Biomimetic Calcium Phosphate Coatings for Bioactivation of Titanium Implant Surfaces: Methodological Approach and In Vitro Evaluation of Biocompatibility
Abstract
:1. Introduction
2. Materials and Methods
2.1. Biomimetic CaP-Coated Ti6Al4V Substrates
2.2. Surface Analysis
2.3. Cell Culture of Primary Human Osteoblasts
2.4. Cell Proliferation
2.5. Cell Morphology
2.6. Quantification of Procollagen Type I Synthesis
2.7. Quantification of Inflammatory Signals
2.8. VEGF
2.9. Total Protein Content
2.10. Statistical Analysis
3. Results
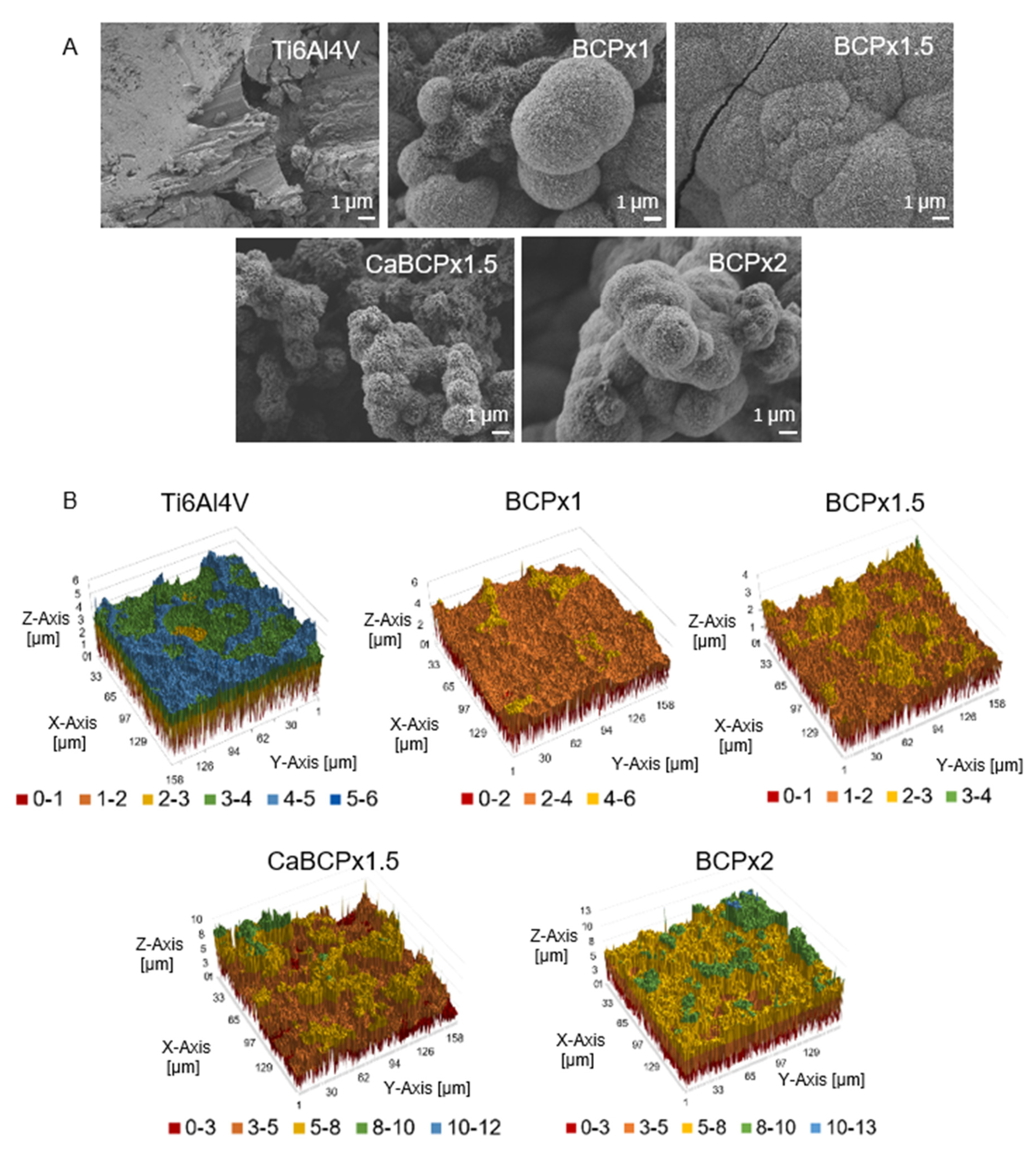
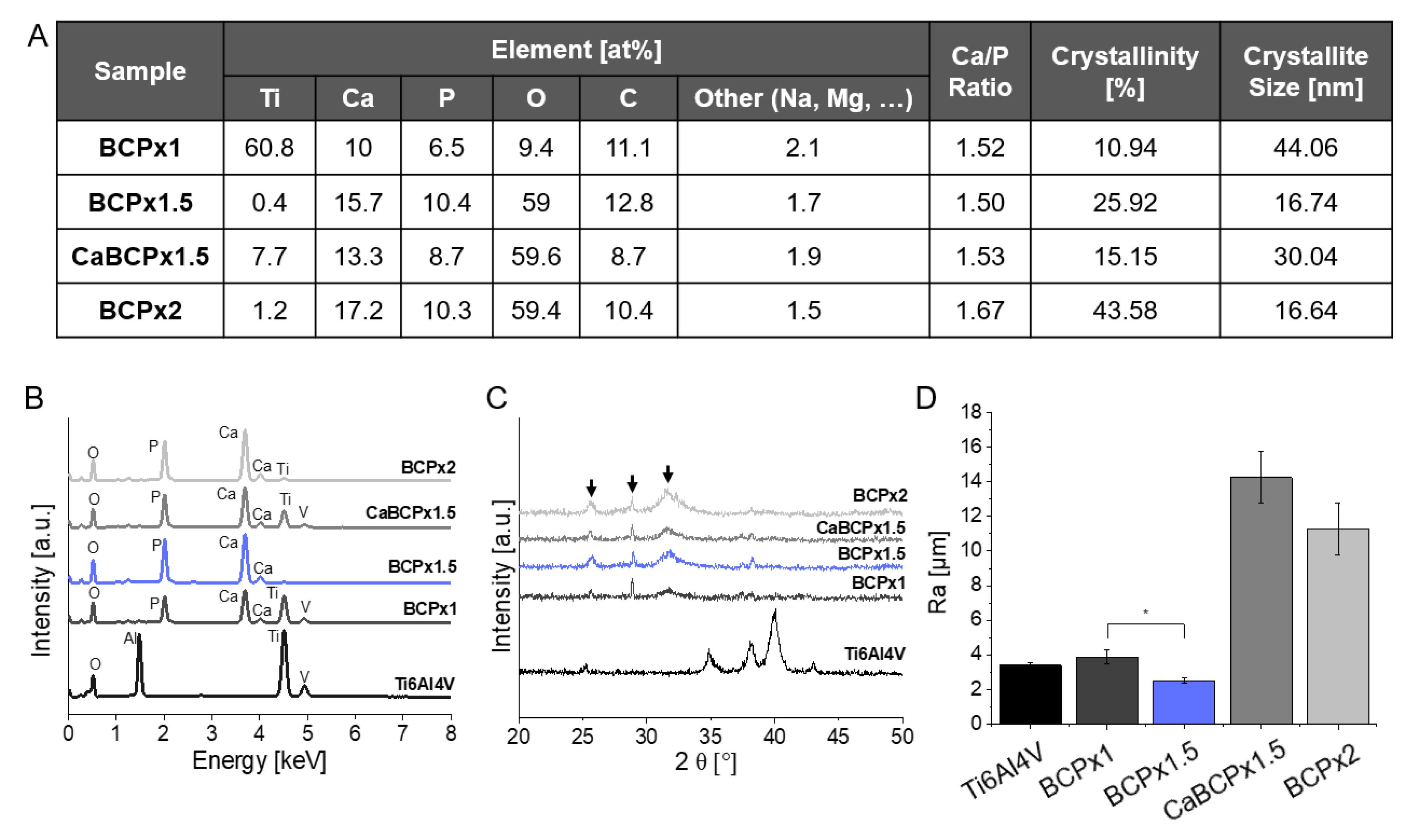
3.1. Structure and Composition of CaP-Coatings
3.2. In Vitro Biocompatibility of CaP-Coatings
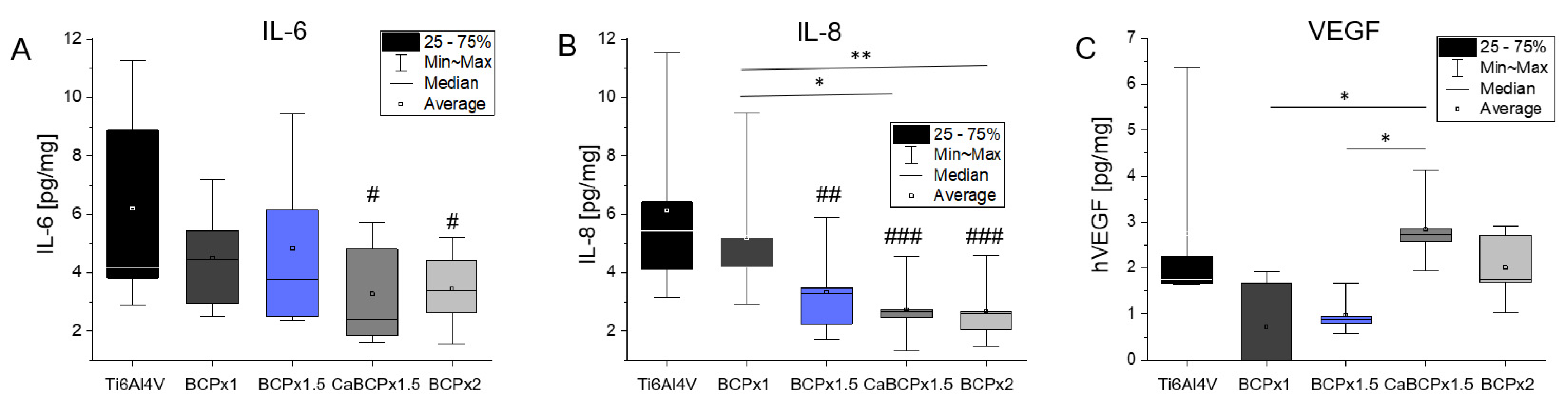
3.3. Induction of Cytokine Release by Human Osteoblasts
4. Discussion
4.1. Structure and Composition of CaP-Coatings
4.2. In Vitro Biocompatibility of CaP-Coatings
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BCP | Biomimetic Calcium Phosphate |
| BMSC | Bone Marrow-Derived Mesenchymal Stem Cells |
| CaP | Calcium Phosphates |
| CaBCPx1.5 | Ti6Al4V chemically pre-treated samples exposed to a CaCl2 treatment before immersion in BCPx1.5 |
| CICP | Type I C-Terminal Collagen Propeptide |
| Cp-Ti | Commercially Pure Titanium |
| HA | Hydroxyapatite |
| HCA | Hydroxyl Carbonated Apatite |
| IL | Interleukine |
| RT | Room Temperature (RT = 22 °C) |
| SBF | Simulated Body Fluid |
| UPW | Ultra Pure Water |
References
- Bauer, S.; Schmuki, P.; Von Der Mark, K.; Park, J. Engineering biocompatible implant surfaces. Part I: Materials and surfaces. Prog. Mater. Sci. 2013, 58, 261–326. [Google Scholar] [CrossRef]
- Calin, M.; Gebert, A.; Ghinea, A.C.; Gostin, P.-F.; Abdi, S.; Mickel, C.; Eckert, J. Designing biocompatible Ti-based metallic glasses for implant applications. Mater. Sci. Eng. C 2013, 33, 875–883. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yang, C.; Zhao, H.; Qu, S.; Li, X.; Li, Y. New Developments of Ti-Based Alloys for Biomedical Applications. Materials 2014, 7, 1709–1800. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saini, M. Implant biomaterials: A comprehensive review. World J. Clin. Cases 2015, 3, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Arifin, A.; Sulong, A.B.; Muhamad, N.; Syarif, J.; Ramli, M.I. Powder injection molding of HA/Ti6Al4V composite using palm stearin as based binder for implant material. Mater. Des. 2015, 65, 1028–1034. [Google Scholar] [CrossRef]
- Mistry, S.; Roy, S.; Maitra, N.J.; Roy, R.; Datta, S.; Chanda, A.; Sarkar, S. Safety and efficacy of additive and subtractive surface modification of Ti6Al4V endosseous implant in goat bone. J. Mech. Behav. Biomed. Mater. 2016, 57, 69–87. [Google Scholar] [CrossRef]
- Kokubo, T.; Takadama, H. How useful is SBF in predicting in vivo bone bioactivity? Biomaterials 2006, 27, 2907–2915. [Google Scholar] [CrossRef]
- Nebe, J.B.; Müller, L.; Lüthen, F.; Ewald, A.; Bergemann, C.; Conforto, E.; Müller, F.A. Osteoblast response to biomimetically altered titanium surfaces. Acta Biomater. 2008, 4, 1985–1995. [Google Scholar] [CrossRef]
