Simulation of Slip-Oxidation Process by Mesh Adaptivity in a Cohesive Zone Framework
Abstract
:1. Introduction
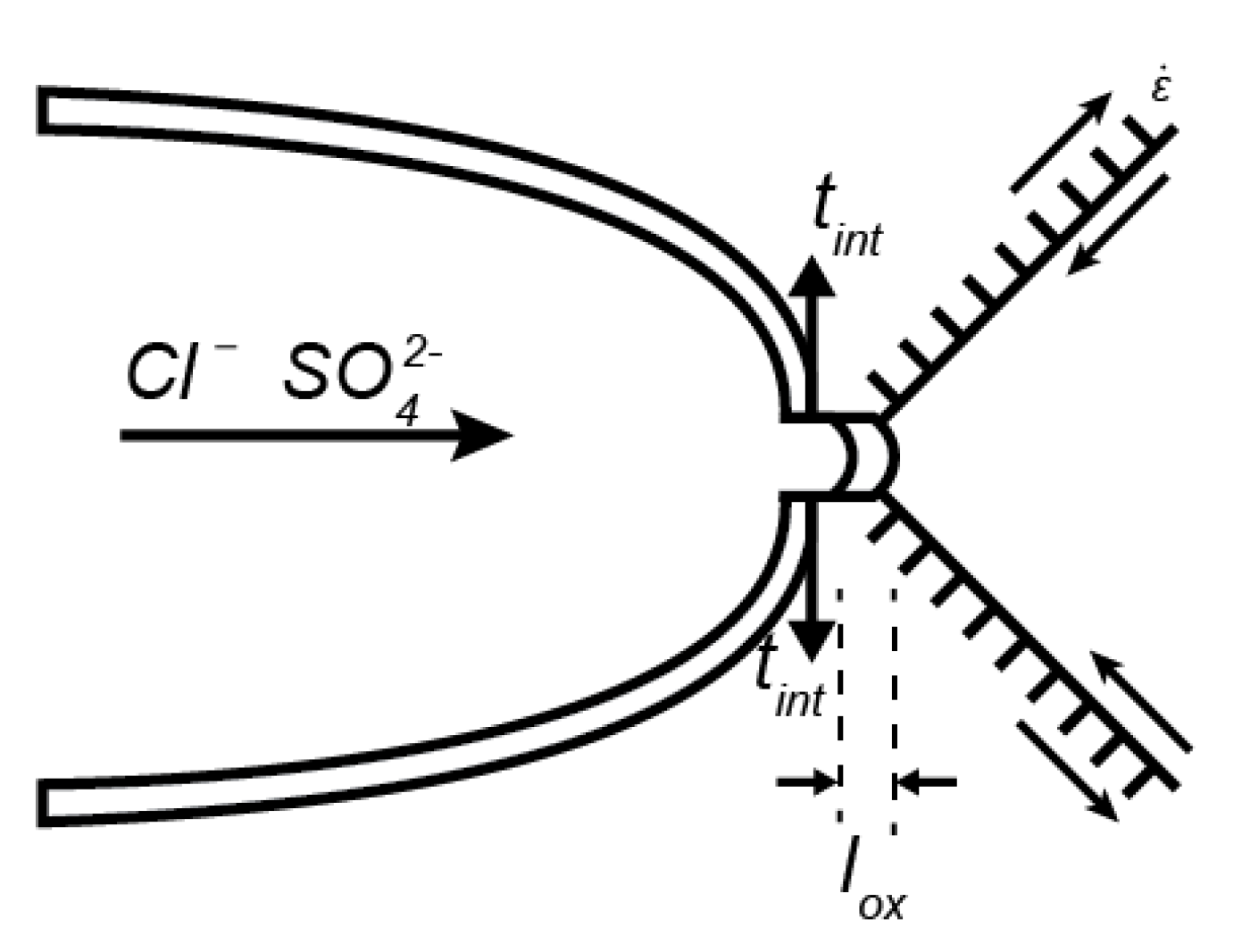
2. Model
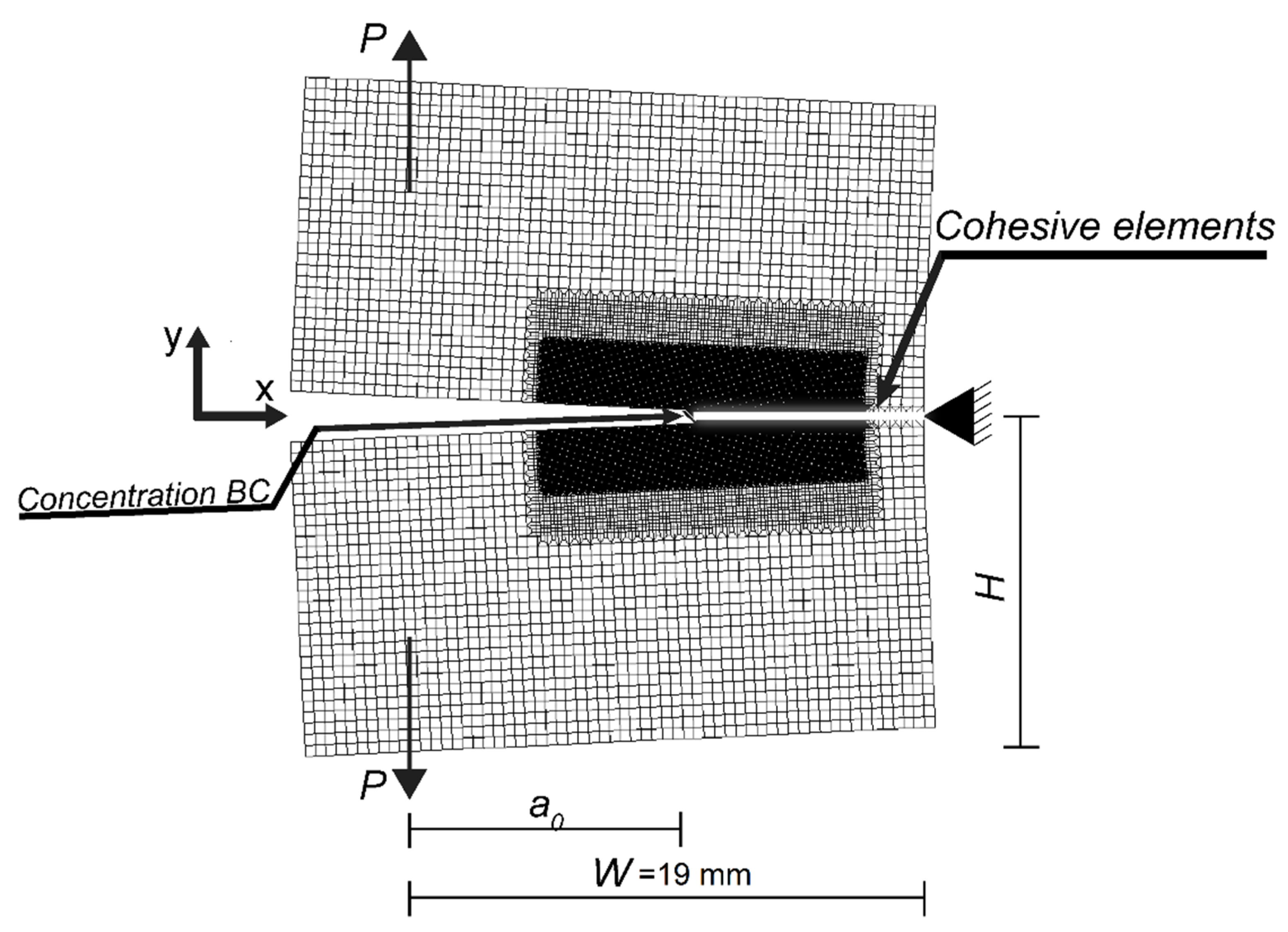
3. Structural Model
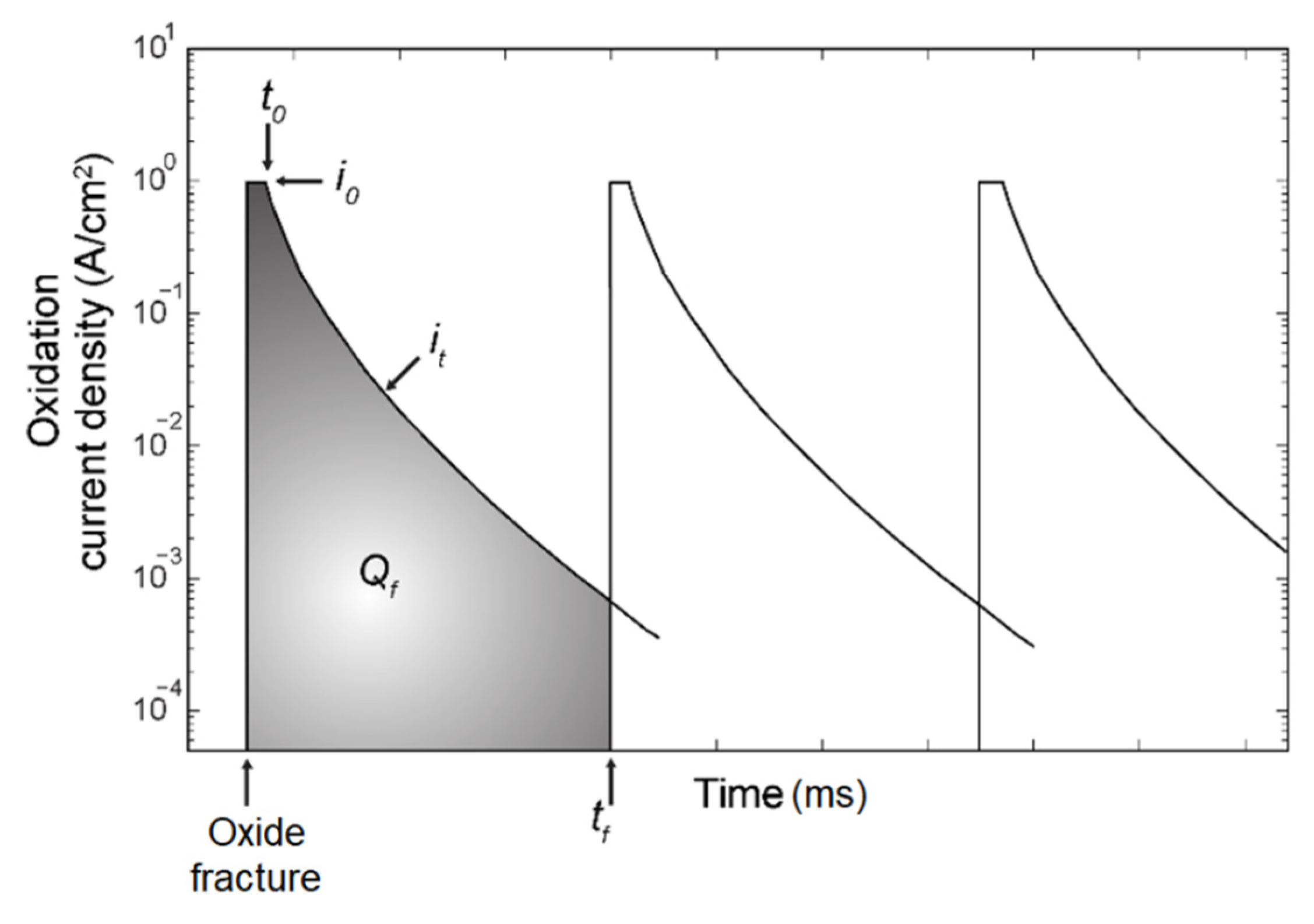
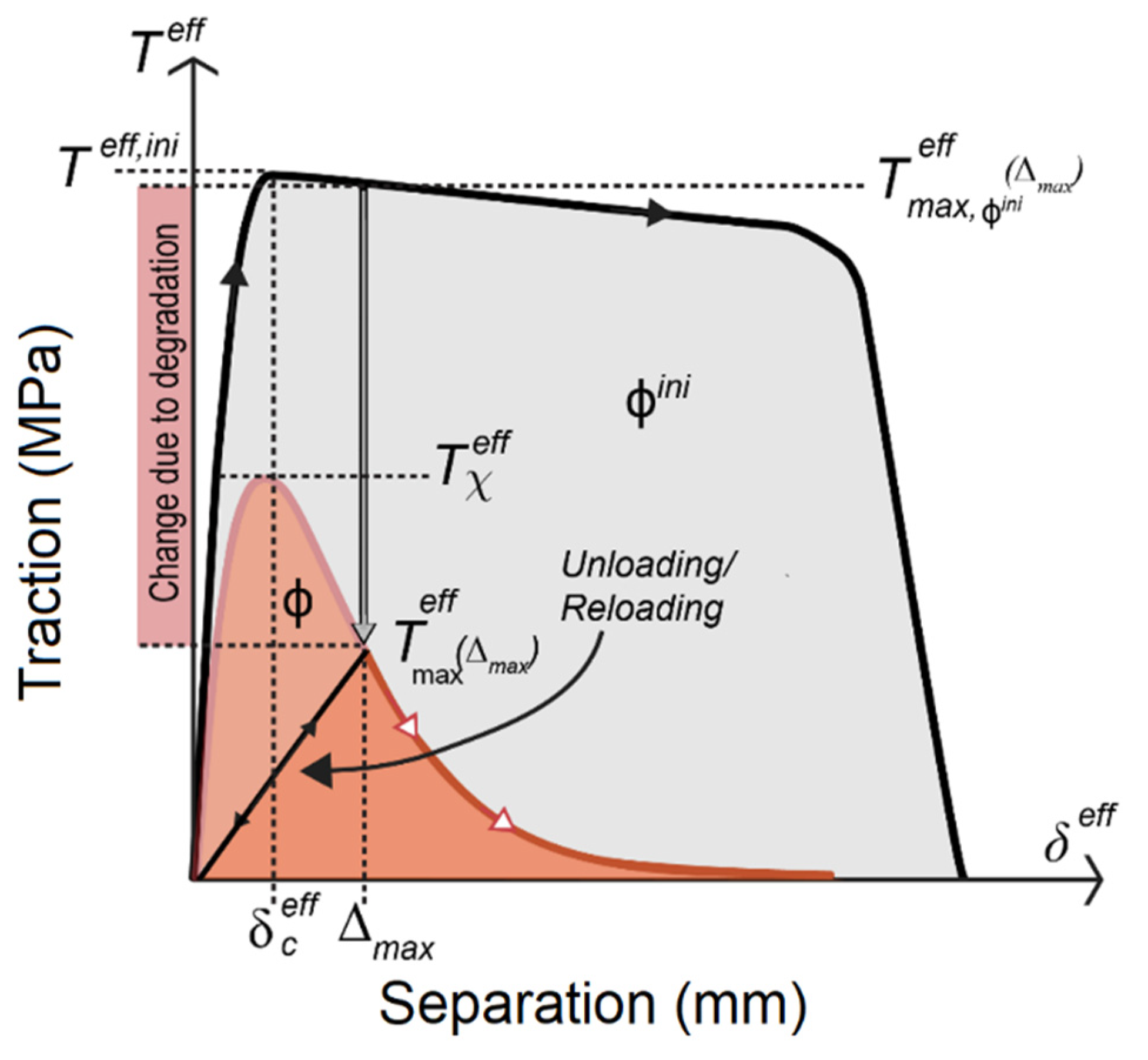
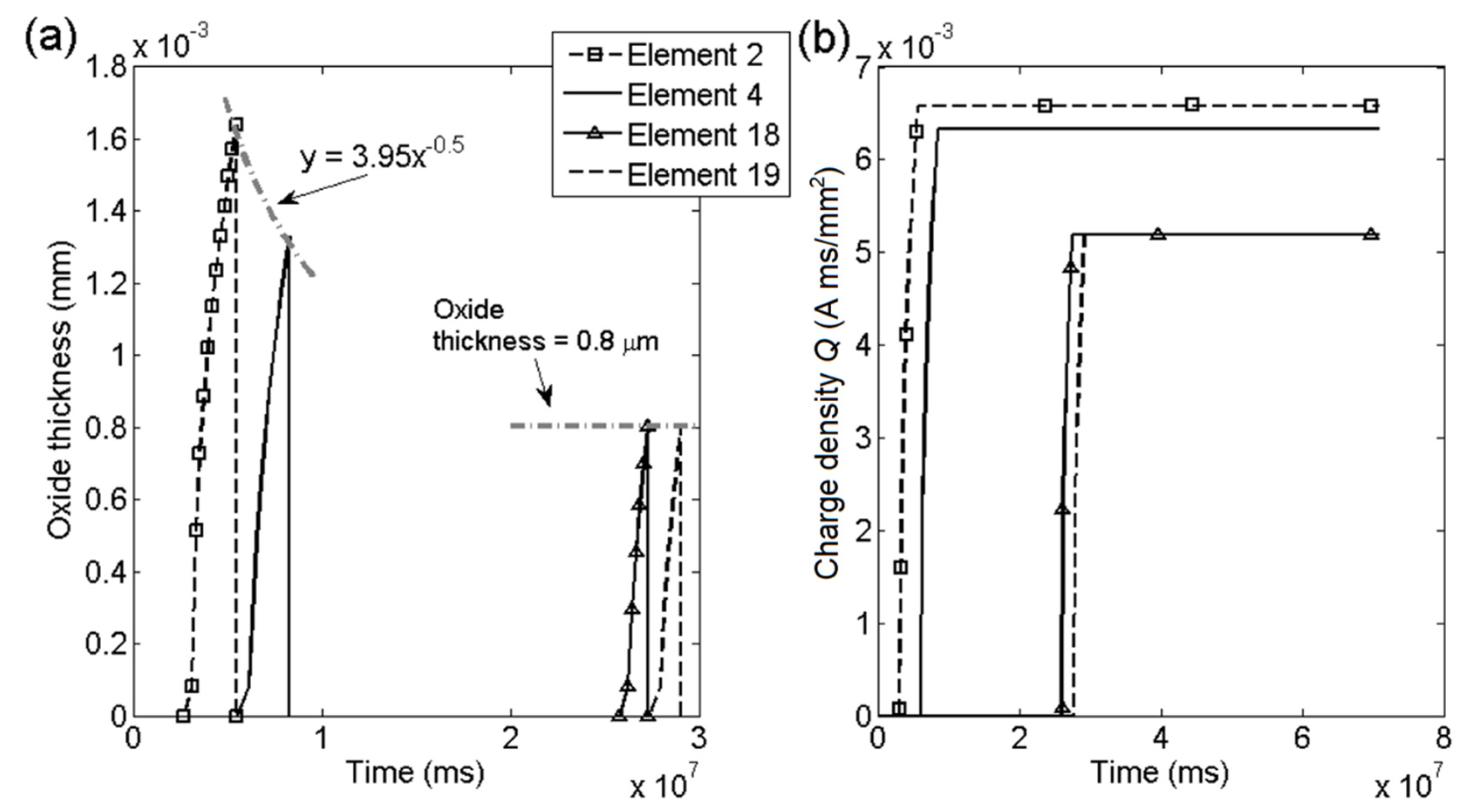
4. Degradation
