Iso- and Anisotropic Etching of Micro Nanofibrillated Cellulose Films by Sequential Oxygen and Nitrogen Gas Plasma Exposure for Tunable Wettability on Crystalline and Amorphous Regions
Abstract
:1. Introduction
2. Materials and Methods
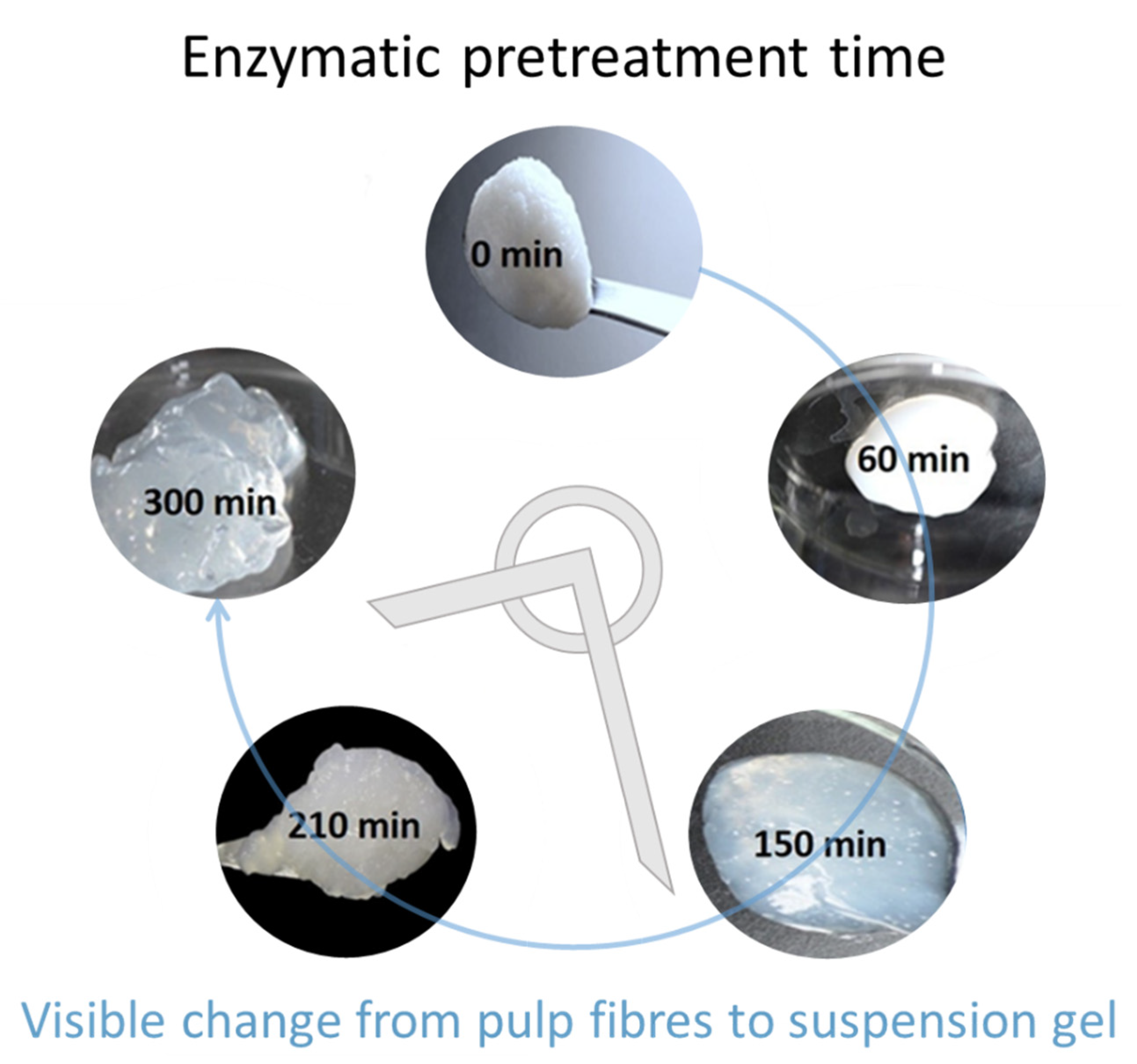
2.1. Preparation of MNFC
2.2. MNFC Suspension Characterisation
2.2.1. Drainage
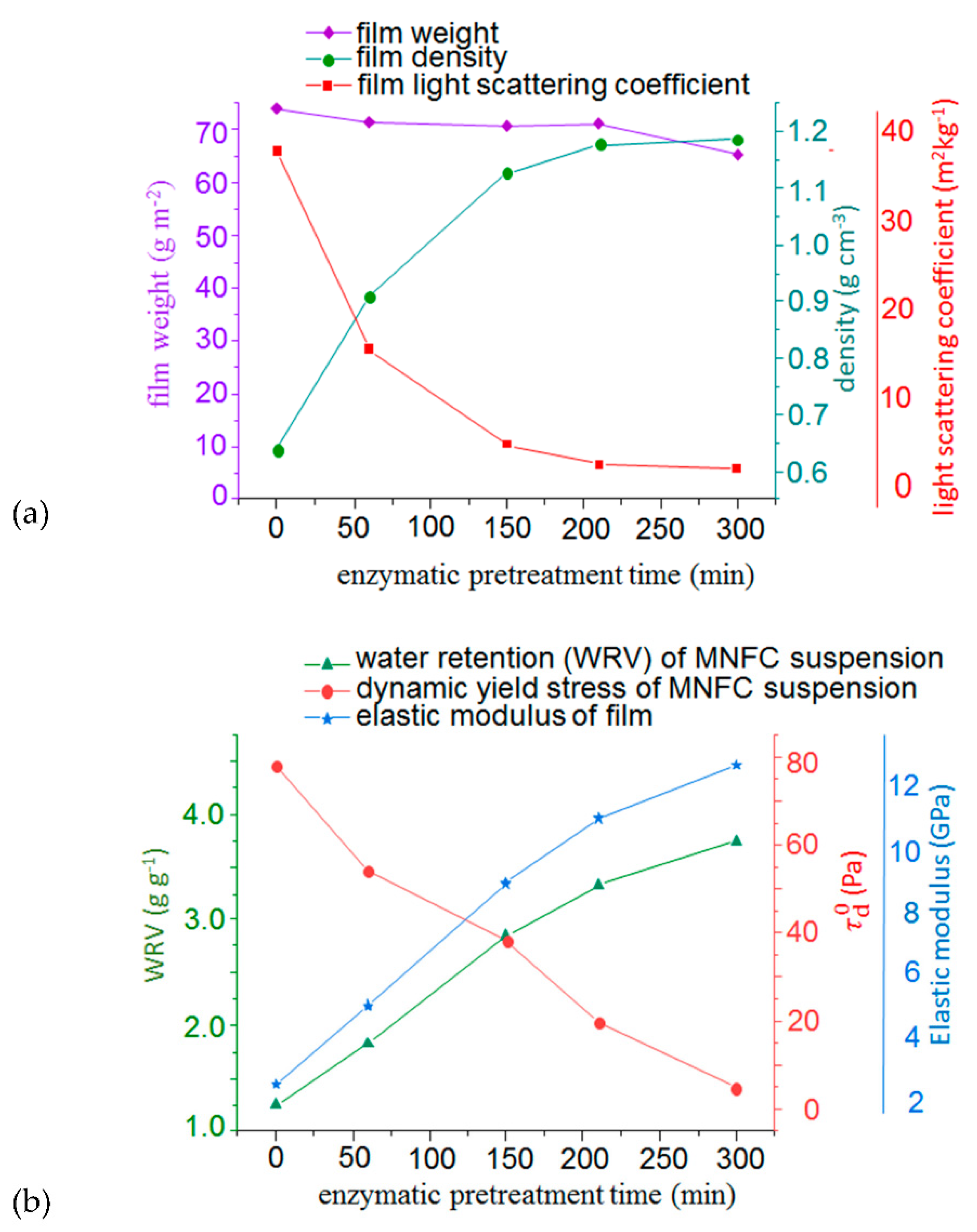
2.2.2. Water Retention
2.3. Rheological Evaluation of MNFC Suspensions
