Novel High-Entropy Aluminide-Silicide Alloy
Abstract
:1. Introduction
2. Materials and Methods
3. Results
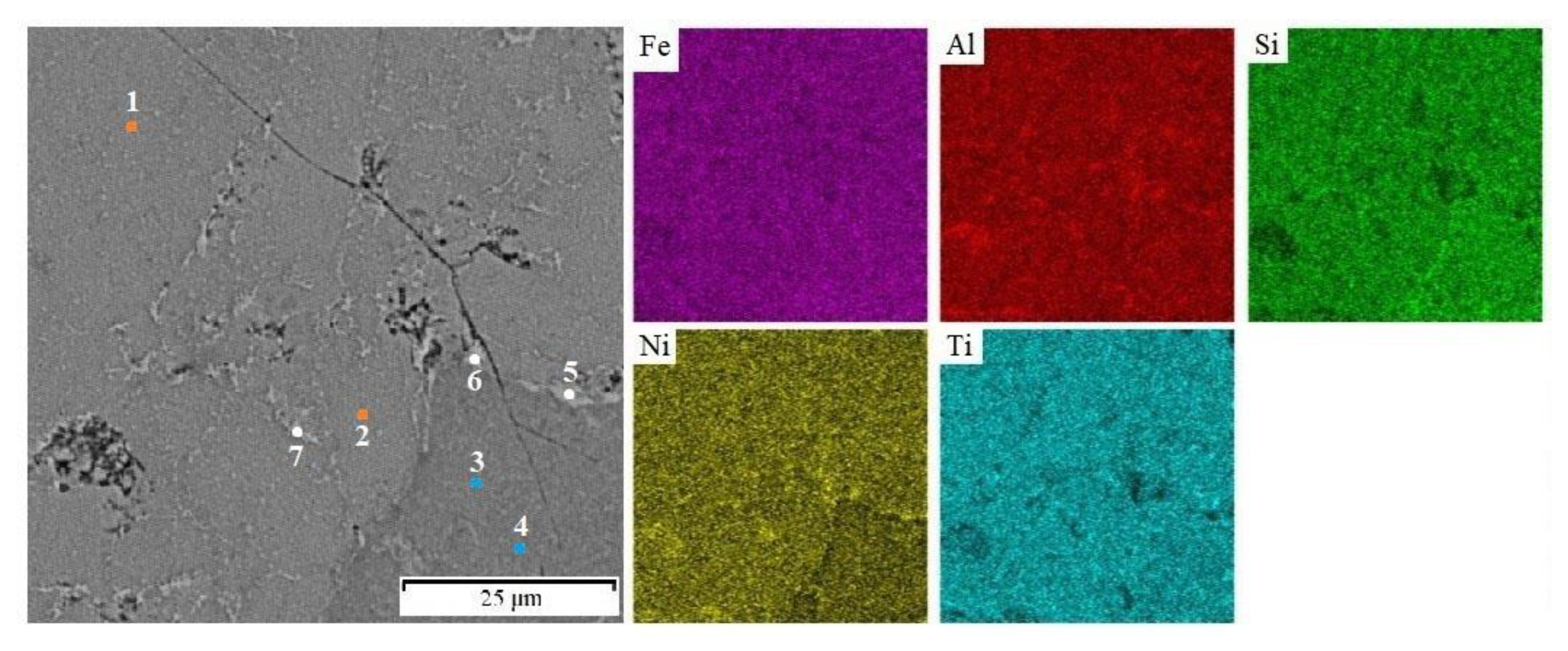
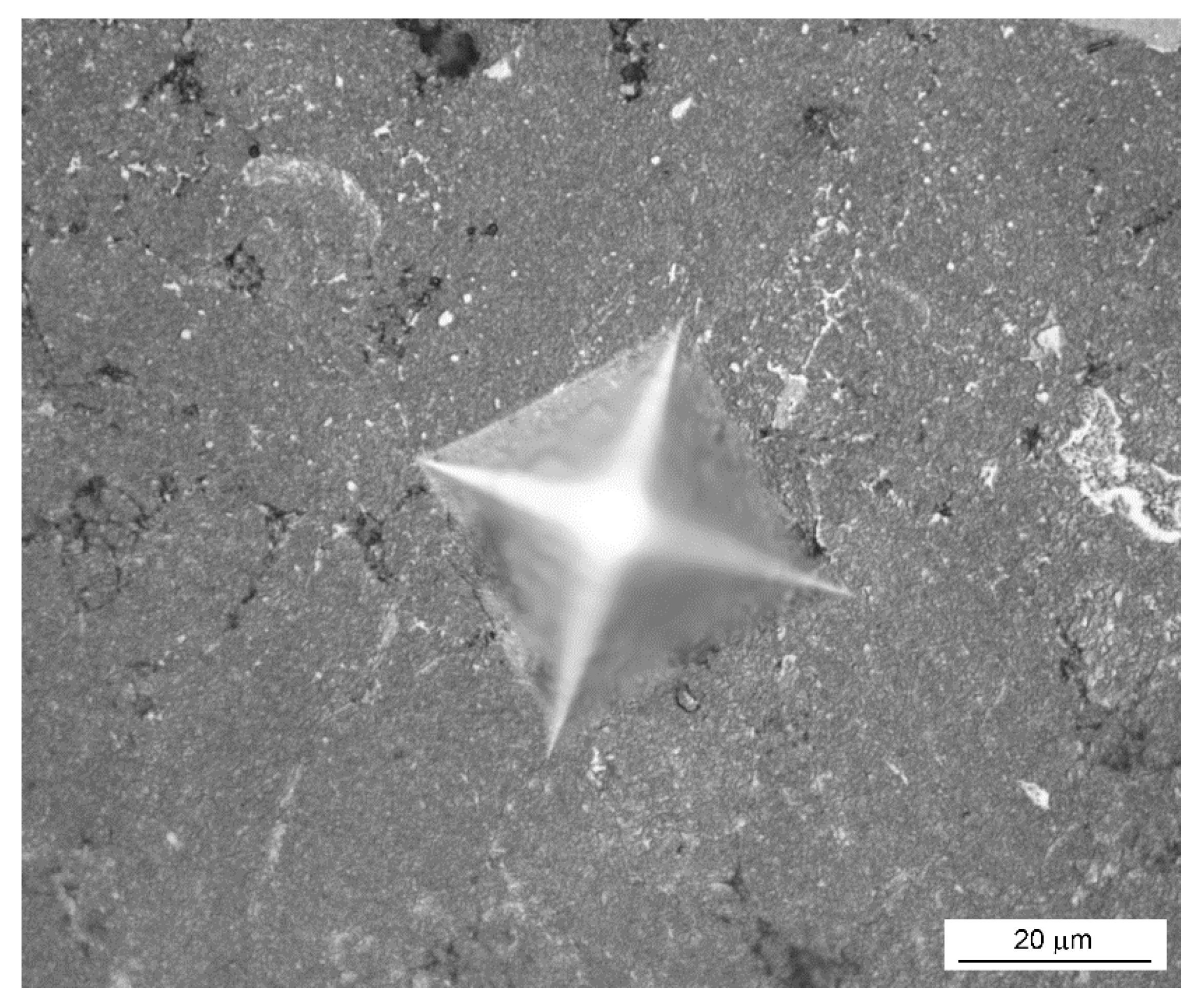
3.1. Phase Composition and Microstructure
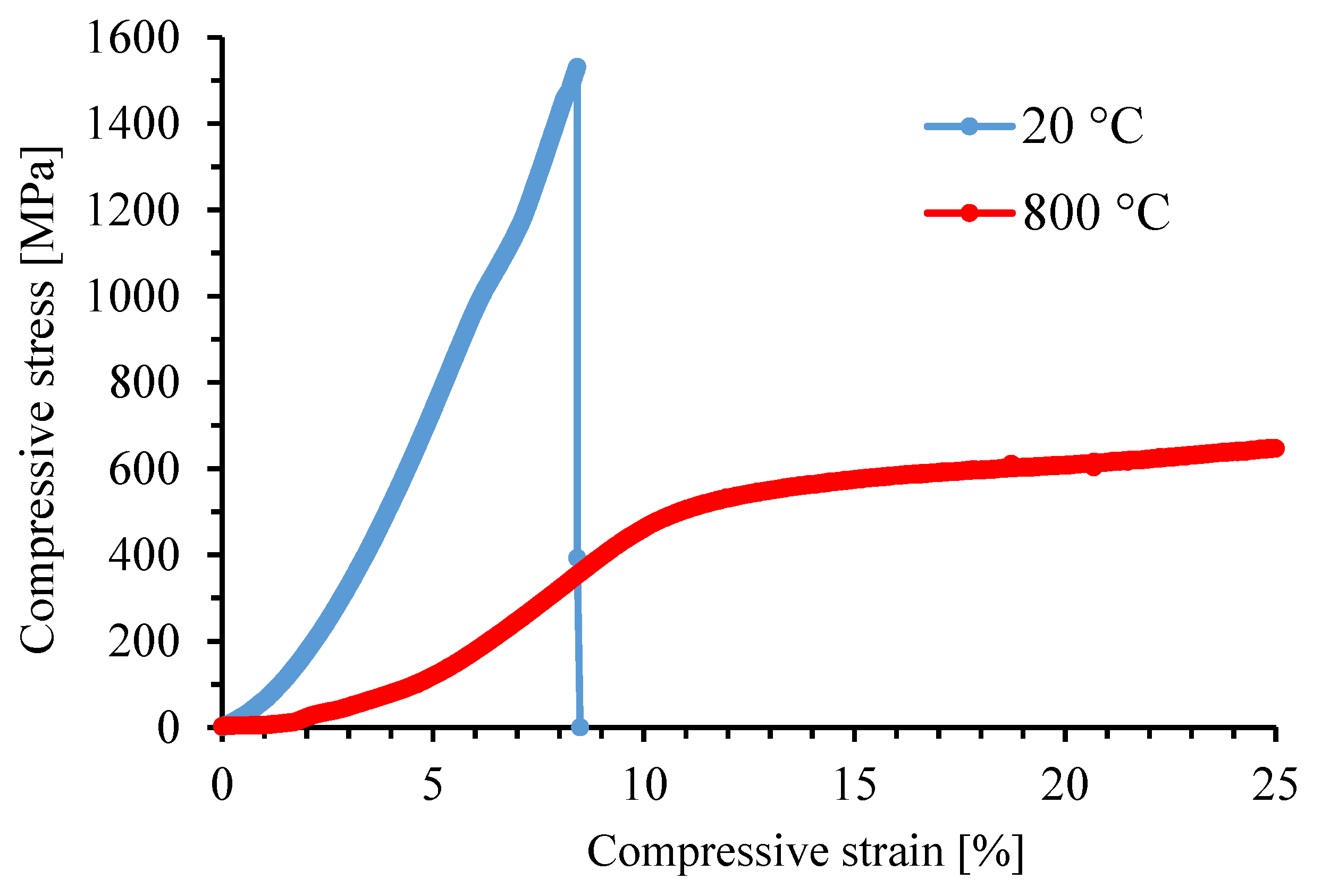
3.2. Mechanical and Tribological Properties
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yeh, J.-W.; Chen, S.-K.; Lin, S.-J.; Gan, J.-Y.; Chin, T.-S.; Shun, T.-T.; Tsau, C.-H.; Chang, S.-Y. Nanostructured high-entropy alloys with multiple principal elements: Novel alloy design concepts and outcomes. Adv. Eng. Mater. 2004, 6, 299–303. [Google Scholar] [CrossRef]
- Cantor, B.; Chang, I.; Knight, P.; Vincent, A. Microstructural development in equiatomic multicomponent alloys. Mater. Sci. Eng. A 2004, 375, 213–218. [Google Scholar] [CrossRef]
- Huang, P.-K.; Yeh, J.-W.; Shun, T.-T.; Chen, S.-K. Multi-Principal-Element Alloys with Improved Oxidation and Wear Resistance for Thermal Spray Coating. Adv. Eng. Mater. 2004, 6, 74–78. [Google Scholar] [CrossRef]
- Miracle, D.B.; Senkov, O.N. A critical review of high entropy alloys and related concepts. Acta Mater. 2017, 122, 448–511. [Google Scholar] [CrossRef] [Green Version]
- Manzoni, A.M.; Glatzel, U. New multiphase compositionally complex alloys driven by the high entropy alloy approach. Mater. Charact. 2019, 147, 512–532. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, Y.; Cai, J.; Ji, S.; Geng, J.; Sun, X.; Li, D. High-strength NbMoTaX refractory high-entropy alloy with low stacking fault energy eutectic phase via laser additive manufacturing. Mater. Des. 2021, 201, 109462. [Google Scholar] [CrossRef]
