in the manufacturing of coatings. The synthesis consisted of esterification of THEIC with DCOFA and subsequent epoxidation of the ester formed (TEFA), followed by fixating CO
2 with oxirane rings within the intermediate product—THEIC–ester of fatty acid (CTEFA). Cyclo-carbonated THEIC–ester of fatty acid (CTEFA) underwent the curing process with either hexamethylene diamine (HMDA), isophorone diamine (IPDA), or diamino diphenyl sulphone (DDS). The type of amine used for curing had a significant influence on the overall properties of obtained coatings. Coatings with aromatic and cyclo-aliphatic amines used as curing agents exhibited superior mechanical, chemical, thermal, and anti-corrosive properties compared to those obtained with the aliphatic amine [
50].
De Aguiar et al., synthesized polydimethylsiloxane hybrid urethane (PDMSUr) coatings by ring-opening polymerization of a PDMS-derived cyclic carbonate (CCPDMS) by 3-aminopropyltriethoxysilane (APTES) alone or with 5-amino-1,3,3-trimethylciclohexano methylamine (IPDA). Poly(dimethylsiloxane) diglycidyl terminated ether (E-PDMS) underwent a carbon dioxide fixation reaction with tetraethylammonium bromide (TEAB) as a catalyst, resulting in the formation of PDMS-derived cyclic carbonate (CCPDMS). The first synthetic route was a two-step reaction—in the first step, CCPDMS and 3-aminopropyltriethoxysilane (APTES) were stirred and heated at 70 °C for 40 min. Next, phosphotungstic acid PWA, added in the amount of 35 wt% or 1 wt%, dissolved beforehand in dimethylacetamide (DMAc), was mixed in. The reactants were stirred at 50 °C for 24 h. Another batch of the hybrid PDMSUr was obtained in three steps. First, CCPDMS and 5-amino-1,3,3-trimethylciclohexano methylamine (IPDA) were mixed at 70 °C for 20 min. Afterward, APTES was mixed in and stirred again for 20 more minutes. Finally, PWA (prepared in the same amount and manner as previously) was added, and the mixture was stirred 50 °C for 24 h, as it was done in the first route. Although authors primarily focused on the physical surface modification of biomedical grade metallic surfaces by oxygen plasma and laser treatment, the hybrid PDMS–urethane coatings were found to be fitting materials for covering metal surfaces utilized in orthopedic or dental implants, characterized by preferable adhesion strength. Additionally, the obtained hybrid-PU layers may serve as anti-corrosion materials for metals directly exposed to physiological media by forming a hydrophobic physical barrier [
51].
The topic of obtaining NIPU composite materials has been actively studied. As conventional polyurethanes can be utilized for composite preparation, their non-isocyanate counterparts also fit this role successfully. There are three distinctive ways to incorporate NIPUs in composite materials. In the conventional approach, NIPUs are used as a polymer matrix and, thus, are modified with different compounds. The useage of nanofillers is especially promising, as even a low amount of nanofiller incorporated in NIPU matrix can improve its properties significantly. Examples include, but are not limited to, graphene oxide or POSS nanoparticles. The second way to incorporate NIPU in a composite material is to use it as an additive to another polymer matrix. The usage of NIPU as a filler improves mechanical properties of such polymer matrices as poly(propylene carbonate) or epoxy resin. The final approach to manufacturing NIPU composite materials is the synthesis of copolymers with NIPU as one of the building blocks. Epoxy compounds receive most attention in terms of copolymers for NIPUs. The copolymerization of non-isocyanate polyurethanes with epoxies allows for overcoming the flaws of the latter in terms of mechanical properties or chemical resistance. Taking into consideration the vast possibilities of combining different materials in the process of composite preparation, the progress in this field is far from over.