- Buser, D.; Broggini, N.; Wieland, M.; Schenk, R.K.; Denzer, A.J.; Cochran, D.L.; Hoffmann, B.; Lussi, A.; Steinemann, S.G. Enhanced Bone Apposition to a Chemically Modified SLA Titanium Surface. J. Dent. Res. 2004, 83, 529–533. [Google Scholar] [CrossRef]
- Wang, H.; Li, Y.; Zuo, Y.; Li, J.; Ma, S.; Cheng, L. Biocompatibility and osteogenesis of biomimetic nano-hydroxyapatite/polyamide composite scaffolds for bone tissue engineering. Biomaterials 2007, 28, 3338–3348. [Google Scholar] [CrossRef]
- Woodard, J.R.; Hilldore, A.J.; Lan, S.K.; Park, C.; Morgan, A.W.; Eurell, J.A.C.; Clark, S.G.; Wheeler, M.B.; Jamison, R.D.; Johnson, A.J.W. The mechanical properties and osteoconductivity of hydroxyapatite bone scaffolds with multi-scale porosity. Biomaterials 2007, 28, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Jarcho, M.; Kay, J.F.; Gumaer, K.I.; Doremus, R.H.; Drobeck, H.P. Tissue, cellular and subcellular events at a bone-ceramic hydroxylapatite interface. J. Bioeng. 1977, 1, 79–92. [Google Scholar] [PubMed]
- Blind, O.; Klein, L.H.; Dailey, B.; Jordan, L. Characterization of hydroxyapatite films obtained by pulsed-laser deposition on Ti and Ti-6AL-4v substrates. Dent. Mater. 2005, 21, 1017–1024. [Google Scholar] [CrossRef] [PubMed]
- Detsch, R.; Uhl, F.; Deisinger, U.; Ziegler, G. 3D-Cultivation of bone marrow stromal cells on hydroxyapatite scaffolds fabricated by dispense-plotting and negative mould technique. J. Mater. Sci. Mater. Electron. 2008, 19, 1491–1496. [Google Scholar] [CrossRef] [PubMed]
- Nishiguchi, S.; Fujibayashi, S.; Kim, H.-M.; Kokubo, T.; Nakamura, T. Biology of alkali- and heat-treated titanium implants. J. Biomed. Mater. Res. 2003, 67, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Lu, R.-J.; Wang, X.; He, H.-X.; E, L.-L.; Li, Y.; Zhang, G.-L.; Li, C.-J.; Ning, C.-Y.; Liu, H.-C. Tantalum-incorporated hydroxyapatite coating on titanium implants: Its mechanical and in vitro osteogenic properties. J. Mater. Sci. Mater. Med. 2019, 30, 111. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Ding, C.; Zhou, Y.; Luo, J.; Li, J. Universal and biocompatible hydroxyapatite coating induced by phytic acid-metal complex multilayer. Colloids Surf. B Biointerfaces 2018, 169, 478–485. [Google Scholar] [CrossRef]
- Borsari, V.; Giavaresi, G.; Fini, M.; Torricelli, P.; Salito, A.; Chiesa, R.; Chiusoli, L.; Volpert, A.; Rimondini, L.; Giardino, R. Physical characterization of different-roughness titanium surfaces, with and without hydroxyapatite coating, and their effect on human osteoblast-like cells. J. Biomed. Mater. Res. Part B Appl. Biomater. 2005, 75, 359–368. [Google Scholar] [CrossRef]
- de Oliveira, P.G.F.P.; Soares, M.S.D.M.; e Souza, A.M.M.S.; Taba, M.T., Jr.; Palioto, D.B.; Messora, M.R.; Ghiraldini, B.; Nunes, F.A.D.S.; de Souza, S.L.S. Influence of nano-hydroxyapatite coating implants on gene expression of osteogenic markers and micro-CT parameters. An in vivo study in diabetic rats. J. Biomed. Mater. Res. Part A 2021, 109, 682–694. [Google Scholar] [CrossRef]