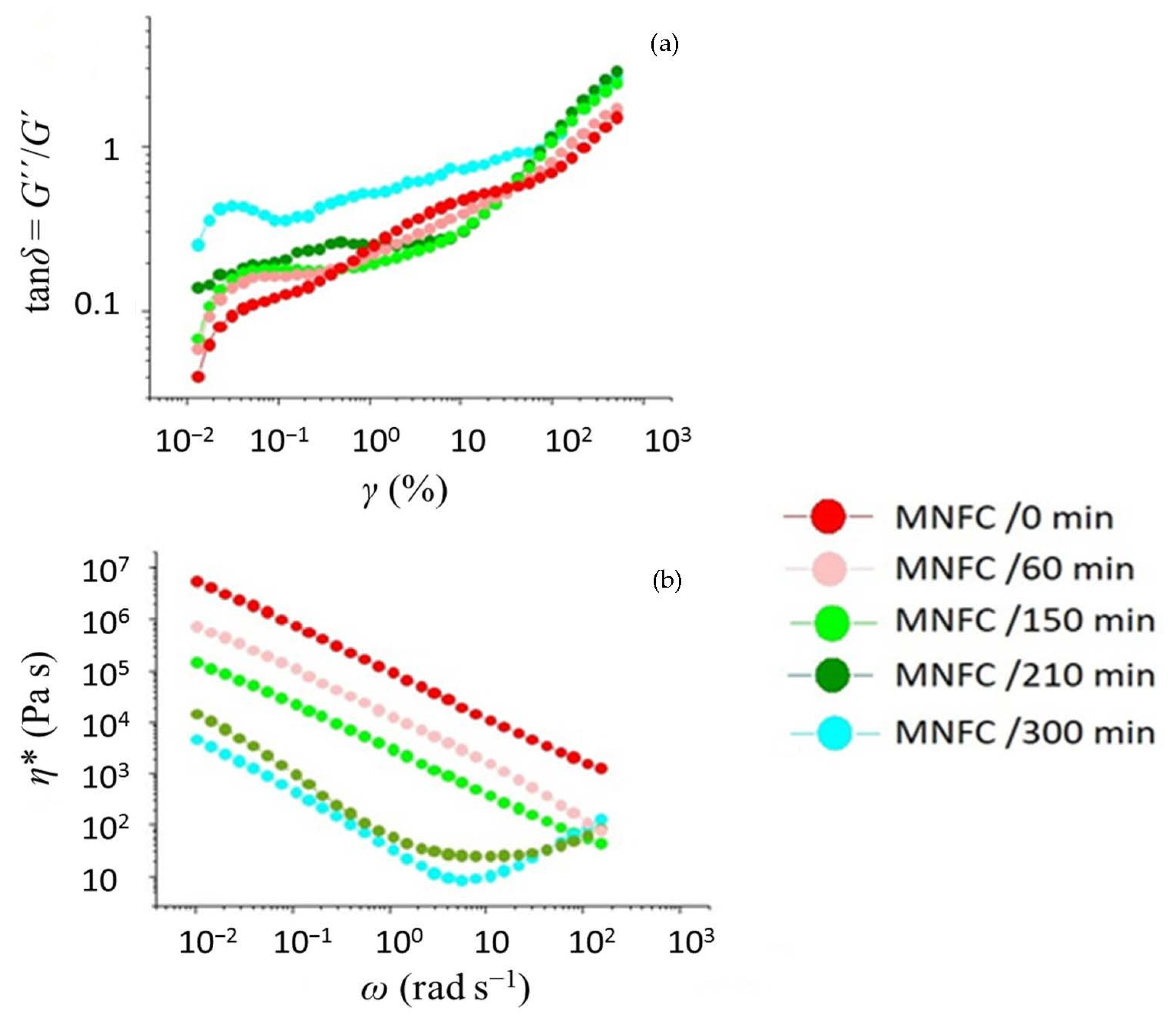
2.3.1. Shear Response
2.3.2. Viscoelastic Response
2.3.3. Yield Stress
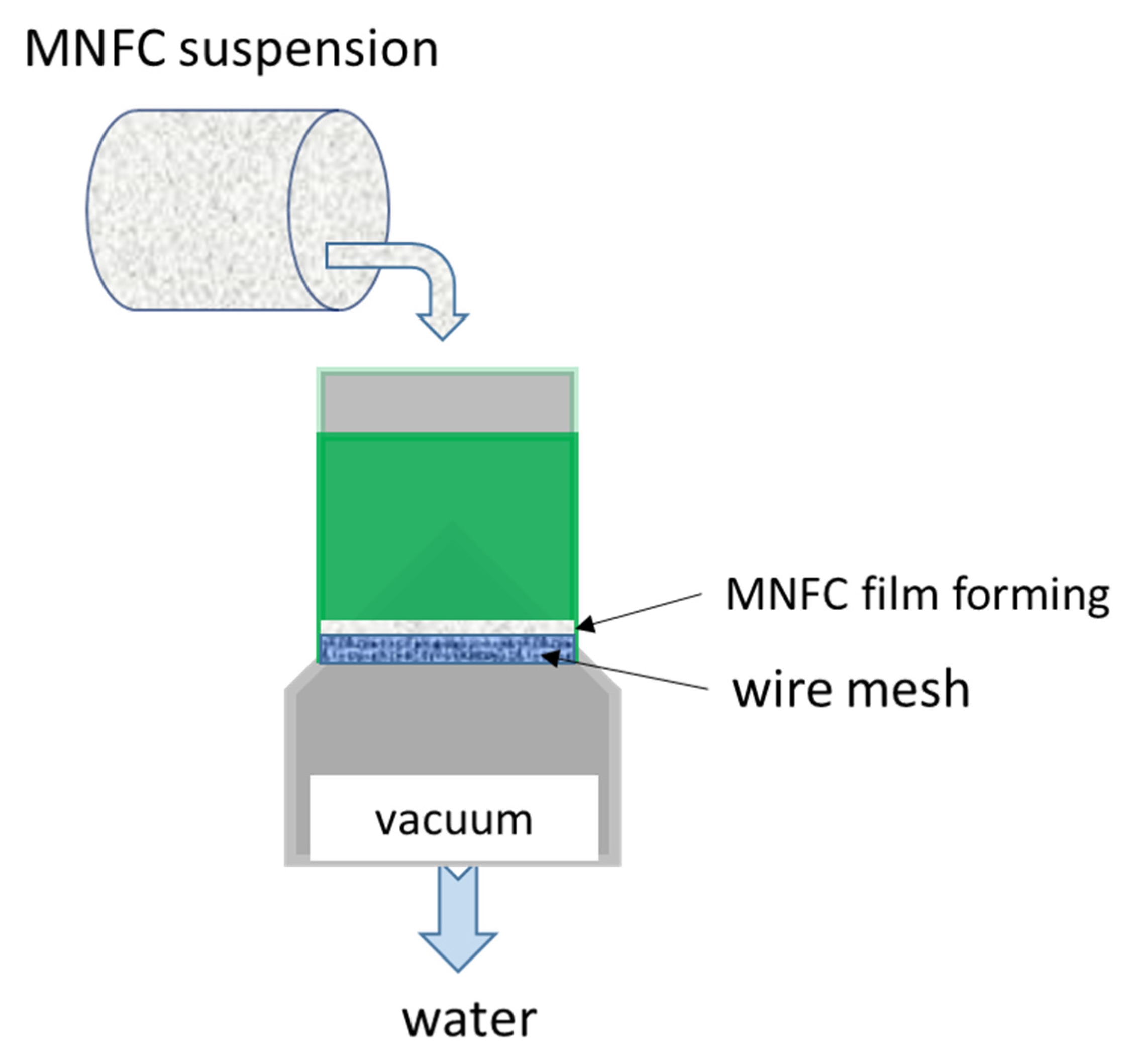
2.4. MNFC Film Preparation
2.5. DBD Plasma Film Exposure
2.6. Material Characterisation