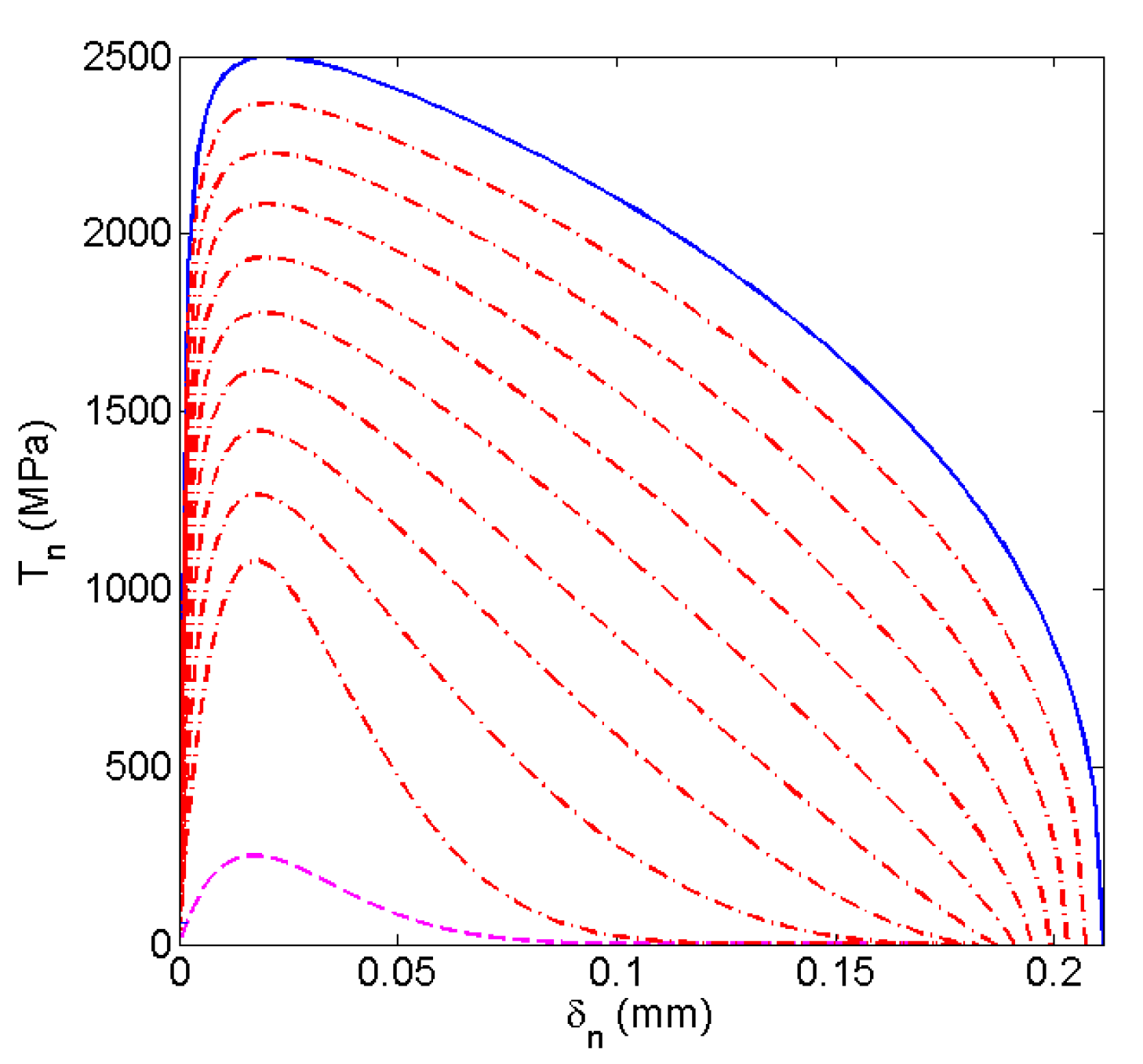
5. Results
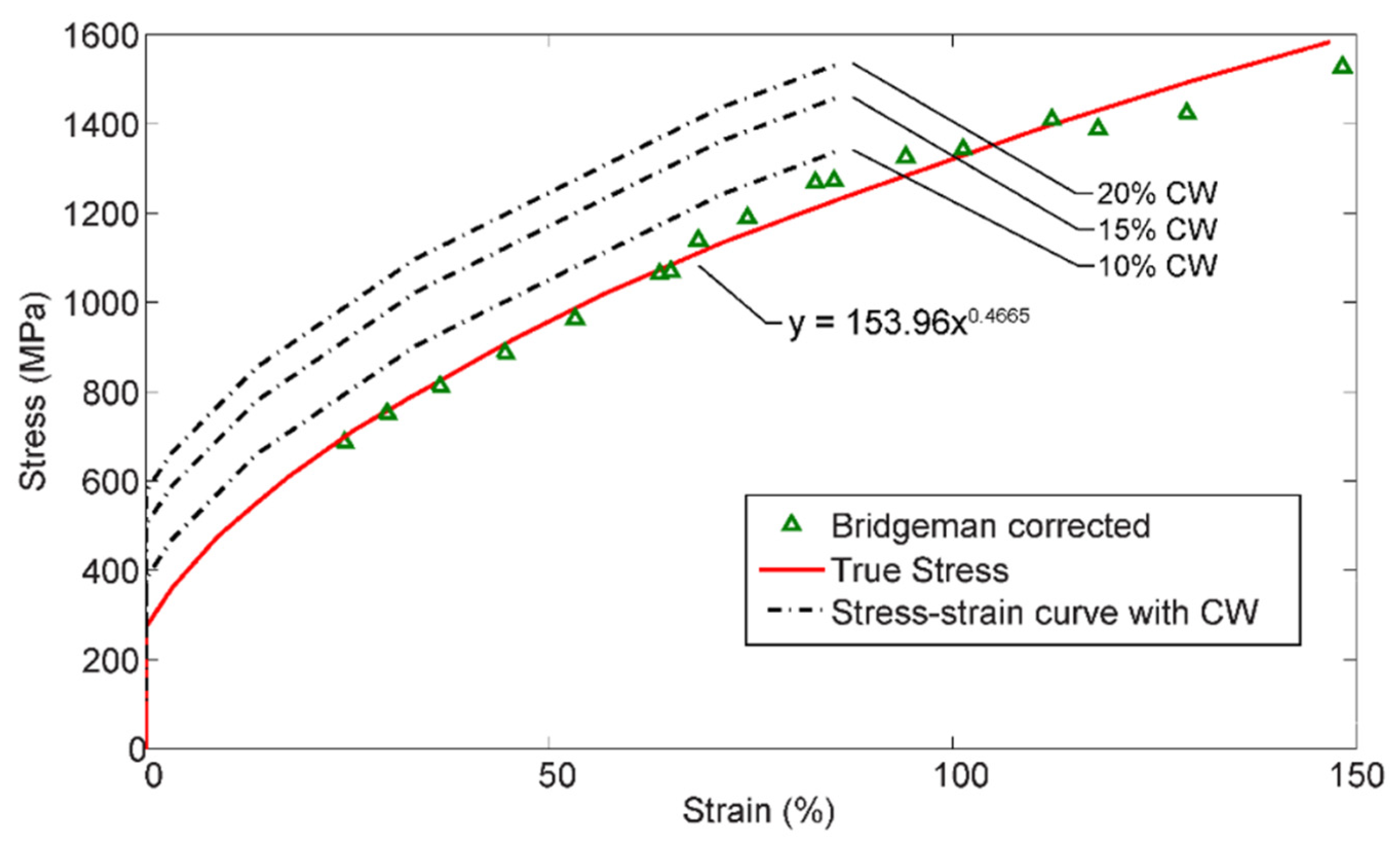
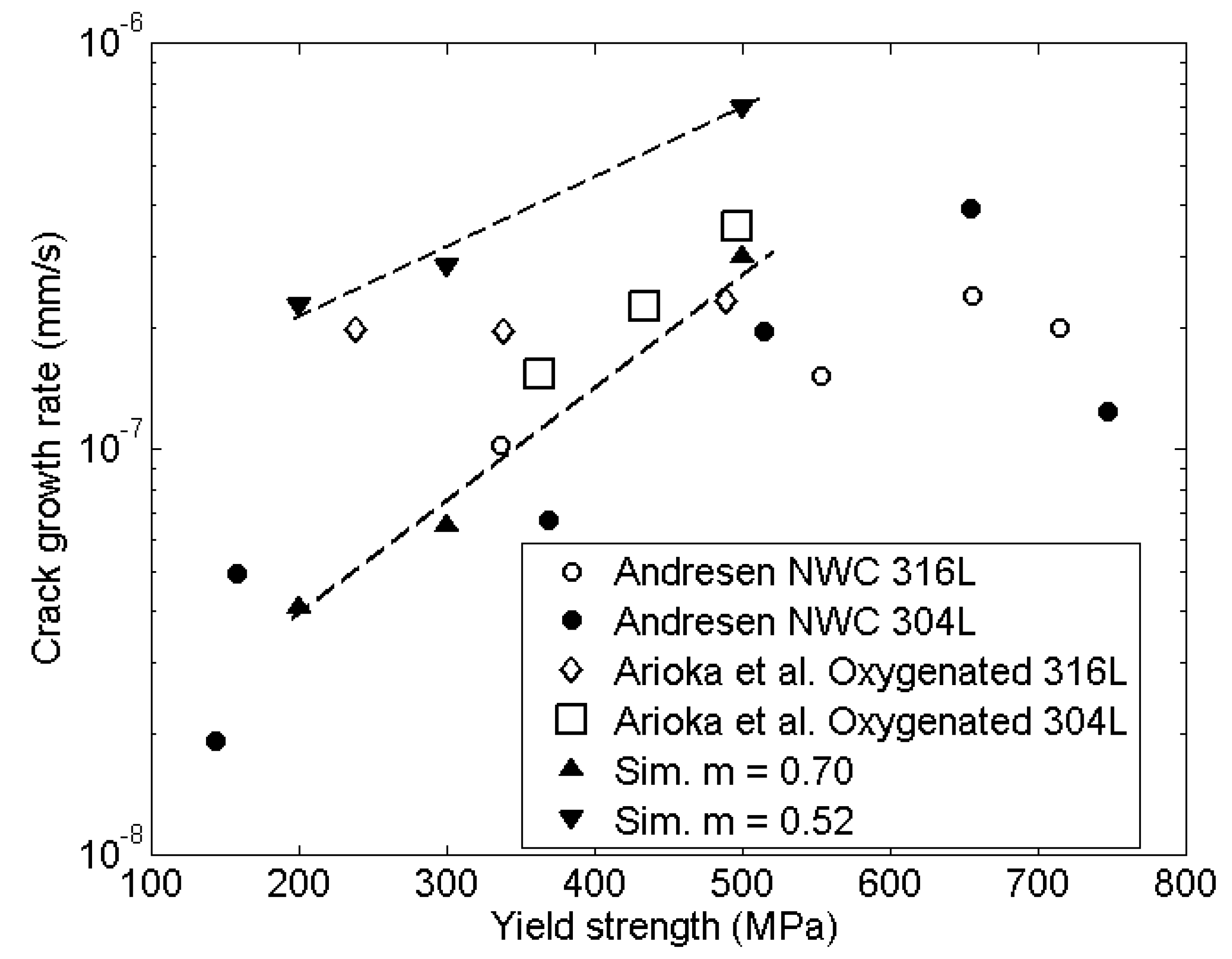
5.1. Yield Strength
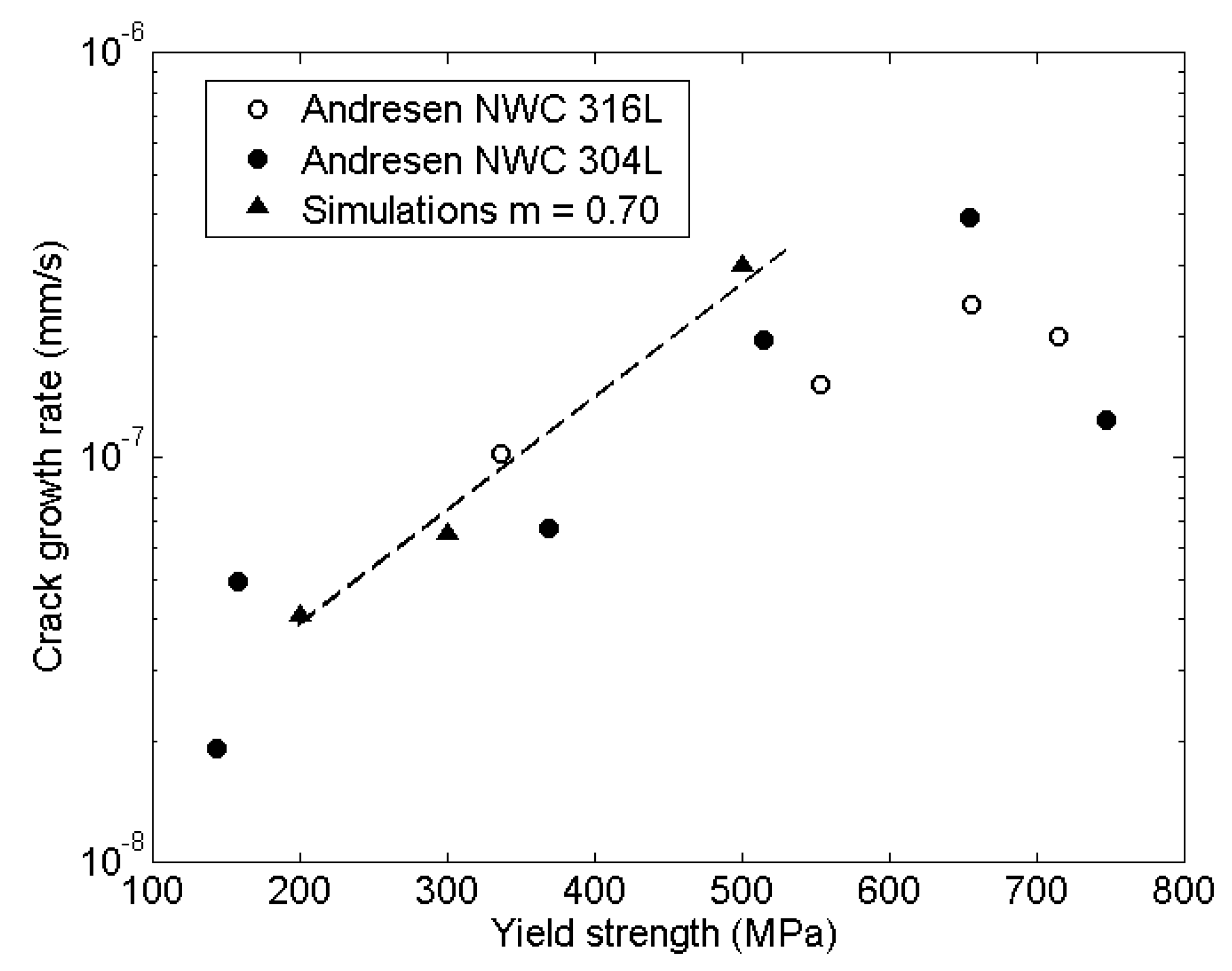
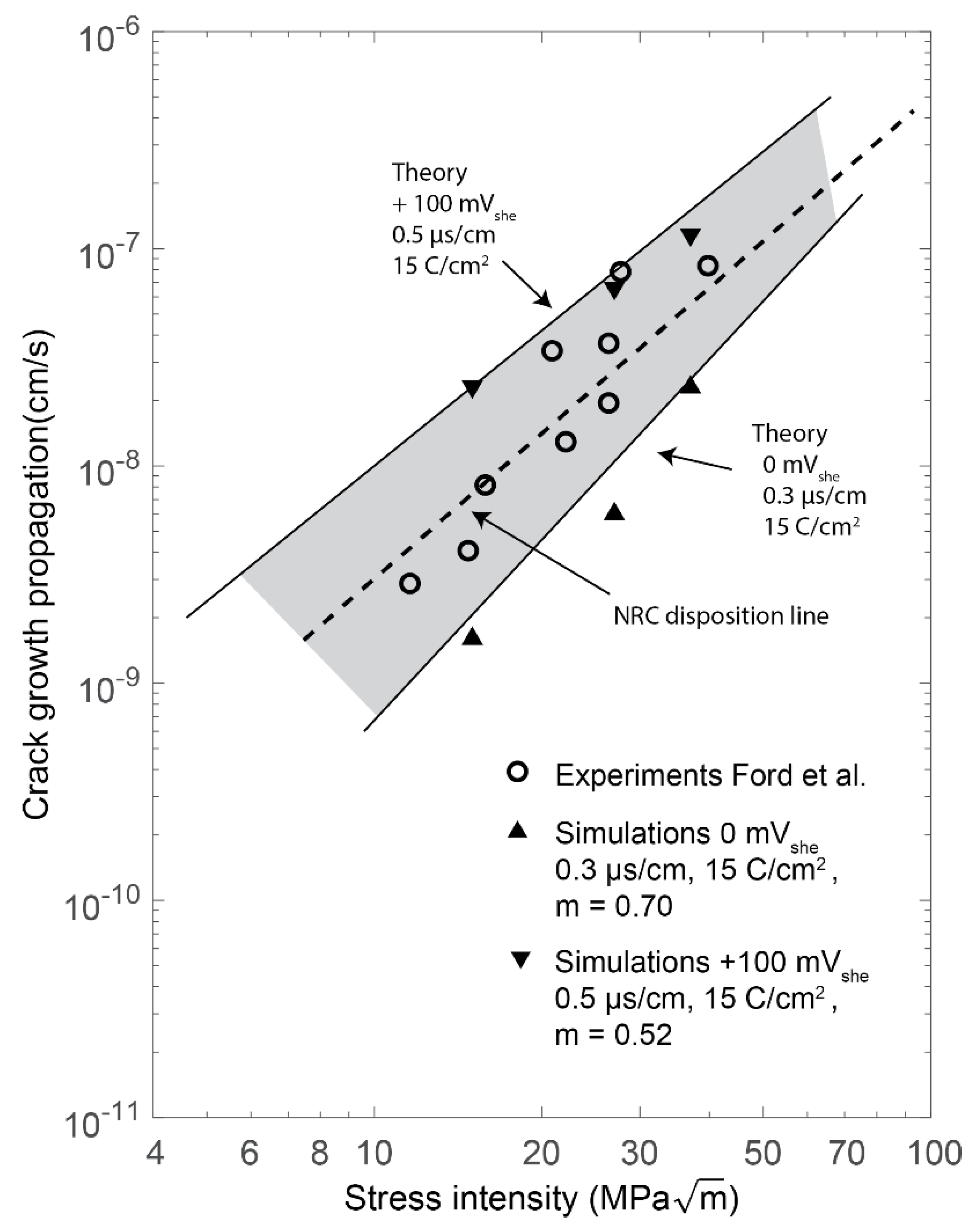
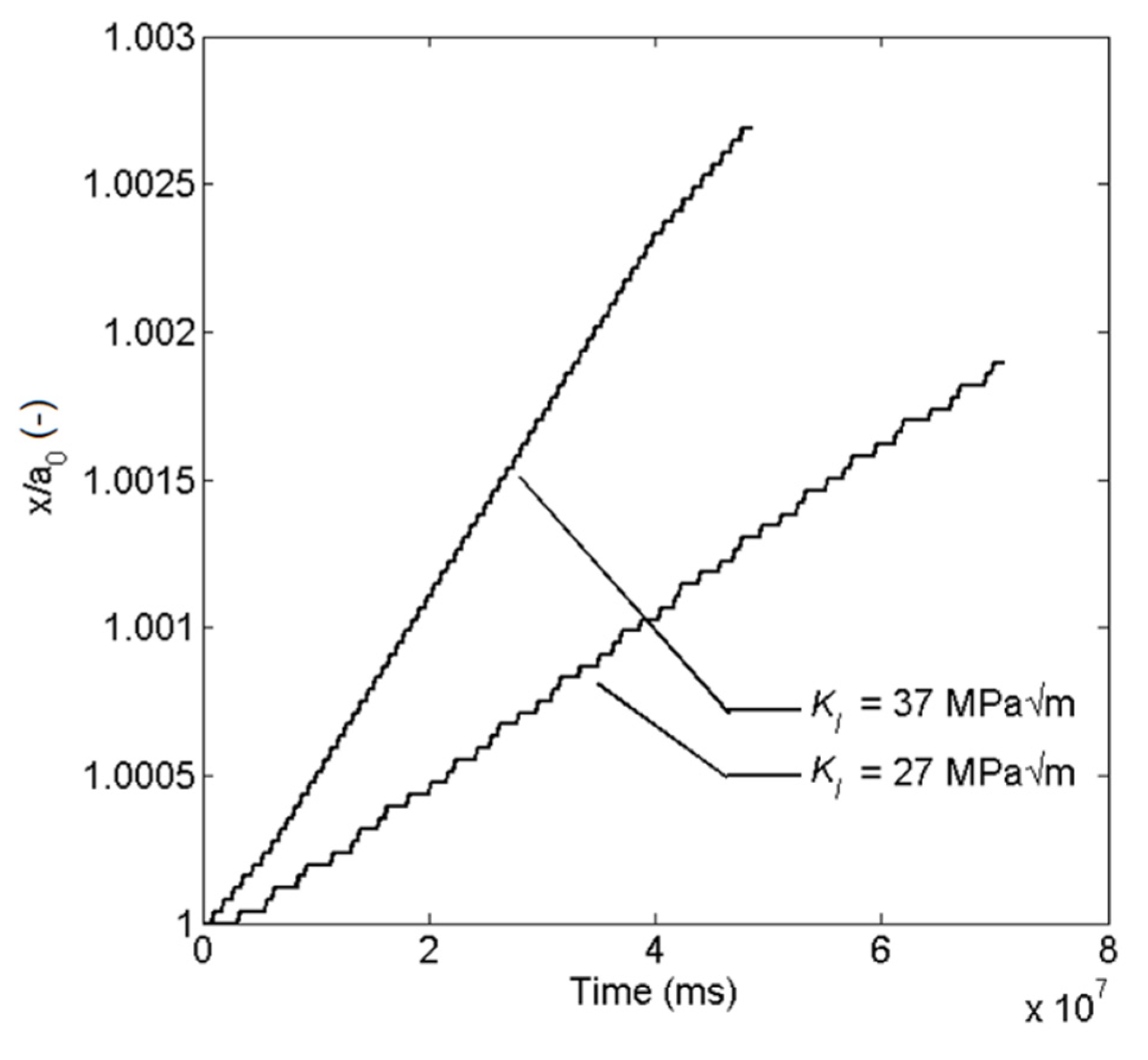
5.2. Stress Intensity Factor
6. Conclusions
- The cyclic physics of the slip oxidation model was