- Jeong, J.; Kim, J.H.; Shim, J.H.; Hwang, N.S.; Heo, C.Y. Bioactive calcium phosphate materials and applications in bone regeneration. Biomater. Res. 2019, 23, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Johnson, K.E.; Wilgus, T.A. Vascular Endothelial Growth Factor and Angiogenesis in the Regulation of Cutaneous Wound Repair. Adv. Wound Care 2014, 3, 647–661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jämsen, E.; Kouri, V.-P.; Ainola, M.; Goodman, S.B.; Nordström, D.C.; Eklund, K.K.; Pajarinen, J. Correlations between macrophage polarizing cytokines, inflammatory mediators, osteoclast activity, and toll-like receptors in tissues around aseptically loosened hip implants. J. Biomed. Mater. Res. Part A 2016, 105, 454–463. [Google Scholar] [CrossRef]
- Liskmann, S.; Vihalemm, T.; Salum, O.; Zilmer, K.; Fischer, K.; Zilmer, M. Correlations between clinical parameters and interleukin-6 and interleukin-10 levels in saliva from totally edentulous patients with peri-implant disease. Int. J. Oral Maxillofac. Implant. 2006, 21, 543–550. [Google Scholar]
- Di Benedetto, A.; Gigante, I.; Colucci, S.; Grano, M. Periodontal Disease: Linking the Primary Inflammation to Bone Loss. Clin. Dev. Immunol. 2013, 2013, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shimauchi, H.; Takayama, S.; Narikawa-Kiji, M.; Shimabukuro, Y.; Okada, H. Production of Interleukin–8 and Nitric Oxide in Human Periapical Lesions. J. Endod. 2001, 27, 749–752. [Google Scholar] [CrossRef]
- Llorente, I.; García-Castañeda, N.; Valero, C.; González-Álvaro, I.; Castañeda, S. Osteoporosis in Rheumatoid Arthritis: Dangerous Liaisons. Front. Med. 2020, 7, 601618. [Google Scholar] [CrossRef]
- Park, Y.-T.; Lee, S.-M.; Kou, X.; Karabucak, B. The Role of Interleukin 6 in Osteogenic and Neurogenic Differentiation Potentials of Dental Pulp Stem Cells. J. Endod. 2019, 45, 1342–1348. [Google Scholar] [CrossRef]
- Xie, Z.; Tang, S.; Ye, G.; Wang, P.; Li, J.; Liu, W.; Li, M.; Wang, S.; Wu, X.; Cen, S.; et al. Interleukin-6/interleukin-6 receptor complex promotes osteogenic differentiation of bone marrow-derived mesenchymal stem cells. Stem Cell Res. Ther. 2018, 9, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Bastidas-Coral, A.P.; Bakker, A.D.; Zandieh-Doulabi, B.; Kleverlaan, C.J.; Bravenboer, N.; Forouzanfar, T.; Klein-Nulend, J. Cytokines TNF-α, IL-6, IL-17F, and IL-4 Differentially Affect Osteogenic Differentiation of Human Adipose Stem Cells. Stem Cells Int. 2016, 2016, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Furlong, R.; Osborn, J. Fixation of hip prostheses by hydroxyapatite ceramic coatings. J. Bone Jt. Surg. Br. Vol. 1991, 73-B, 741–745. [Google Scholar] [CrossRef] [Green Version]
- Eliaz, N.; Metoki, N. Calcium Phosphate Bioceramics: A Review of Their History, Structure, Properties, Coating Technologies and Biomedical Applications. Materials 2017, 10, 334. [Google Scholar] [CrossRef] [Green Version]
- Habibovic, P.; Barrère, F.; Van Blitterswijk, C.; Groot, K.; Layrolle, P. Biomimetic Hydroxyapatite Coating on Metal Implants. J. Am. Ceram. Soc. 2004, 85, 517–522. [Google Scholar] [CrossRef] [Green Version]