- Pang, J.; Zhang, H.; Zhang, L.; Zhu, Z.; Fu, H.; Li, H.; Wang, A.; Li, Z.; Zhang, H. Ductile Ti1.5ZrNbAl0.3 refractory high entropy alloy with high specific strength. Mater. Lett. 2021, 290, 129428. [Google Scholar] [CrossRef]
- Hua, N.; Wang, W.; Wang, Q.; Ye, Y.; Lin, S.; Zhang, L.; Guo, Q.; Brechtl, J.; Liaw, P.K. Mechanical, corrosion, and wear properties of biomedical Ti-Zr-Nb-Ta-Mo high entropy alloys. J. Alloy. Compd. 2021, 861, 157997. [Google Scholar] [CrossRef]
- Zhang, Y.; Zuo, T.T.; Tang, Z.; Gao, M.C.; Dahmen, K.A.; Liaw, P.K.; Lu, Z.P. Microstructures and properties of high-entropy alloys. Prog. Mater. Sci. 2014, 61, 1–93. [Google Scholar] [CrossRef]
- Grilli, M.L.; Bellezze, T.; Gamsjäger, E.; Rinaldi, A.; Novak, P.; Balos, S.; Piticescu, R.R.; Ruello, M.L. Solutions for Critical Raw Materials under Extreme Conditions: A Review. Materials 2017, 10, 285. [Google Scholar] [CrossRef] [Green Version]
- Moravcik, I.; Gamanov, S.; Moravcikova-Gouvea, L.; Kovacova, Z.; Kitzmantel, M.; Neubauer, E.; Dlouhy, I. Influence of Ti on the Tensile Properties of the High-Strength Powder Metallurgy High Entropy Alloys. Materials 2020, 13, 578. [Google Scholar] [CrossRef] [Green Version]
- Koch, C.C.; Whittenberger, J.D. Mechanical milling/alloying of intermetallics. Intermetallics 1996, 4, 339–355. [Google Scholar] [CrossRef]
- Froes, F.H.S.; Suryanarayana, C.; Russell, K.; Li, C.-G. Synthesis of intermetallics by mechanical alloying. Mater. Sci. Eng. A 1995, 192, 612–623. [Google Scholar]