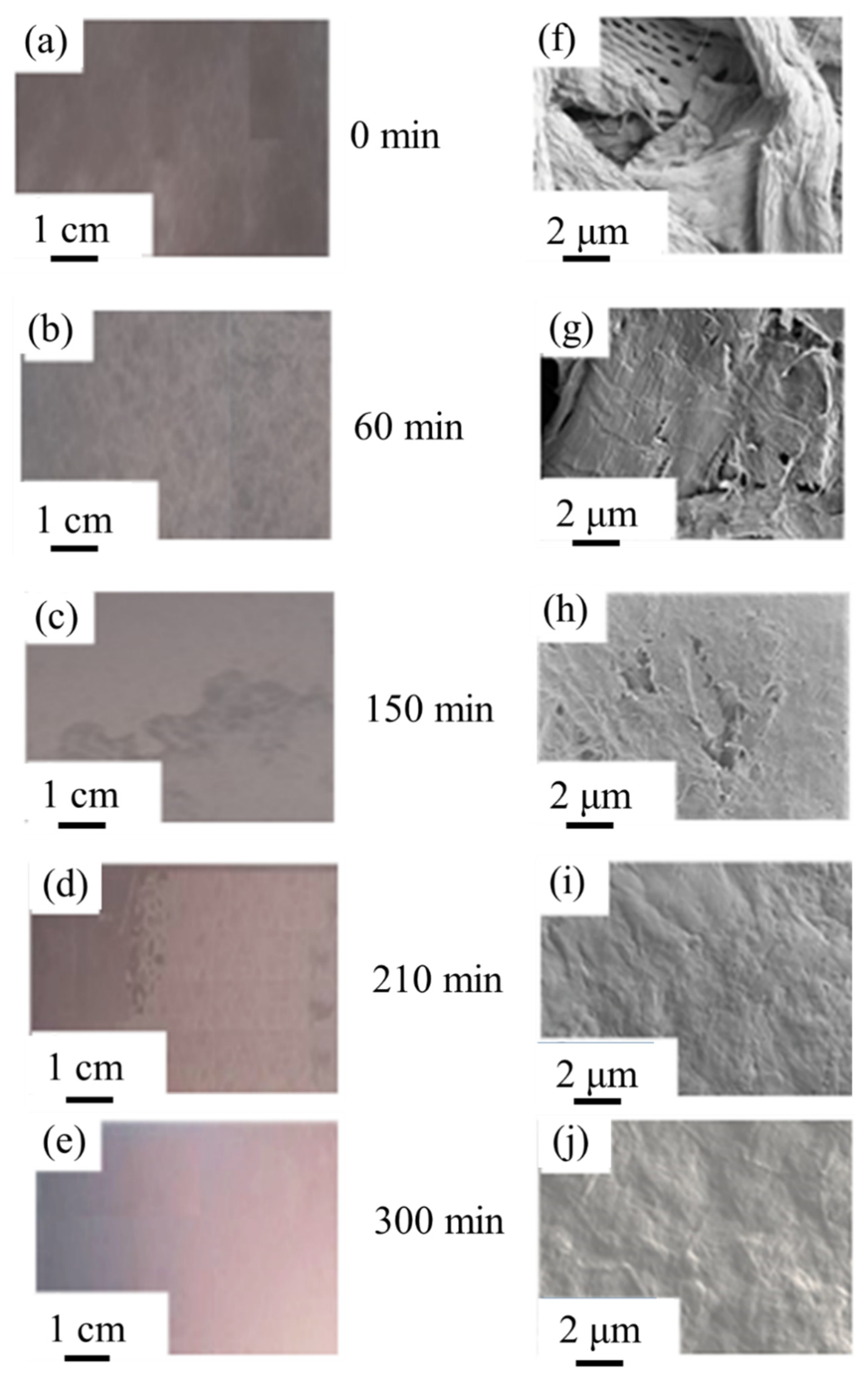
2.6.1. Digital Imaging and Optical Microscopy
2.6.2. Scanning Electron Microscopy (SEM)
2.6.3. Permeability—Air Flow Technique
2.6.4. Surface Chemical Composition
2.6.5. Surface Roughness