- Jonášová, L.; Müller, F.A.; Helebrant, A.; Strnad, J.; Greil, P. Biomimetic apatite formation on chemically treated titanium. Biomaterials 2004, 25, 1187–1194. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Kangasniemi, I.; Groot, K.; Kokubo, T. Bonelike Hydroxyapatite Induction by a Gel-Derived Titania on a Titanium Substrate. J. Am. Ceram. Soc. 1994, 77, 1307–1312. [Google Scholar] [CrossRef]
- Li, P.; Ohtsuki, C.; Kokubo, T.; Nakanishi, K.; Soga, N.; De Groot, K. The role of hydrated silica, titania, and alumina in inducing apatite on implants. J. Biomed. Mater. Res. 1994, 28, 7–15. [Google Scholar] [CrossRef]
- Nývlt, J. Kinetics of nucleation in solutions. J. Cryst. Growth 1968, 3-4, 377–383. [Google Scholar] [CrossRef]
- Tas, A.C.; Bhaduri, S.B. Rapid coating of Ti6Al4V at room temperature with a calcium phosphate solution similar to 10× simulated body fluid. J. Mater. Res. 2004, 19, 2742–2749. [Google Scholar] [CrossRef]
- Zhu, P.; Masuda, Y.; Yonezawa, T.; Koumoto, K. Investigation of Apatite Deposition onto Charged Surfaces in Aqueous Solutions Using a Quartz-Crystal Microbalance. J. Am. Ceram. Soc. 2003, 86, 782–790. [Google Scholar] [CrossRef]
- Lochner, K.; Fritsche, A.; Jonitz, A.; Hansmann, D.; Mueller, P.; Mueller-Hilke, B.; Bader, R. The potential role of human osteoblasts for periprosthetic osteolysis following exposure to wear particles. Int. J. Mol. Med. 2011, 28, 1055–1063. [Google Scholar] [CrossRef]
- Reiter, T.; Panick, T.; Schuhladen, K.; Roether, J.A.; Hum, J.; Boccaccini, A.R. Bioactive glass based scaffolds coated with gelatin for the sustained release of icariin. Bioactive Mater. 2019, 4, 1–7. [Google Scholar] [CrossRef]
- Saleem, O.; Wahaj, M.; Akhtar, M.A.; Rehman, M.A.U. Fabrication and Characterization of Ag–Sr-Substituted Hydroxyapatite/Chitosan Coatings Deposited via Electrophoretic Deposition: A Design of Experiment Study. ACS Omega 2020, 5, 22984–22992. [Google Scholar] [CrossRef]
- Ureña, J.; Tsipas, S.; Jiménez-Morales, A.; Gordo, E.; Detsch, R.; Boccaccini, A. In-vitro study of the bioactivity and cytotoxicity response of Ti surfaces modified by Nb and Mo diffusion treatments. Surf. Coat. Technol. 2018, 335, 148–158. [Google Scholar] [CrossRef] [Green Version]
- Müller, F.A.; Müller, L.; Caillard, D.; Conforto, E. Preferred growth orientation of biomimetic apatite crystals. J. Cryst. Growth 2007, 304, 464–471. [Google Scholar] [CrossRef]
- Müller, L.; Müller, F.A. Preparation of SBF with different HCO−3 content and its influence on the composition of biomimetic apatites. Acta Biomater. 2006, 2, 181–189. [Google Scholar] [CrossRef]
- Gritsch, L.; Maqbool, M.; Mouriño, V.; Ciraldo, F.E.; Cresswell, M.; Jackson, P.R.; Lovell, C.; Boccaccini, A.R. Chitosan/hydroxyapatite composite bone tissue engineering scaffolds with dual and decoupled therapeutic ion delivery: Copper and strontium. J. Mater. Chem. B 2019, 7, 6109–6124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Furko, M.; Havasi, V.; Kónya, Z.; Grünewald, A.; Detsch, R.; Boccaccini, A.R.; Balázsi, C. Development and characterization of multi-element doped hydroxyapatite bioceramic coatings on metallic implants for orthopedic applications. Bol. Soc. Española Cerámica Vidr. 2018, 57, 55–65. [Google Scholar] [CrossRef] [Green Version]