- Mamedov, V. Spark plasma sintering as advanced PM sintering method. Powder Metall. 2002, 45, 322–328. [Google Scholar] [CrossRef]
- Kratochvíl, P. The history of the search and use of heat resistant Pyroferal© alloys based on FeAl. Intermetallics 2008, 16, 587–591. [Google Scholar] [CrossRef]
- Lu, Y.; Wang, X.; Zhang, Y.; Wang, J.; Kim, M.J.; Zhang, H. Aluminum carbide hydrolysis induced degradation of thermal conductivity and tensile strength in diamond/aluminum composite. J. Compos. Mater. 2018, 52, 2709–2717. [Google Scholar] [CrossRef]
- Hotař, A.; Palm, M.; Kratochvíl, P.; Vodičková, V.; Daniš, S. High-temperature oxidation behaviour of Zr alloyed Fe3Al-type iron aluminide. Corros. Sci. 2012, 63, 71–81. [Google Scholar] [CrossRef]
- Kratochvíl, P.; Vodičková, V.; Král, R.; Švec, M. The Effect of Laves Phase (Fe,Al)2Zr on the High-Temperature Strength of Carbon-Alloyed Fe3Al Aluminide. Metall. Mater. Trans. A 2016, 47, 1128–1131. [Google Scholar] [CrossRef]
- Kejzlar, P.; Kratochvíl, P.; Král, R.; Vodičková, V. Phase Structure and High-Temperature Mechanical Properties of Two-Phase Fe-25Al-xZr Alloys Compared to Three-Phase Fe-30Al-xZr Alloys. Metall. Mater. Trans. A 2014, 45, 335–342. [Google Scholar] [CrossRef]
- Haušild, P.; Karlík, M.; Sima, V.; Alexander, D. Microstructure and mechanical properties of hot rolled Fe–40 at-%Al intermetallic alloys with Zr and B addition. Mater. Sci. Technol. 2011, 27, 1448–1452. [Google Scholar] [CrossRef]
- Novák, P.; Vanka, T.; Nová, K.; Stoulil, J.; Průša, F.; Kopeček, J.; Haušild, P.; Laufek, F. Structure and Properties of Fe-Al-Si Alloy Prepared by Mechanical Alloying. Materials 2019, 12, 2463. [Google Scholar] [CrossRef] [Green Version]