replicated. In the model, the thickness of the oxide was taken into consideration as the physical length of the cohesive element. The cyclic process was modelled with oxide film growth, oxide rupture, and re-passivation.
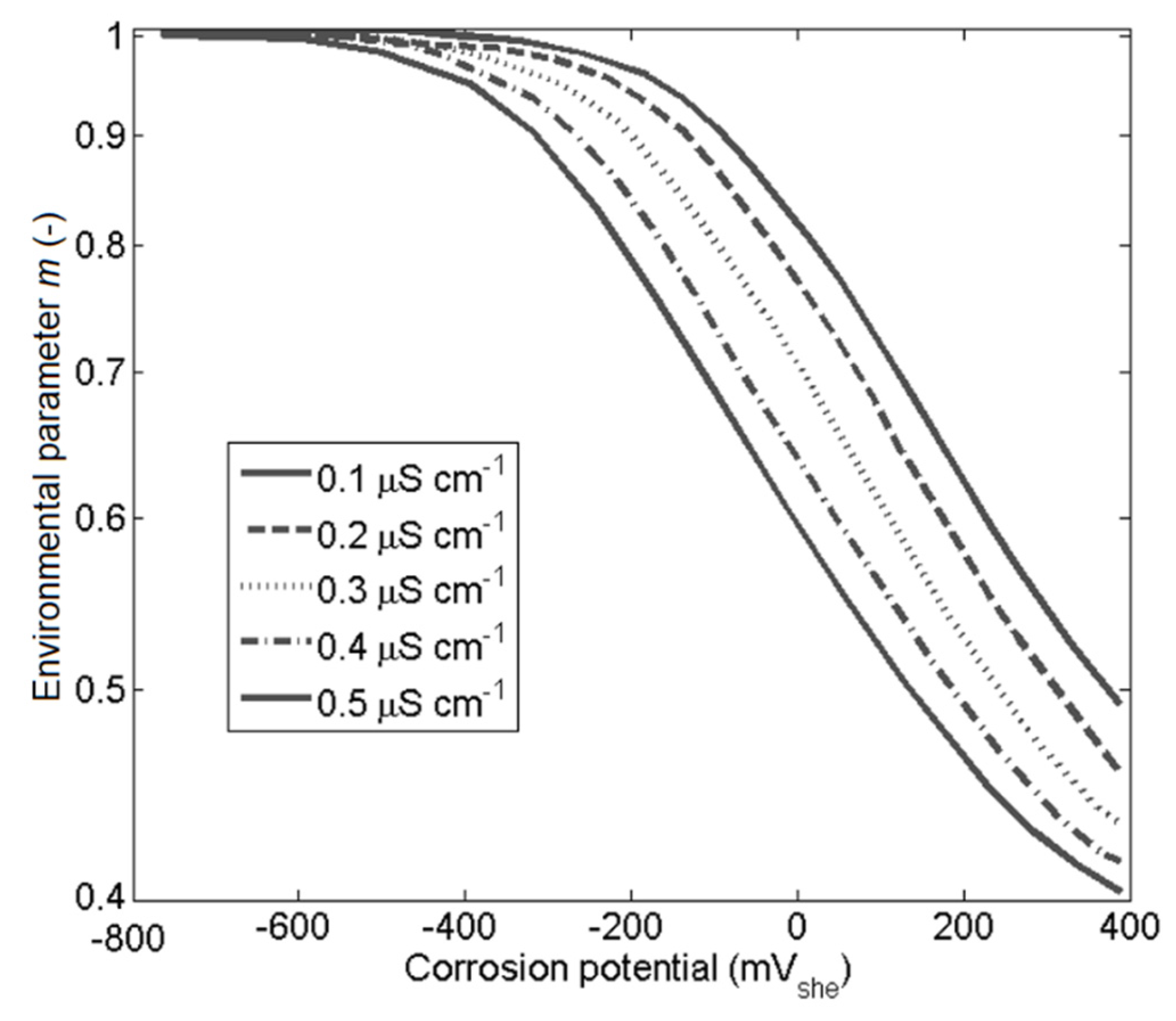
- The model shows good agreements with experiments in the literature for changes in stress intensity factor, yield stress representing cold work, and environmental factors such as conductivity and corrosion potential.
- When comparing the stress intensity factor simulations to the theoretical upper limit, the simulations gave an underestimation by a mean factor of 1.15. The lower limit was underestimated by a factor of 2.61. The mean deviation during all stress intensity simulations was calculated to .
- Cold works simulations gave good agreement also with experiments in oxidizing environment. In the experimental data the non-oxidized to oxidized environment increased the crack growth rate by a factor of 4.3 at yield strength 370 MPa and 1.8 at 490 MPa. The simulations gave an increase of 4 at yield strength 370 MPa and 2.3 at 490 MPa.