- Nishio, K.; Neo, M.; Akiyama, H.; Nishiguchi, S.; Kim, H.; Kokubo, T.; Nakamura, T. The effect of alkali- and heat-treated titanium and apatite-formed titanium on osteoblastic differentiation of bone marrow cells. J. Biomed. Mater. Res. 2000, 52, 652–661. [Google Scholar] [CrossRef]
- Kokubo, T.; Yamaguchi, S. Novel bioactive materials developed by simulated body fluid evaluation: Surface-modified Ti metal and its alloys. Acta Biomater. 2016, 44, 16–30. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, S.; Takadama, H.; Matsushita, T.; Nakamura, T.; Kokubo, T. Cross-sectional analysis of the surface ceramic layer developed on Ti metal by NaOH-heat treatment and soaking in SBF. J. Ceram. Soc. Jpn. 2009, 117, 1126–1130. [Google Scholar] [CrossRef] [Green Version]
- Kokubo, T.; Yamaguchi, S. Biomimetic surface modification of metallic biomaterials. Surf. Coat. Modif. Metal. Biomater. 2015, 219–246. [Google Scholar] [CrossRef]
- Yamaguchi, S.; Akeda, K.; Murata, K.; Takegami, N.; Goto, M.; Sudo, A.; Matsushita, T.; Kokubo, T. Chemical and Heat Treatments for Inducing Bone-Bonding Ability of Ti-6Al-4V Pedicle Screw. Key Eng. Mater. 2014, 631, 225–230. [Google Scholar] [CrossRef]
- Wei, M.; Kim, H.-M.; Kokubo, T.; Evans, J. Optimising the bioactivity of alkaline-treated titanium alloy. Mater. Sci. Eng. C 2002, 20, 125–134. [Google Scholar] [CrossRef]
- Kim, H.-M.; Kim, Y.; Park, S.-J.; Rey, C.; Lee, H.; Glimcher, M.J.; Ko, J.S. Thin film of low-crystalline calcium phosphate apatite formed at low temperature. Biomaterials 2000, 21, 1129–1134. [Google Scholar] [CrossRef]
- Chen, X.; Li, Y.; Hodgson, P.D.; Wen, C. Microstructures and bond strengths of the calcium phosphate coatings formed on titanium from different simulated body fluids. Mater. Sci. Eng. C 2009, 29, 165–171. [Google Scholar] [CrossRef]
- Crane, N.J.; Popescu, V.; Morris, M.D.; Steenhuis, P.; Ignelzi, M.A. Raman spectroscopic evidence for octacalcium phosphate and other transient mineral species deposited during intramembranous mineralization. Bone 2006, 39, 434–442. [Google Scholar] [CrossRef] [PubMed]
- Olszta, M.J.; Cheng, X.; Jee, S.S.; Kumar, R.; Kim, Y.-Y.; Kaufman, M.J.; Douglas, E.P.; Gower, L.B. Bone structure and formation: A new perspective. Mater. Sci. Eng. R Rep. 2007, 58, 77–116. [Google Scholar] [CrossRef]
- Dey, A.; Bomans, P.; Muller, F.; Will, J.; Frederik, P.; De With, G.; Sommerdijk, N. The role of prenucleation clusters in surface-induced calcium phosphate crystallization. Nat. Mater. 2010, 9, 1010–1014. [Google Scholar] [CrossRef]
- Dorozhkin, S.V.; Epple, M. Die biologische und medizinische Bedeutung von Calciumphosphaten. Angew. Chem. 2002, 114, 3260–3277. [Google Scholar] [CrossRef]
- Kim, H.-M.; Takadama, H.; Miyaji, F.; Kokubo, T.; Nishiguchi, S.; Nakamura, T. Formation of bioactive functionally graded structure on Ti-6Al-4V alloy by chemical surface treatment. J. Mater. Sci. Mater. Electron. 2000, 11, 555–559. [Google Scholar] [CrossRef]