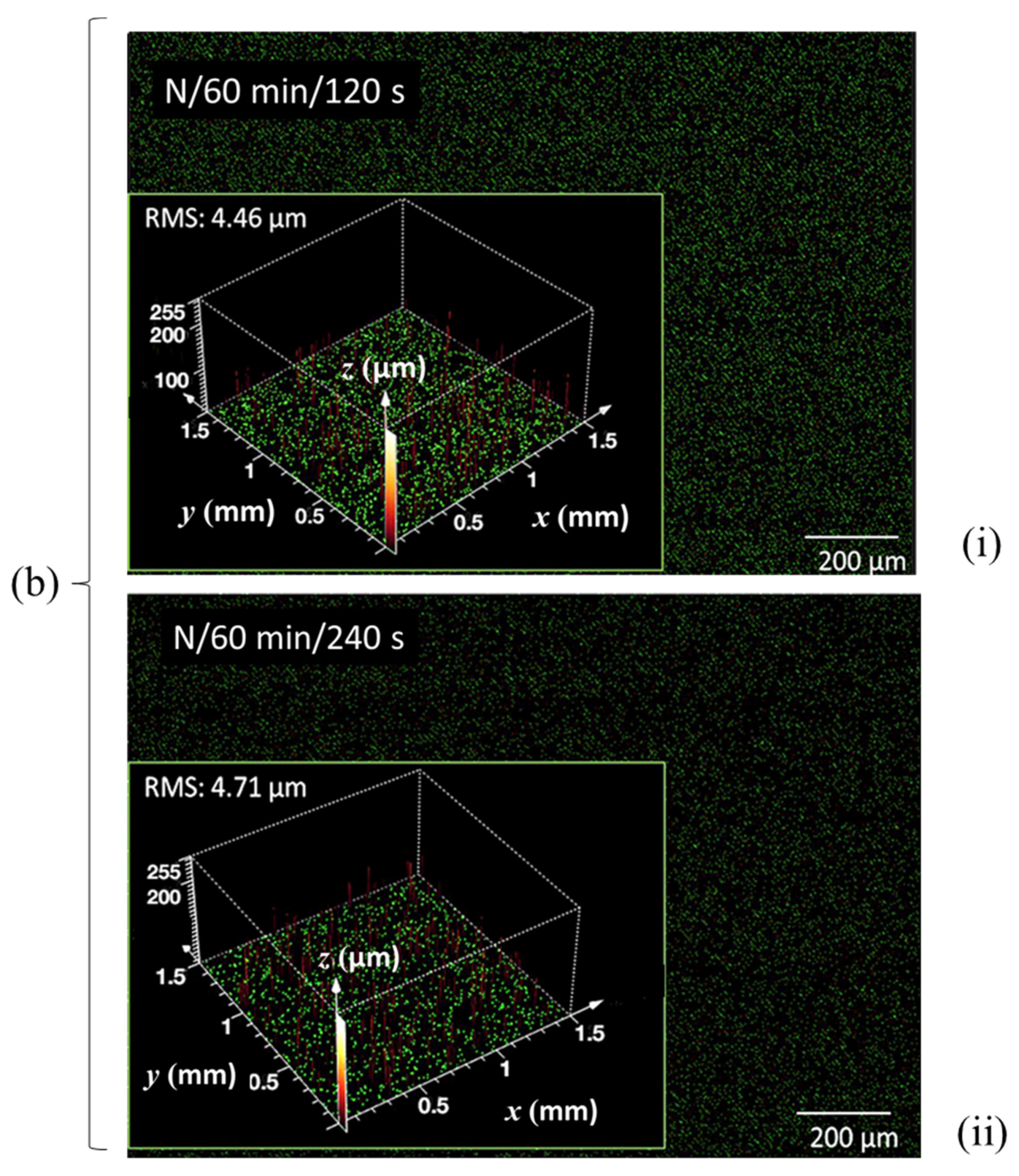
Microroughness—Confocal Laser Microscope (CLSM)
Nanoroughness—Atomic Force Microscope (AFM)
MNFC Film Physical Testing
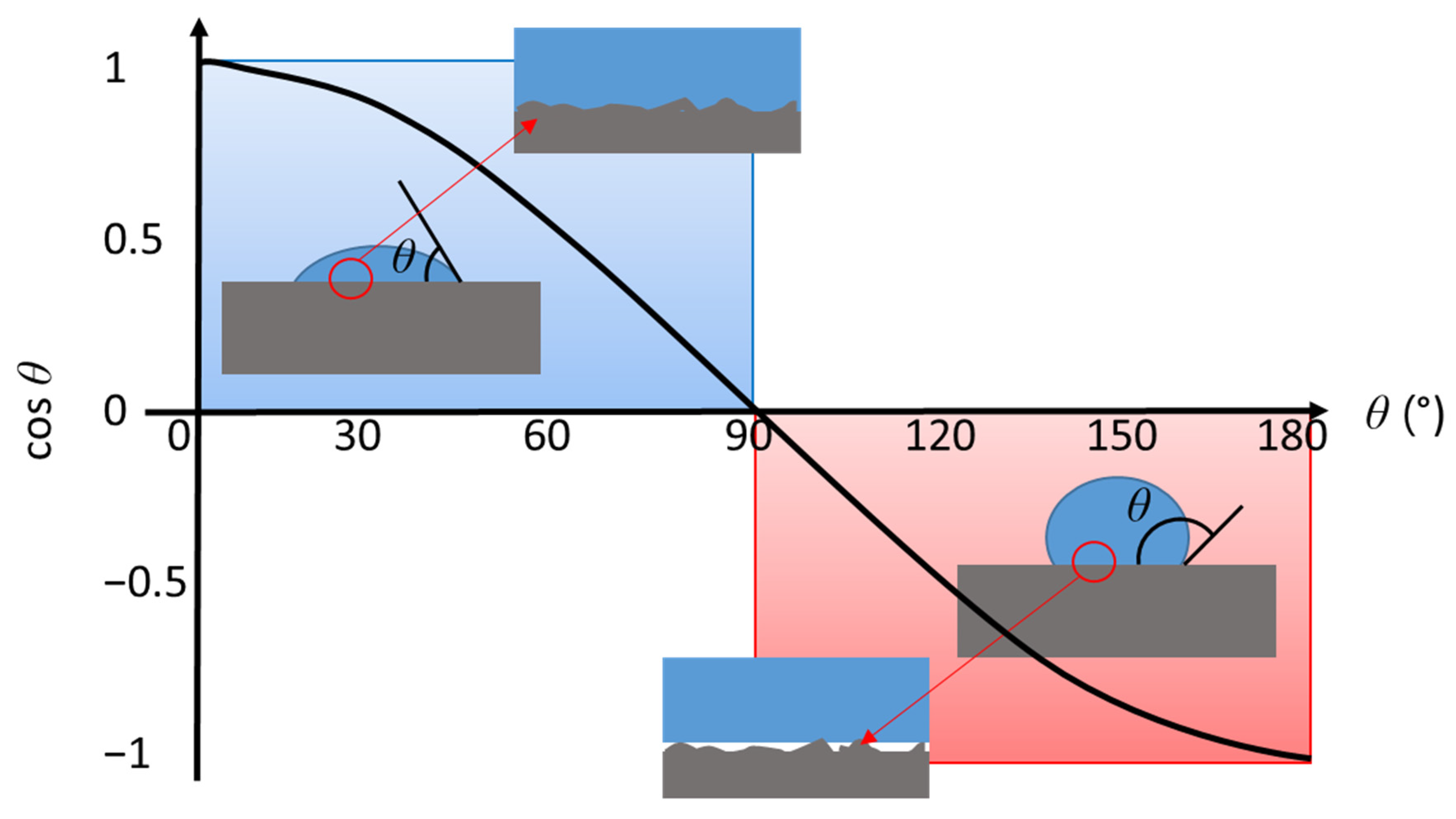