- Milekhine, M.I.; Onsøien, M.I.; Solberg, J.K.; Skaland, T. Mechanical properties of FeSi (ε), FeSi2 (ζα) and Mg2Si. Intermetallics 2002, 10, 743–750. [Google Scholar] [CrossRef]
- Kubošová, A.; Karlík, M.; Haušild, P.; Prahl, J. Fracture Behaviour of Fe3Al and FeAl Type Iron Aluminides. Mater. Sci. Forum 2007, 567–568, 349–352. [Google Scholar] [CrossRef]
- Ivanov, E.G. Thermodynamic analysis of phase transformations during aluminizing. Met. Sci. Heat Treat. 1979, 21, 449–452. [Google Scholar] [CrossRef]
- Novák, P.; Barták, Z.; Nová, K.; Průša, F. Effect of Nickel and Titanium on Properties of Fe-Al-Si Alloy Prepared by Mechanical Alloying and Spark Plasma Sintering. Materials 2020, 13, 800. [Google Scholar] [CrossRef] [Green Version]
- Umakoshi, Y.; Yamaguchi, M. Deformation of FeAl single crystals at high temperatures. Philos. Mag. A 1980, 41, 573–588. [Google Scholar] [CrossRef]
- Czichos, H. Tribology: A Systems Approach to the Science and Technology of Friction, Lubrication, and Wear, 1st ed.; Elsevier Scientific Publishing Company: Amsterdam, The Netherlands, 1978; 399p. [Google Scholar]
- Massalski, T.B. Binary Alloy Phase Diagrams; ASM, Materials Park: Novelty, OH, USA, 1990. [Google Scholar]
- Novák, P.; Knotek, V.; Šerák, J.; Michalcová, A.; Vojtěch, D. Synthesis of Fe-Al-Si intermediary phases by reactive sintering. Powder Metall. 2011, 54, 167–171. [Google Scholar] [CrossRef]
- Zhao, P.; Morris, D.; Munoz, M. Forging, textures, and deformation systems in a B2 FeAl alloy. J. Mater. Res. 1999, 14, 715–728. [Google Scholar] [CrossRef] [Green Version]