- Yamaguchi, S.; Takadama, H.; Matsushita, T.; Nakamura, T.; Kokubo, T. Preparation of bioactive Ti-15Zr-4Nb-4Ta alloy from HCl and heat treatments after an NaOH treatment. J. Biomed. Mater. Res. Part A 2011, 97, 135–144. [Google Scholar] [CrossRef]
- Bigi, A. Relationship between triple-helix content and mechanical properties of gelatin films. Biomaterials 2004, 25, 5675–5680. [Google Scholar] [CrossRef] [PubMed]
- Bütev, E.; Ziya, E.S.; Şakir, B.O. Characterization of Ti6Al7Nb alloy foams surface treated in aqueous NaOH and CaCl 2 solutions. J. Mech. Behav. Biomed. Mater. 2016, 60, 127–138. [Google Scholar] [CrossRef] [PubMed]
- Hata, K.; Kokubo, T.; Nakamura, T.; Yamamuro, T. Growth of a Bonelike Apatite Layer on a Substrate by a Biomimetic Process. J. Am. Ceram. Soc. 1995, 78, 1049–1053. [Google Scholar] [CrossRef]
- Strnad, G.; Chirila, N. Corrosion Rate of Sand Blasted and Acid Etched Ti6Al4V for Dental Implants. Procedia Technol. 2015, 19, 909–915. [Google Scholar] [CrossRef] [Green Version]
- Wennerberg, A.; Hallgren, C.; Johansson, C.; Danelli, S. A histomorphometric evaluation of screw-shaped implants each prepared with two surface roughnesses. Clin. Oral Implant. Res. 1998, 9, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J. Surface Property and Biocompatibility of Ti and Ti6Al4V for Dental Implants. In Proceedings of the 2014 International Conference on Mechatronics, Electronic, Industrial and Control Engineering, Shenyang, China, 15–17 November 2014. [Google Scholar] [CrossRef] [Green Version]
- De Yoreo, J.J. Principles of Crystal Nucleation and Growth. Rev. Miner. Geochem. 2003, 54, 57–93. [Google Scholar] [CrossRef] [Green Version]
- Boskey, A. Bone mineral crystal size. Osteoporos. Int. 2003, 14, 16–21. [Google Scholar] [CrossRef]
- Wen, C.; Xu, W.; Hu, W.; Hodgson, P. Hydroxyapatite/titania sol–gel coatings on titanium–zirconium alloy for biomedical applications. Acta Biomater. 2007, 3, 403–410. [Google Scholar] [CrossRef]
- Maxian, S.H.; Zawadsky, J.P.; Dunn, M.G. Mechanical and histological evaluation of amorphous calcium phosphate and poorly crystallized hydroxyapatite coatings on titanium implants. J. Biomed. Mater. Res. 1993, 27, 717–728. [Google Scholar] [CrossRef]
- Rho, J.-Y.; Kuhn-Spearing, L.; Zioupos, P. Mechanical properties and the hierarchical structure of bone. Med. Eng. Phys. 1998, 20, 92–102. [Google Scholar] [CrossRef]
- Tadic, D.; Peters, F.; Epple, M. Continuous synthesis of amorphous carbonated apatites. Biomaterials 2002, 23, 2553–2559. [Google Scholar] [CrossRef]
- Pan, H.; Zhao, X.; Darvell, B.W.; Lu, W.W. Apatite-formation ability—Predictor of “bioactivity”? Acta Biomater. 2010, 6, 4181–4188. [Google Scholar] [CrossRef]
- Rydén, L.; Omar, O.; Johansson, A.; Jimbo, R.; Palmquist, A.; Thomsen, P. Inflammatory cell response to ultra-thin amorphous and crystalline hydroxyapatite surfaces. J. Mater. Sci. Mater. Electron. 2016, 28, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Müller, W.E.; Ackermann, M.; Al-Nawas, B.; Righesso, L.A.; Muñoz-Espí, R.; Tolba, E.; Neufurth, M.; Schröder, H.C.; Wang, X. Amplified morphogenetic and bone forming activity of amorphous versus crystalline calcium phosphate/polyphosphate. Acta Biomater. 2020, 118, 233–247. [Google Scholar] [CrossRef] [PubMed]