2.7. Droplet Analysis of Surface Energy-Dependent Wetting
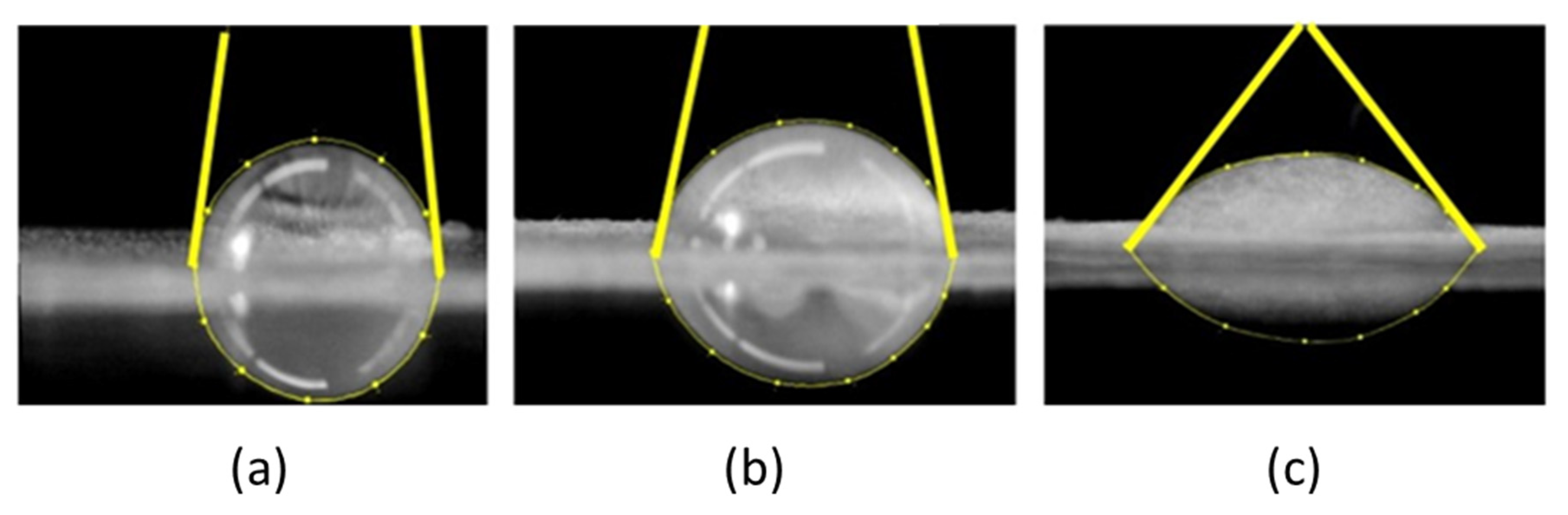
2.7.1. Contact Angle and Free Surface Area
2.7.2. Printing
3. Results and Discussion
3.1. Dewatering and Rheological Evaluation of Suspensions