- The model was computational and cost effective for predicting IGSCC, which is useful for optimization situations.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Nomenclature
| model parameter (C mm3) | |
| crack length, initial crack length (mm) | |
| concentration (ppb) | |
| diffusivity, in liquid and in solid, respectively (mm2/ms) | |
| Young’s modulus, (GPa) | |
| Faraday’s constant (C/mol) | |
| half height of specimen (mm) | |
| initial-, slope current density, current density (A/mm2) | |
| KI | stress intensity factor (MPa) |
| energy proportion constants (N mm/(A ms)) | |
| Atomic weight (g/Mol) | |
| environmental parameter | |
| process parameter, (MPa mm2/g) | |
| force (kN) | |
| energy proportion constant | |
| charge density, charge density for one cycle (A ms/mm2) | |
| effective traction, current maximum traction and initial traction, respectively (MPa) | |
| normal and tangential initial tractions (MPa) | |
| normal and tangential fully degraded TSL tractions (MPa) | |
| current tractions: effective, normal and tangential, respectively (MPa) | |
| cohesive traction (MPa) | |
| time (ms) | |
| time for repassivation, time between ruptures (ms) | |
| oxide thickness (mm) | |
| length of CT-specimen (mm) | |
| thickness (mm) | |
| spatial coordinate (mm) | |
| fitted equation for oxide thickness (mm) | |
| electron exchanged | |
| initial and fully degraded normal softening parameter | |
| initial and fully degraded tangential softening parameter | |
| constant (1/s) | |
| cohesive element separation (mm) | |
| maximum effective separation (mm) | |
| incremental time step (ms) | |
| normal and tangential current separation (mm) | |
| critical effective separation (mm) | |
| effective separation (mm) | |
| virtual separation (mm) | |
| internal virtual work (Nmm) | |
| surface film rupture strain | |
| crack tip strain rate (1/s) | |
| damage parameter | |
| normal and tangential initial slope indicators | |
| normal and tangential fully degraded slope indicators | |
| mass density (g/mm3) | |
| yield stress (MPa) | |
| Poisson’s ratio | |
| degradation parameter | |
| fracture energy at the crossing point (N/mm) | |
| normal and tangential fracture energies (N/mm) | |
| normal and tangential initial fracture energies (N/mm) | |
| normal and tangential fully degraded fracture energies (N/mm) |
Appendix A
Appenidx A.1. Environment Parameters
| Parameters | Standard Value |
|---|---|
| Atomic weight, M (g/Mol) | 55.382 |
| Oxidation current density, i0 (A/cm2) | 0.12 |
| Number of electrons exchanged, z | 2.67 |
| Faraday’s constant, F (C/mol) | 96,500 |
| Mass density, ρ (g/mm3) | 0.00786 |
Appenidx A.2. Degradation Parameters
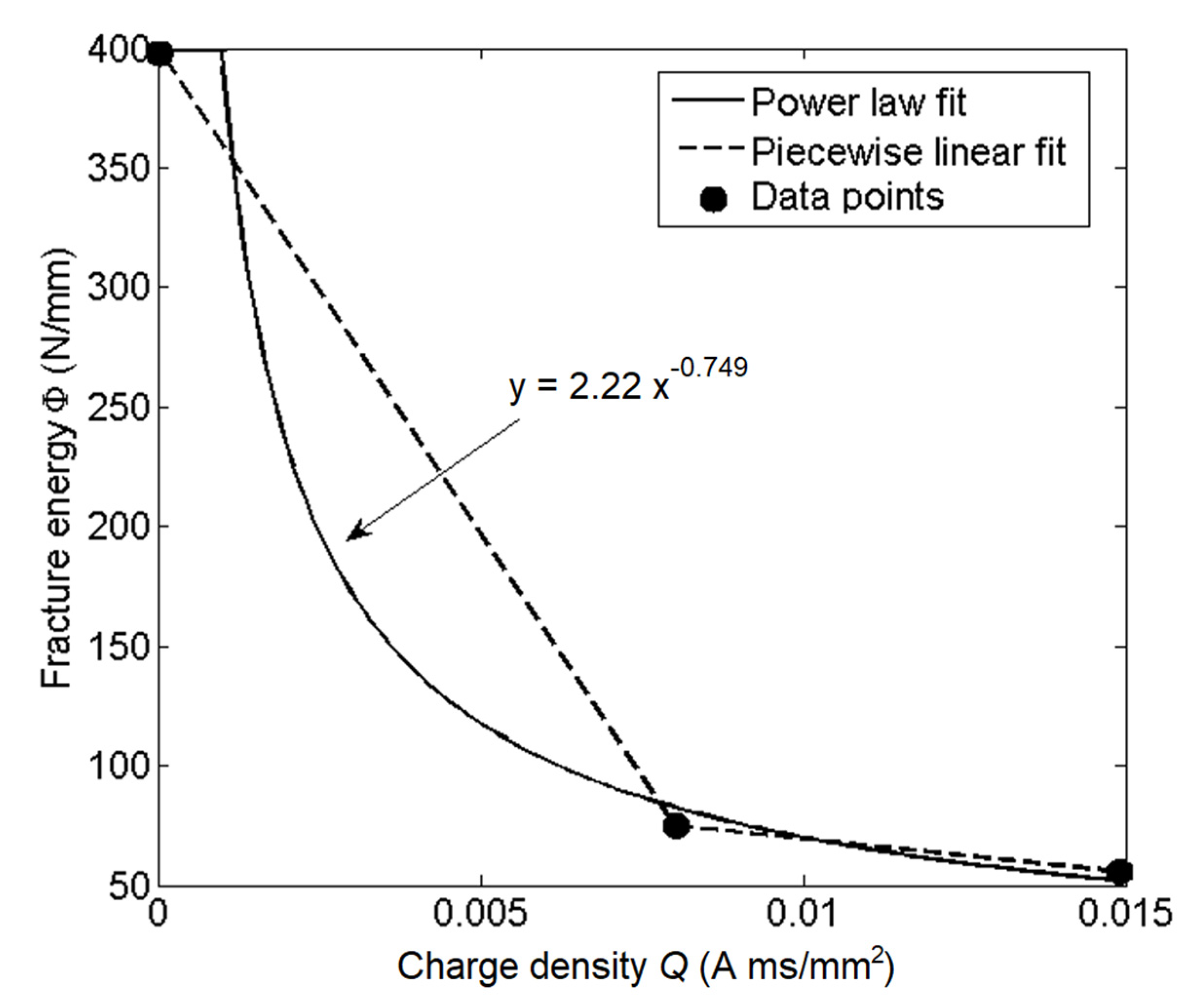
Appendix B
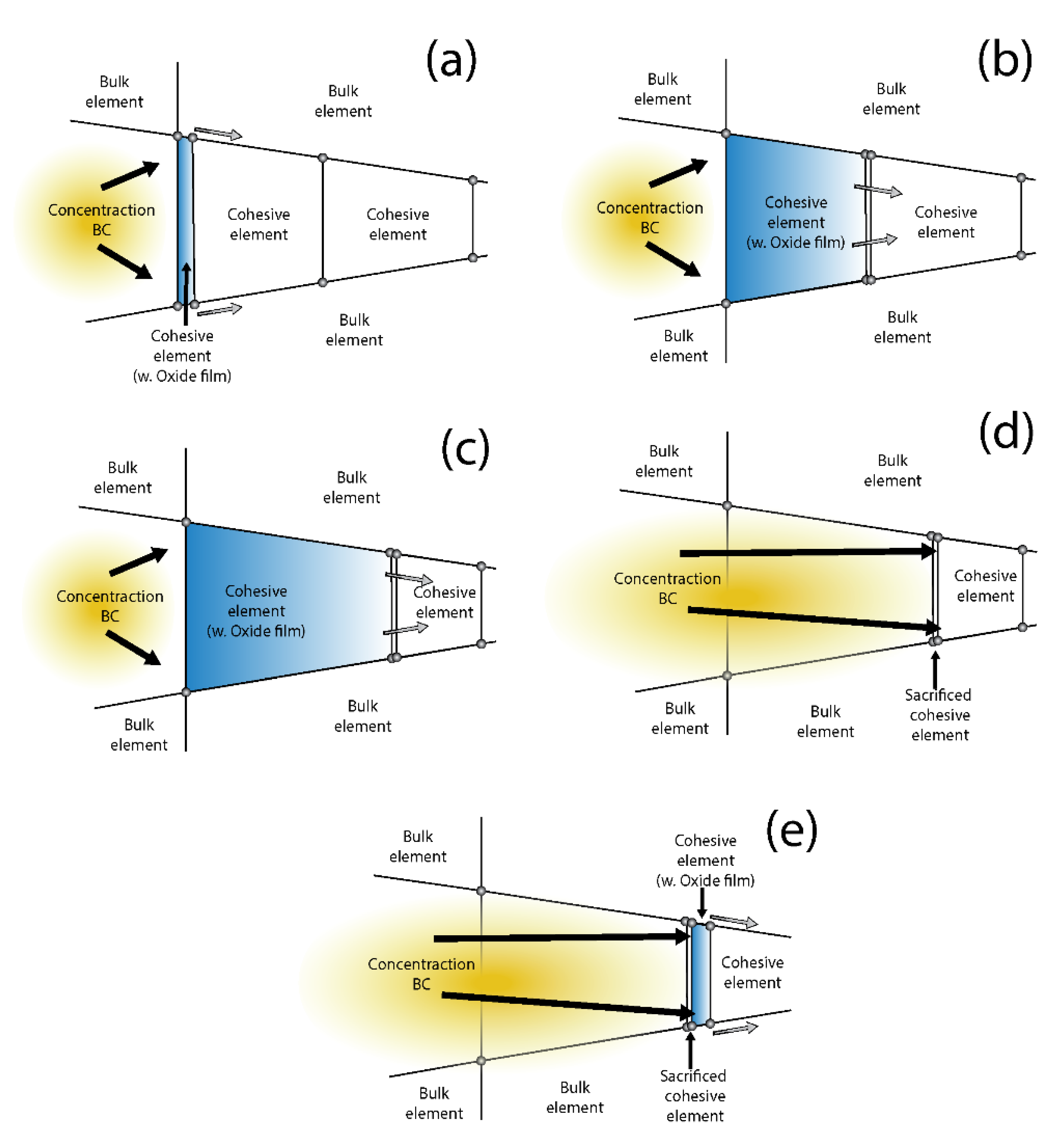
Appendix C
Appenidx C.1. Diffusion Element Formulation
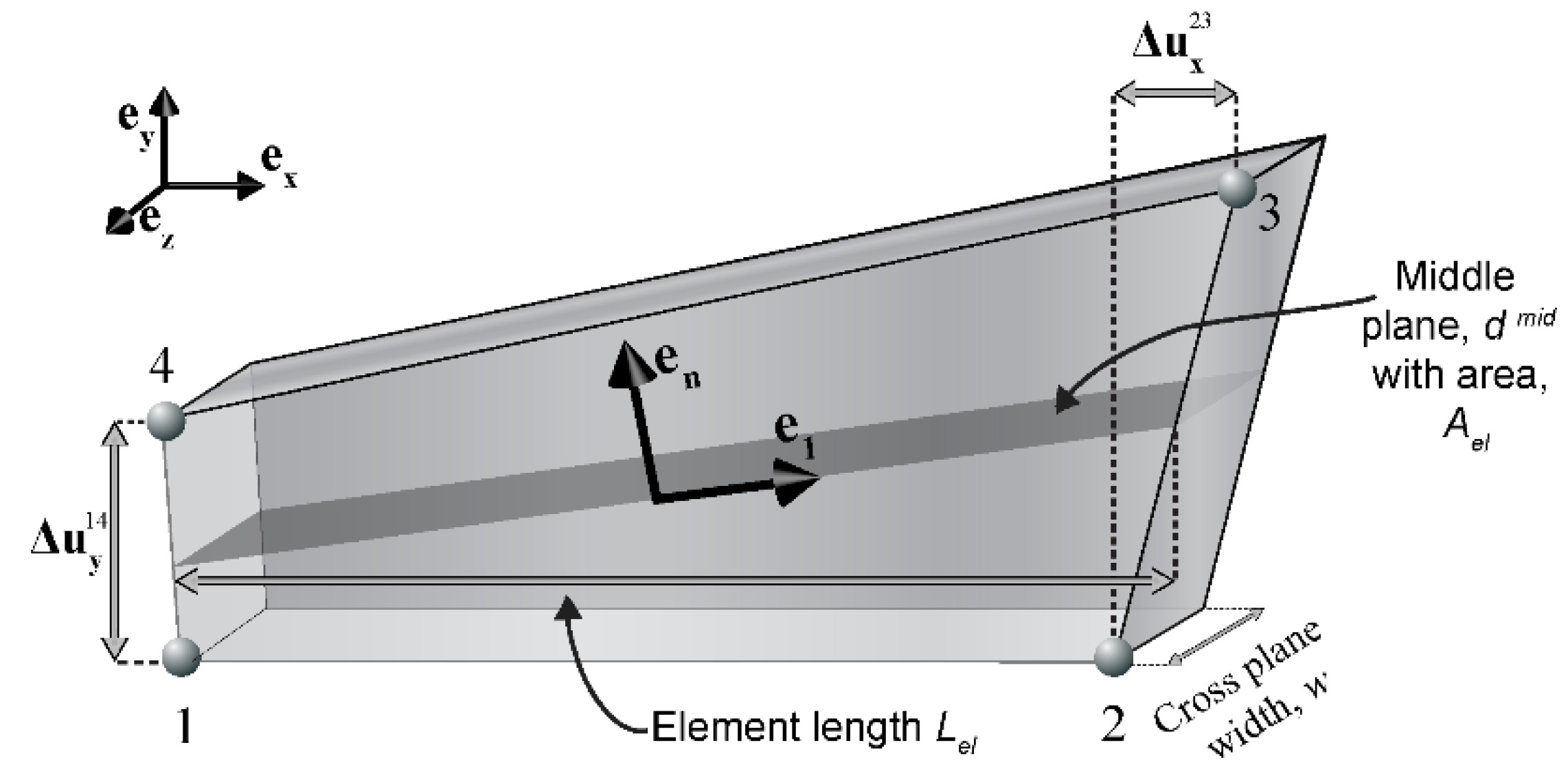
Appenidx C.2. Structural Element Formulation
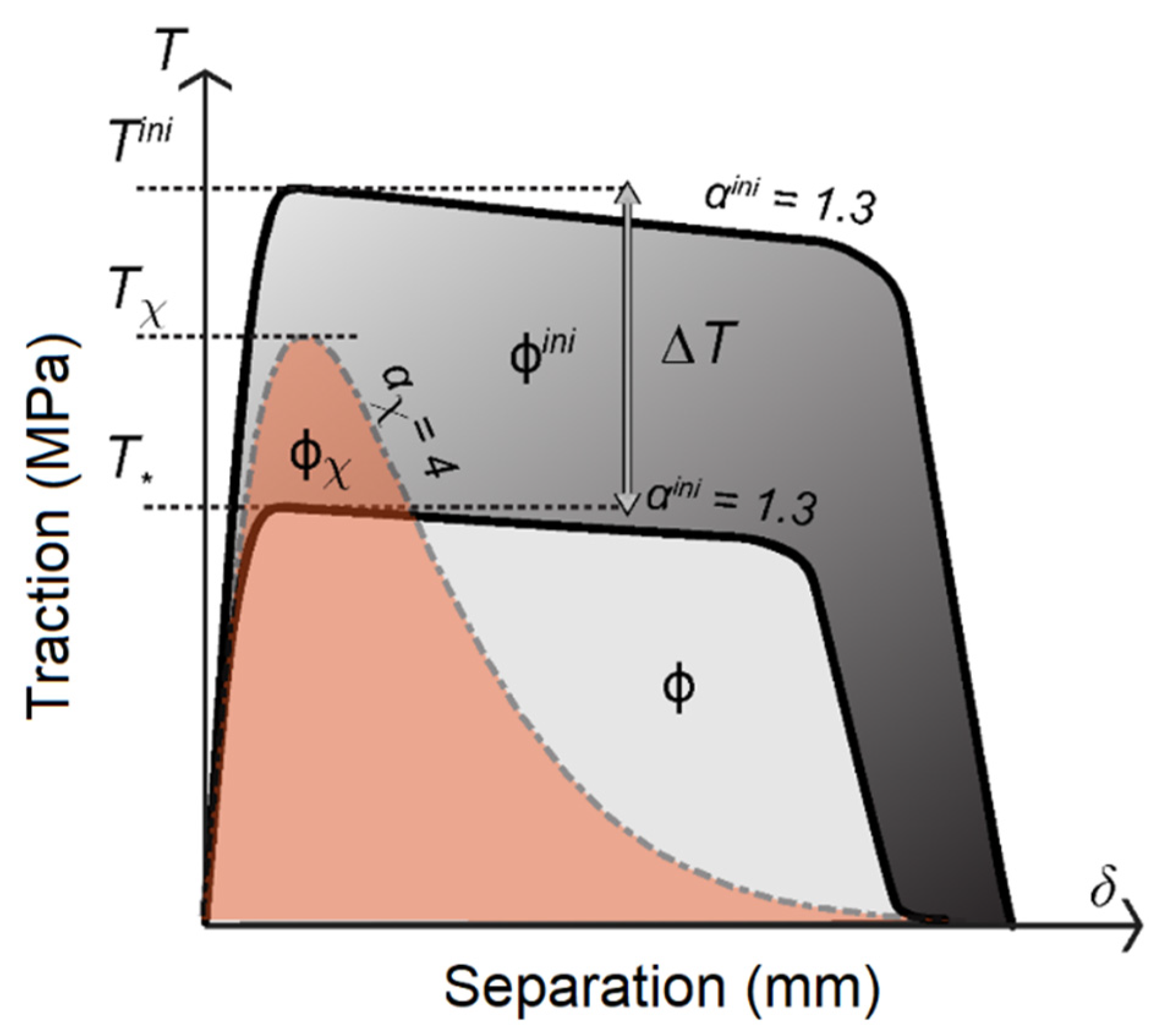
Appendix D
References
- Huang, Y.; Ouyang, Y.; Weng, S.; Xuan, F.-Z. Effect of loading mode on fracture behavior of CrNiMoV steel welded joint in simulated environment of low pressure nuclear steam turbine. Eng. Fract. Mech. 2019, 205, 81–93. [Google Scholar] [CrossRef]