- Blair, H.C.; Larrouture, Q.C.; Li, Y.; Lin, H.; Beer-Stoltz, D.; Liu, L.; Tuan, R.S.; Robinson, L.J.; Schlesinger, P.H.; Nelson, D.J. Osteoblast Differentiation and Bone Matrix Formation In Vivo and In Vitro. Tissue Eng. Part B Rev. 2017, 23, 268–280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Korovessis, P.G.; Deligianni, D.D. Role of Surface Roughness of Titanium Versus Hydroxyapatite on Human Bone Marrow Cells Response. J. Spinal Disord. Tech. 2002, 15, 175–183. [Google Scholar] [CrossRef]
- Fini, M.; Giardino, R.; Borsari, V.; Torricelli, P.; Rimondini, L.; Giavaresi, G.; Aldini, N.N. In Vitro Behaviour of Osteoblasts Cultured on Orthopaedic Biomaterials with Different Surface Roughness, Uncoated and Fluorohydroxyapatite-Coated, Relative to the in Vivo Osteointegration Rate. Int. J. Artif. Organs 2003, 26, 520–528. [Google Scholar] [CrossRef]
- Barbeck, M.; Schröder, M.-L.; Alkildani, S.; Jung, O.; Unger, R. Exploring the Biomaterial-Induced Secretome: Physical Bone Substitute Characteristics Influence the Cytokine Expression of Macrophages. Int. J. Mol. Sci. 2021, 22, 4442. [Google Scholar] [CrossRef]
- Ishimi, Y.; Miyaura, C.; Jin, C.H.; Akatsu, T.; Abe, E.; Nakamura, Y.; Yamaguchi, A.; Yoshiki, S.; Matsuda, T.; Hirano, T. IL-6 is produced by osteoblasts and induces bone resorption. J. Immunol. 1990, 145, 3297–3303. [Google Scholar]
- Matsukawa, A.; Yoshimura, T.; Maeda, T.; Ohkawara, S.; Takagi, K.; Yoshinaga, M. Neutrophil accumulation and activation by homologous IL-8 in rabbits. IL-8 induces destruction of cartilage and production of IL-1 and IL-1 receptor antagonist in vivo. J. Immunol. 1995, 154, 5418–5425. [Google Scholar]
- Franchimont, N.; Rydziel, S.; Delany, A.M.; Canalis, E. Interleukin-6 and Its Soluble Receptor Cause a Marked Induction of Collagenase 3 Expression in Rat Osteoblast Cultures. J. Biol. Chem. 1997, 272, 12144–12150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Legendre, F.; Dudhia, J.; Pujol, J.-P.; Bogdanowicz, P. JAK/STAT but Not ERK1/ERK2 Pathway Mediates Interleukin (IL)-6/Soluble IL-6R Down-regulation of Type II Collagen, Aggrecan Core, and Link Protein Transcription in Articular Chondrocytes. J. Biol. Chem. 2003, 278, 2903–2912. [Google Scholar] [CrossRef] [Green Version]
- Landgraeber, S.; Jäger, M.; Jacobs, J.J.; Hallab, N.J. The Pathology of Orthopedic Implant Failure Is Mediated by Innate Immune System Cytokines. Mediat. Inflamm. 2014, 2014, 185150. [Google Scholar] [CrossRef] [PubMed]
- Aniket; Young, A.; Marriott, I. El-Ghannam, A. Promotion of pro-osteogenic responses by a bioactive ceramic coating. J. Biomed. Mater. Res. Part A 2012, 100A, 3314–3325. [Google Scholar] [CrossRef] [PubMed]
- Nicoletti, A.; Torricelli, P.; Bigi, A.; Fornasari, P.; Fini, M.; Moroni, L. Incorporation of nanostructured hydroxyapatite and poly(N-isopropylacrylamide) in demineralized bone matrix enhances osteoblast and human mesenchymal stem cell activity. Biointerphases 2015, 10, 041001. [Google Scholar] [CrossRef] [PubMed]