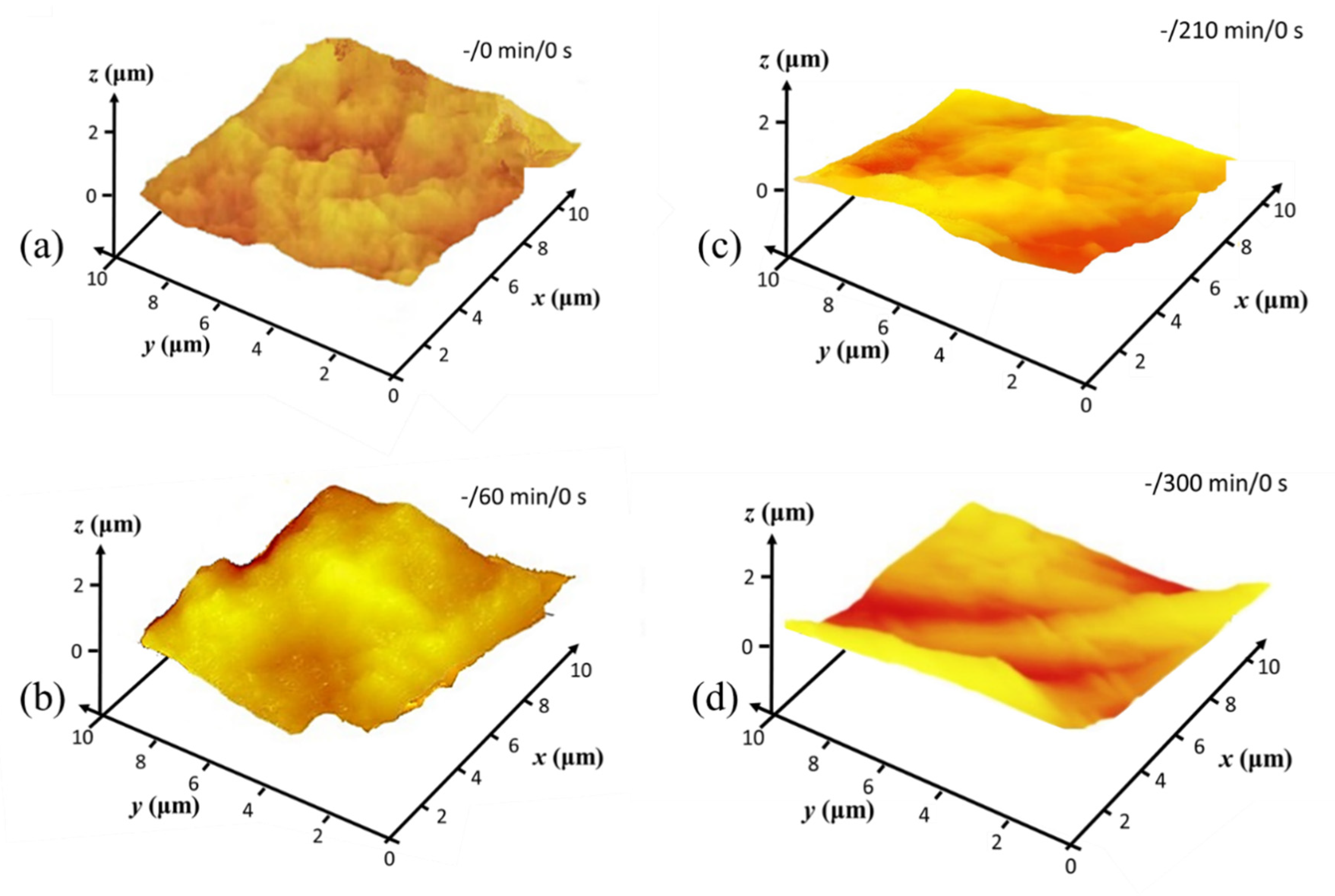
3.2. Morphological and Mechanical Evaluation of MNFC Films
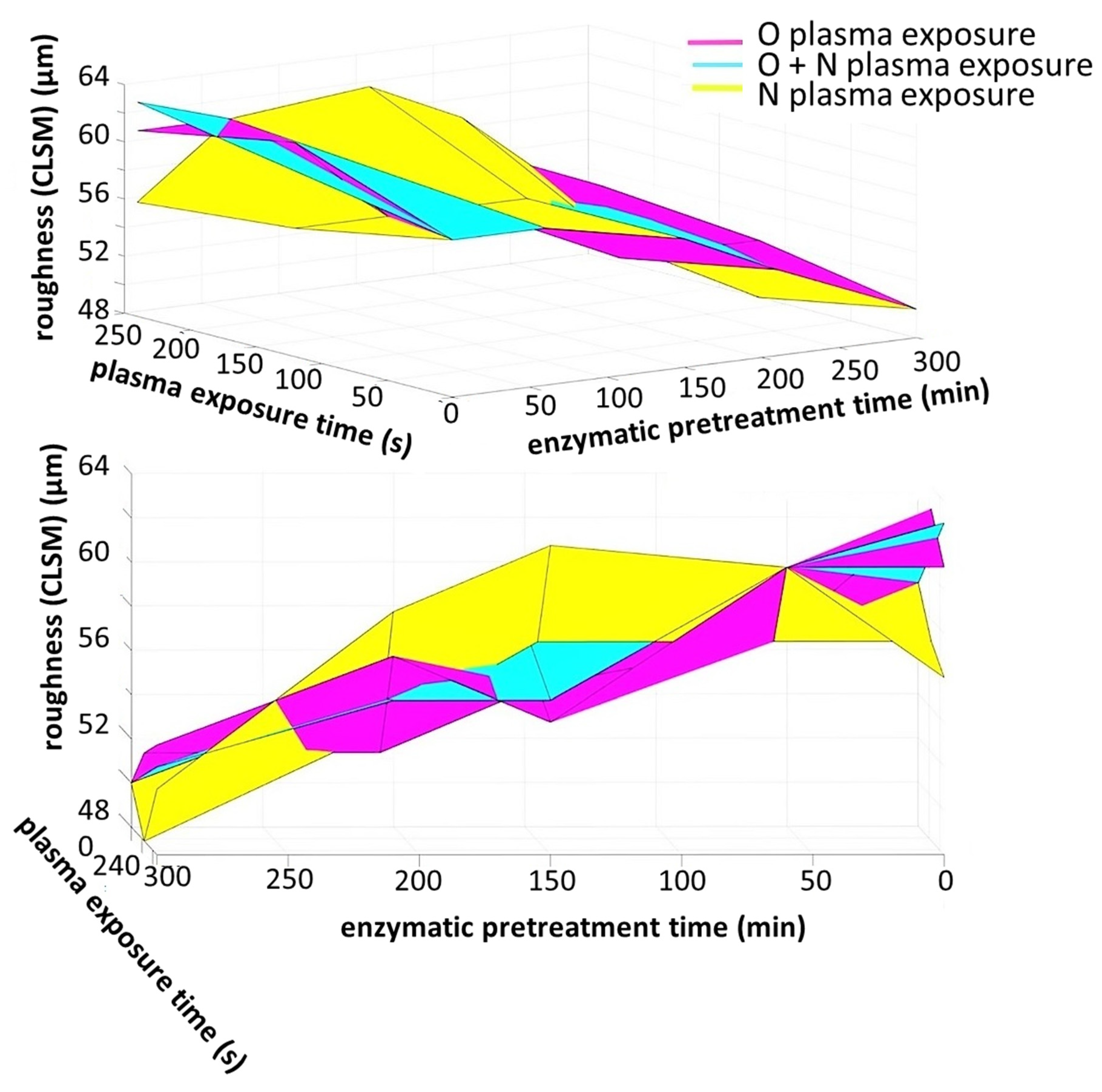
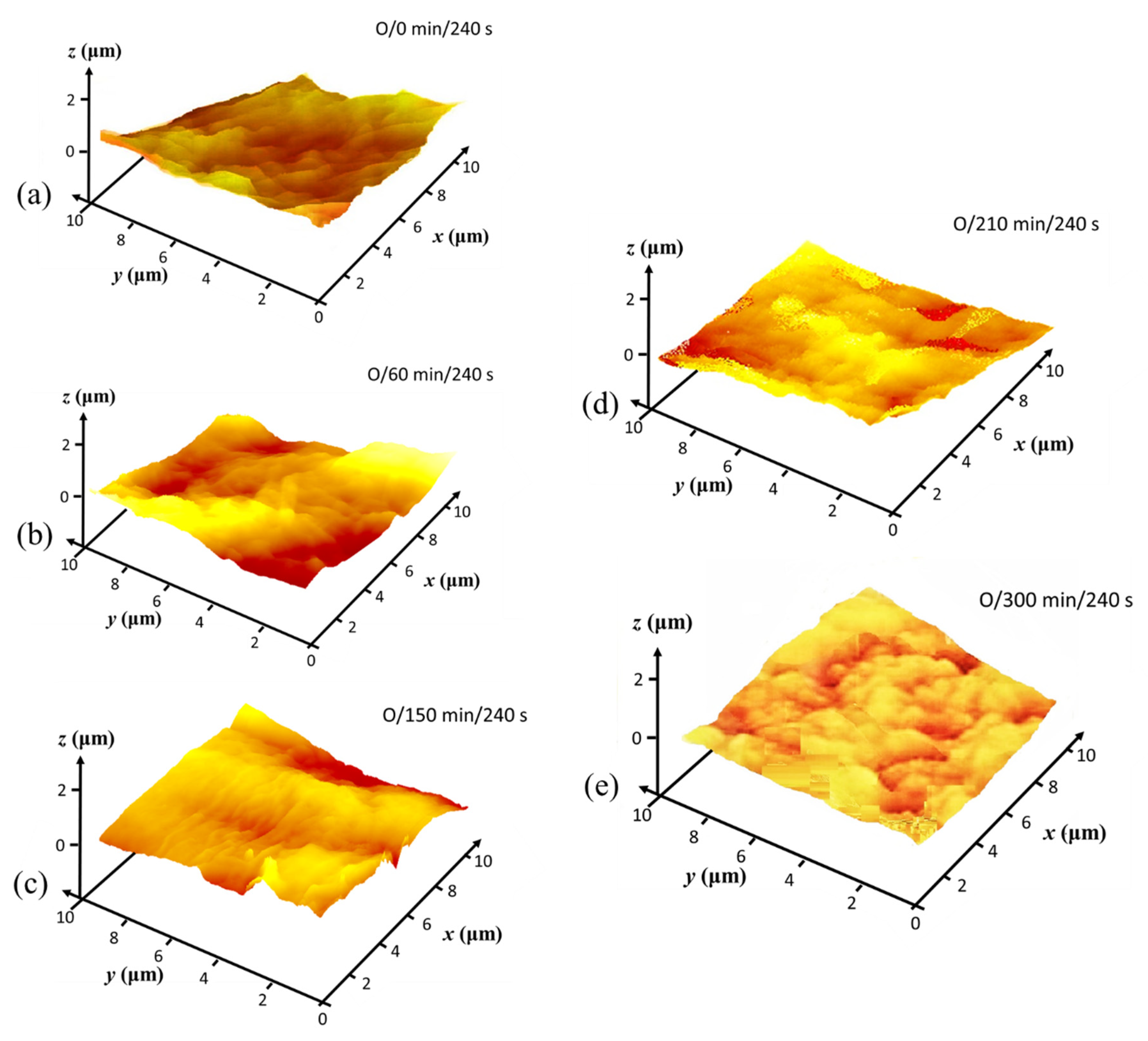
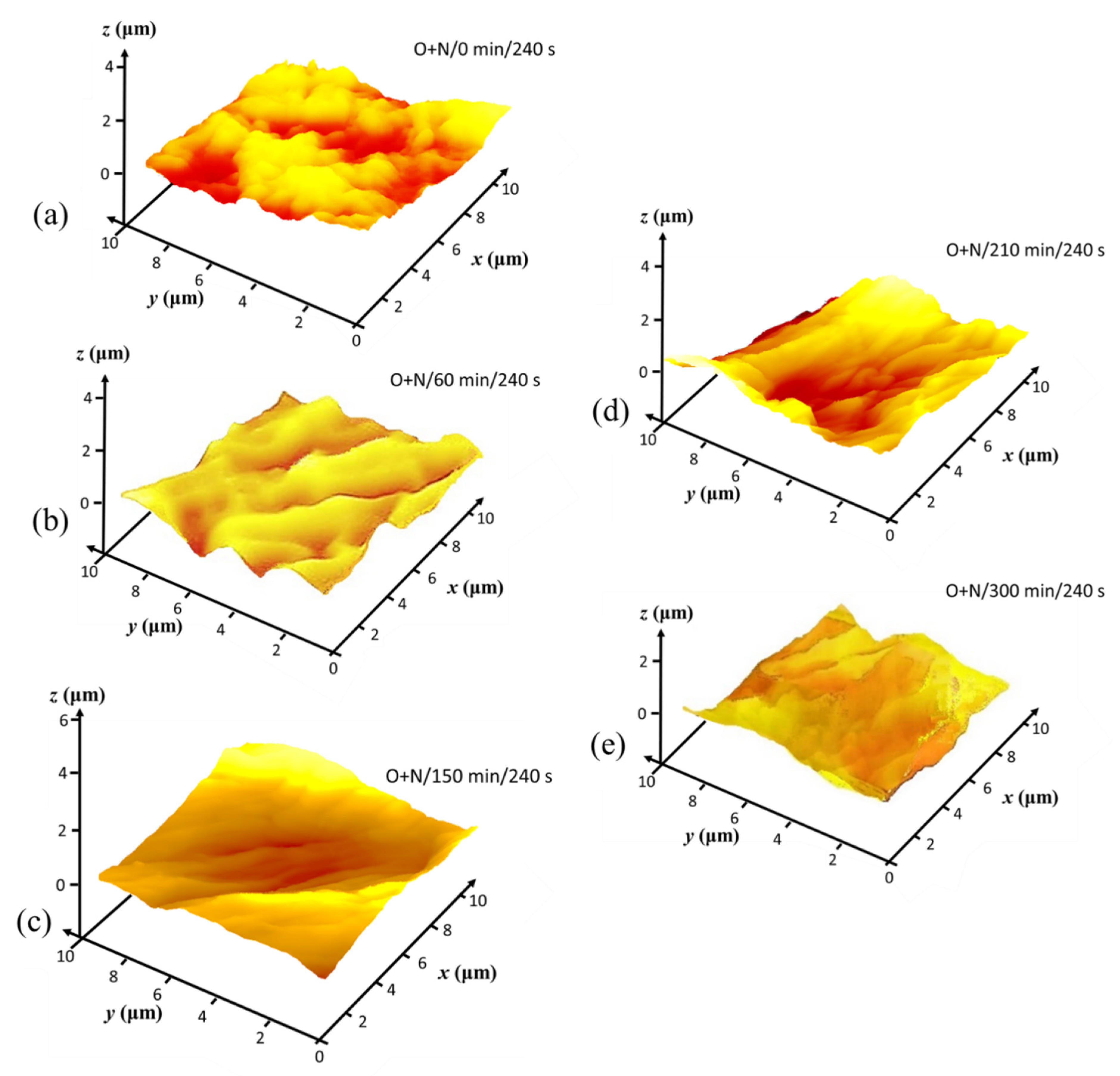