- Fujii, T.; Tohgo, K.; Kawamori, M.; Shimamura, Y. Characterization of stress corrosion crack growth in austenitic stainless steel under variable loading in small- and large-scale yielding conditions. Eng. Fract. Mech. 2019, 205, 94–107. [Google Scholar] [CrossRef]
- Andresen, P.L. Fracture Mechanics Data and Modeling of Environmental Cracking of Nickel-Base Alloys in High-Temperature Water. Corrosion 1991, 47, 917–938. [Google Scholar] [CrossRef]
- Electric Power Research Institute. Corrosion-Assisted Cracking of Stainless and Low-Alloy Steels in LWR Environmens; EPRI NP-5064M 1987; Electric Power Research Institute: Palo Alto, CA, USA, 1987. [Google Scholar]
- Gangloff, R.P.; Ives, M.B. Environment-Induced Cracking of Metals. In Proceedings of the International Conference 1st, Held Kohler, WI, USA, 2–7 October 1988; p. 1990. [Google Scholar]
- Bianchi, G.; Galvele, J. Stress corrosion cracking of pure copper and pure silver in gaseous environments. Corros. Sci. 1993, 34, 1411–1417. [Google Scholar] [CrossRef]
- Shoji, T.; Lu, Z.; Murakami, H. Formulating stress corrosion cracking growth rates by combination of crack tip mechanics and crack tip oxidation kinetics. Corros. Sci. 2010, 52, 769–779. [Google Scholar] [CrossRef]
- Shoji, T.; Yamamoto, T.; Watanabe, K.; Lu, Z. 3D-FEM Simulation of EAC Crack Growth Based on the Deformation/Oxidation Mechanism. In Proceedings of the 11th International Conference on Environmental Degradation of Materials in Nuclear Power Systems, Stevenson, WA, USA, 10–14 August 2003. [Google Scholar]
- Couvant, T.; Legras, L.; Pokor, C.; Vaillant, F.; Brechet, Y.; Boursier, J.M.; Moulart, P. Investigations on the mechanisms of PWSCC of strain hardening austenitic stainless steels. In Proceedings of the 13th International Conference on Environmental Degradation of Materials in Nuclear Power Systems, Whistler, CA, Canada, 19–23 April 2007. [Google Scholar]
- Sedlak, M.; Alfredsson, B.; Efsing, P. A coupled diffusion and cohesive zone model for intergranular stress corrosion cracking in 316L stainless steel exposed to cold work in primary water conditions. Eng. Fract. Mech. 2019, 217. [Google Scholar] [CrossRef]
- Jivkov, A.; Stevens, N.; Marrow, T. A two-dimensional mesoscale model for intergranular stress corrosion crack propagation. Acta Mater. 2006, 54, 3493–3501. [Google Scholar] [CrossRef]
- Jivkov, A.; Stevens, N.; Marrow, T. A three-dimensional computational model for intergranular cracking. Comput. Mater. Sci. 2006, 38, 442–453. [Google Scholar] [CrossRef]
- Bashir, R.; Xue, H.; Zhang, J.; Guo, R.; Hayat, N.; Li, G.; Bi, Y. Effect of XFEM mesh density (mesh size) on stress intensity factors (K), strain gradient (dεdr) and stress corrosion cracking (SCC) growth rate. Structures 2020, 25, 593–602. [Google Scholar] [CrossRef]
- Öijerholm, J.; Jenssen, A. Interkristallin Spänningskorrosion I Rostfritt Stål I BWR-Miljö—En Sammanställning Av Kunskapsläget Med. Fokus På Erfarenheter Av Studier Genomförda I Sverige; Swedish Radiation Safety Authority: Stockholm, Sweden, 2015.