- Čech, J.; Haušild, P.; Materna, A. Deformation of Fe3Si single-crystals under nanoindentation. Acta Polytech. CTU Proc. 2018, 17, 1–5. [Google Scholar] [CrossRef]




| Content | Element | ||||
|---|---|---|---|---|---|
| Fe | Al | Si | Ni | Ti | |
| at. [%] | 20.0 | 20.0 | 20.0 | 20.0 | 20.0 |
| wt. [%] | 25.7 | 12.4 | 12.9 | 27.0 | 22.0 |
| Element Points | Fe | Al | Si | Ni | Ti | Corresponding Phase |
|---|---|---|---|---|---|---|
| at. [%] | ||||||
| 1 | 20.4 | 22.4 | 18 | 20.3 | 18.9 | FeTiSi |
| 2 | 21.4 | 21.1 | 19 | 19.2 | 19.3 | FeTiSi |
| 3 | 24.2 | 19.9 | 20.8 | 15.7 | 19.4 | FeSi |
| 4 | 23.2 | 21.1 | 20.7 | 15.5 | 19.5 | FeSi |
| 5 | 21.2 | 29.8 | 11.6 | 26.0 | 11.4 | FeAl |
| 6 | 17.8 | 32.8 | 12.7 | 27.0 | 9.7 | FeAl |
| 7 | 17.6 | 31.6 | 13.9 | 25.4 | 11.5 | FeAl |
| FeAlSiNiTi | HV30 [-] | UCS [MPa] |
| 629 ± 82 | 1528 |
| FeAlSiNiTi | Ra [μm] | w [mm3N−1m−1] | f [-] |
| 0.0375 | 24.44 × 10−6 | 0.674 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Novák, P.; Nová, K. Novel High-Entropy Aluminide-Silicide Alloy. Materials 2021, 14, 3541. https://doi.org/10.3390/ma14133541
Novák P, Nová K. Novel High-Entropy Aluminide-Silicide Alloy. Materials. 2021; 14(13):3541. https://doi.org/10.3390/ma14133541
Chicago/Turabian StyleNovák, Pavel, and Kateřina Nová. 2021. "Novel High-Entropy Aluminide-Silicide Alloy" Materials 14, no. 13: 3541. https://doi.org/10.3390/ma14133541
APA StyleNovák, P., & Nová, K. (2021). Novel High-Entropy Aluminide-Silicide Alloy. Materials, 14(13), 3541. https://doi.org/10.3390/ma14133541