- Hong, L.; Sharp, T.; Khorsand, B.; Fischer, C.; Eliason, S.; Salem, A.; Akkouch, A.; Brogden, K.; Amendt, B.A. MicroRNA-200c Represses IL-6, IL-8, and CCL-5 Expression and Enhances Osteogenic Differentiation. PLoS ONE 2016, 11, e0160915. [Google Scholar] [CrossRef]
- Jung, Y.D.; Liu, W.; Reinmuth, N.; Ahmad, S.A.; Fan, F.; Gallick, G.E.; Ellis, L.M. Vascular endothelial growth factor is upregulated by interleukin-1β in human vascular smooth muscle cells via the P38 mitogen-activated protein kinase pathway. Angiogenesis 2001, 4, 155–162. [Google Scholar] [CrossRef]
- Tzeng, H.-E.; Tsai, C.-H.; Chang, Z.-L.; Su, C.-M.; Wang, S.-W.; Hwang, W.-L.; Tang, C.-H. Interleukin-6 induces vascular endothelial growth factor expression and promotes angiogenesis through apoptosis signal-regulating kinase 1 in human osteosarcoma. Biochem. Pharmacol. 2013, 85, 531–540. [Google Scholar] [CrossRef] [PubMed]
- Hu, K.; Olsen, B.R. Osteoblast-derived VEGF regulates osteoblast differentiation and bone formation during bone repair. J. Clin. Investig. 2016, 126, 509–526. [Google Scholar] [CrossRef] [Green Version]
- Aldridge, S.; Lennard, T.; Williams, J.; Birch, M. Vascular endothelial growth factor receptors in osteoclast differentiation and function. Biochem. Biophys. Res. Commun. 2005, 335, 793–798. [Google Scholar] [CrossRef] [PubMed]
- Zittermann, S.I.; Issekutz, A.C. Endothelial growth factors VEGF and bFGF differentially enhance monocyte and neutrophil recruitment to inflammation. J. Leukoc. Biol. 2006, 80, 247–257. [Google Scholar] [CrossRef] [PubMed]


| Ion Concentrations [mM] | ||||
|---|---|---|---|---|
| Blood Plasma | BCPx1 | BCPx1.5 | BCPx2 | |
| Na+ | 142.0 | 142.0 | 213.0 | 284.0 |
| K+ | 5.0 | 5.0 | 7.5 | 10.0 |
| Mg2+ | 1.5 | 1.5 | 2.25 | 3.0 |
| Ca2+ | 2.5 | 1.99 | 2.98 | 3.98 |
| Cl− | 103.0 | 147.8 | 221.7 | 295.6 |
| HCO3− | 27.0 | 4.2 | 6.3 | 8.4 |
| HPO42− | 1.0 | 1.0 | 1.5 | 2.0 |
| SO42− | 0.5 | 0.5 | 0.75 | 1.0 |
| pH | 7.2–7.4 | 7.4 | 7.4 | 7.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kreller, T.; Sahm, F.; Bader, R.; Boccaccini, A.R.; Jonitz-Heincke, A.; Detsch, R. Biomimetic Calcium Phosphate Coatings for Bioactivation of Titanium Implant Surfaces: Methodological Approach and In Vitro Evaluation of Biocompatibility. Materials 2021, 14, 3516. https://doi.org/10.3390/ma14133516
Kreller T, Sahm F, Bader R, Boccaccini AR, Jonitz-Heincke A, Detsch R. Biomimetic Calcium Phosphate Coatings for Bioactivation of Titanium Implant Surfaces: Methodological Approach and In Vitro Evaluation of Biocompatibility. Materials. 2021; 14(13):3516. https://doi.org/10.3390/ma14133516
Chicago/Turabian StyleKreller, Thomas, Franziska Sahm, Rainer Bader, Aldo R. Boccaccini, Anika Jonitz-Heincke, and Rainer Detsch. 2021. "Biomimetic Calcium Phosphate Coatings for Bioactivation of Titanium Implant Surfaces: Methodological Approach and In Vitro Evaluation of Biocompatibility" Materials 14, no. 13: 3516. https://doi.org/10.3390/ma14133516