- Liu, P.; Gu, Z.; Peng, X.; Zheng, J. Finite element analysis of the influence of cohesive law parameters on the multiple delamination behaviors of composites under compression. Compos. Struct. 2015, 131, 975–986. [Google Scholar] [CrossRef]
- Rozylo, P. Experimental-numerical study into the stability and failure of compressed thin-walled composite profiles using progressive failure analysis and cohesive zone model. Compos. Struct. 2021, 257, 113303. [Google Scholar] [CrossRef]
- Panettieri, E.; Fanteria, D.; Danzi, F. Delaminations growth in compression after impact test simulations: Influence of cohesive elements parameters on numerical results. Compos. Struct. 2016, 137, 140–147. [Google Scholar] [CrossRef]
- Barenblatt, G. The Mathematical Theory of Equilibrium Cracks in Brittle Fracture. Adv. Appl. Mech. 1962, 7, 55–129. [Google Scholar] [CrossRef]
- Dugdale, D.S. Yeilding of steel sheets containing slits. J. Mech. Phys. Solids 1960, 8, 100–104. [Google Scholar] [CrossRef]
- Hillerborg, A.; Modéer, M.; Petersson, P.-E. Analysis of crack formation and crack growth in concrete by means of fracture mechanics and finite elements. Cem. Concr. Res. 1976, 6, 773–781. [Google Scholar] [CrossRef]
- Park, K.; Paulino, G.H.; Roesler, J.R. A unified potential-based cohesive model of mixed-mode fracture. J. Mech. Phys. Solids 2009, 57, 891–908. [Google Scholar] [CrossRef]
- Sedlak, M.; Alfredsson, B.; Efsing, P. A cohesive element with degradation controlled shape of the traction separation curve for simulating stress corrosion and irradiation cracking. Eng. Fract. Mech. 2018, 193, 172–196. [Google Scholar] [CrossRef]
- Andresen, P. Stress corrosion cracking (SCC) of austenitic stainless steels in high temperature light water reactor (LWR) environments. Underst. Mitigating Ageing Nucl. Power Plants 2010, 236–307. [Google Scholar] [CrossRef]
- Terachi, T.; Yamada, T.; Miyamoto, T.; Arioka, K. SCC growth behaviors of austenitic stainless steels in simulated PWR primary water. J. Nucl. Mater. 2012, 426, 59–70. [Google Scholar] [CrossRef]
- Castano Marin, M.L.; Garcia Redondo, M.S.; Velasco, G.D. Crack growth rate of hardened austenitic stainless steels in BWR and PWR environments. In Proceedings of the 11th International Conference on Environmental Degradation of Materials in Nuclear Power Systems-Water Reactors, Stevenson, WA, USA, 10–14 August 2003. [Google Scholar]
- Shoji, T.; Li, G.; Kwon, J.; Matsushima, S.; Lu, Z. Quantification of yield strength effects on IGSCC of austenitic stainless steels in high temperature water. In Proceedings of the 11th International Symposium on Environmental Degradation of Materials in Nuclear Power System-Water Reactors, Stevenson, WA, USA, 10–14 August 2003. [Google Scholar]
- Andresen, P.L.; Angeliu, T.M.; Catlin, W.R.; Young, L.M. Effect of deformation on SCC of unsensitized stainless steel. Corrosion 2000, 2000, 203. [Google Scholar]
- Andresen, P.L.; Morra, M.M. IGSCC of non-sensitized stainless steels in high temperature water. J. Nucl. Mater. 2008, 383, 97–111. [Google Scholar] [CrossRef]
- Gomez-Briceno, D.; Sol Garcia, M.J.L. SCC behavior of austenitic stainless steels in high temperature water effect of cold work, water chemistry and type of materials. In Proceedings of the14th International Conference on Environmental Degradation of Materials in Nuclear Power Systems, Virginia Beach, VA, USA, 23–27 August 2009. [Google Scholar]
- Ford, F.P. Quantitative Prediction of Environmentally Assisted Cracking. Corrosion 1996, 52, 375–395. [Google Scholar] [CrossRef] [Green Version]
- Andresen, P.L. Modeling of Water and Material Chemistry Effects on Crack Tip Chemistry and Resultsing Crack Growth Kinetics. In Proceedings of the 3rd International Conference on Environmental Degradation of Materials in Nuclear Power Systems–Water Reactors, Traverse City, MI, USA, 30 August–3 September 1987; TMS-AIME: Pittsburgh, PA, USA; pp. 301–314. [Google Scholar]
- Electric Power Research Institute. BWR Vessel and Internals Project—BWR Water Chemistry Guidelines; BWRVIP-130; Electric Power Research Institute: Palo Alto, CA, USA, 2004. [Google Scholar]
- Sedlak, M. Simulation of Slip-Oxidation Process by Mesh Adaptivity in a Cohesive Zone Framework. Mendeley Data 2020, V1. [Google Scholar] [CrossRef]
- Sedlak Mosesson, M.; Alfredsson, B.; Efsing, P. A duplex oxide cohesive zone model to simulate intergranular stress corrosion cracking. Int. J. Mech. Sci. 2021, 197, 106260. [Google Scholar] [CrossRef]
- Ortiz, M.; Pandolfi, A. Finite-deformation irreversible cohesive elements for three-dimensional crack-propagation analysis. Int. J. Numer. Methods Eng. 1999, 44, 1267–1282. [Google Scholar] [CrossRef]
- van Eeten, P.; Nilsson, F. Constant and variable amplitude cyclic plasticity in 316L stainless steel. J. Test. Eval. 2006, 34, 298–311. [Google Scholar]
- Li, K.; Peng, J. Mechanical Behavior of 316L Stainless Steel after Strain Hardening. MATEC Web Conf. 2017, 114, 2003. [Google Scholar] [CrossRef] [Green Version]
- Mills, W.J. Fracture toughness of type 304 and 316 stainless steels and their welds. Int. Mater. Rev. 1997, 42, 45–82. [Google Scholar] [CrossRef]
- O’Connell, J.P.; Prausnitz, J.M.; Poling, B.E. The Properties of Gases and Liquids, 5th ed.; McGraw-Hill: New York, NY, USA, 2001. [Google Scholar]
- Andresen, P.L. Similarity of Cold Work and Radiation Hardening in Enhancing Yield Strength and SCC Growth of Stainless Steel in Hot Water. Corrosion 2002. [Google Scholar]
- Arioka, K.; Yamada, T.; Terachi, T.; Chiba, G. Cold Work and Temperature Dependence of Stress Corrosion Crack Growth of Austenitic Stainless Steels in Hydrogenated and Oxygenated High-Temperature Water. Corrosion 2007, 63, 1114–1123. [Google Scholar] [CrossRef]
- Ford, F.P.; Andresen, P.L. Corrosion in Nuclear Systems: Environmentally Assisted Cracking in Light Water Reactors. Corrosion 2002, 52, 605–664. [Google Scholar]
- USNRC. Generic Letter 88-NRC Position on Intergranular Stress Corrosion Cracking in BWR Austenitic Stainless Steel Piping. Available online: https://www.nrc.gov/reading-rm/doc-collections/gen-comm/gen-letters/1988/gl88001s1.html (accessed on 20 May 2021).



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sedlak Mosesson, M.; Alfredsson, B.; Efsing, P. Simulation of Slip-Oxidation Process by Mesh Adaptivity in a Cohesive Zone Framework. Materials 2021, 14, 3509. https://doi.org/10.3390/ma14133509
Sedlak Mosesson M, Alfredsson B, Efsing P. Simulation of Slip-Oxidation Process by Mesh Adaptivity in a Cohesive Zone Framework. Materials. 2021; 14(13):3509. https://doi.org/10.3390/ma14133509
Chicago/Turabian StyleSedlak Mosesson, Michal, Bo Alfredsson, and Pål Efsing. 2021. "Simulation of Slip-Oxidation Process by Mesh Adaptivity in a Cohesive Zone Framework" Materials 14, no. 13: 3509. https://doi.org/10.3390/ma14133509