3.3. Hierarchical Roughness and Structure Regimes
3.4. Microroughness Determined by CLSM Scanning
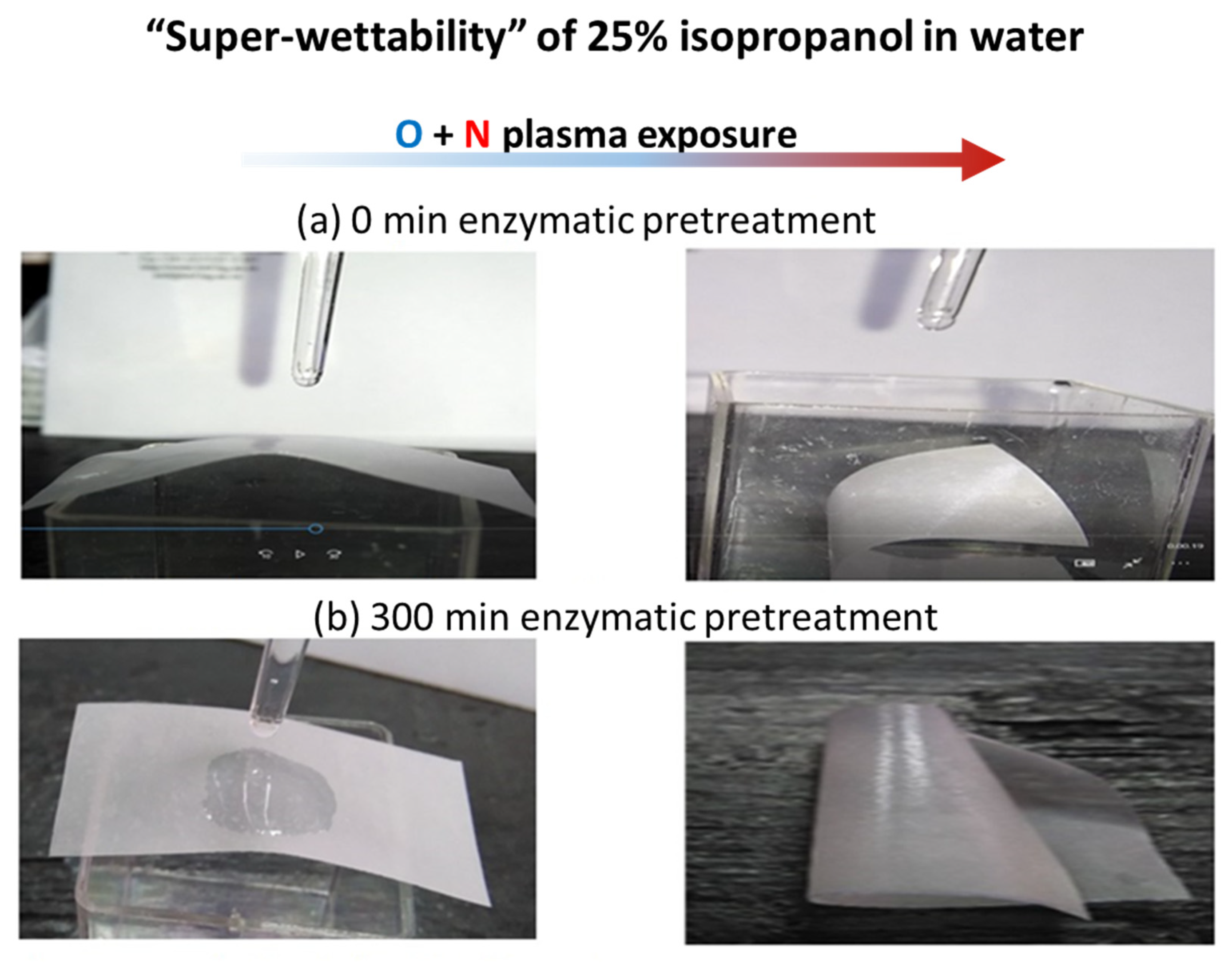
3.5. Contact Angle and Surface Free Energy (SFE)
3.6. Elemental Analysis of Plasma Treated Films (XPS)—Impact on SFE
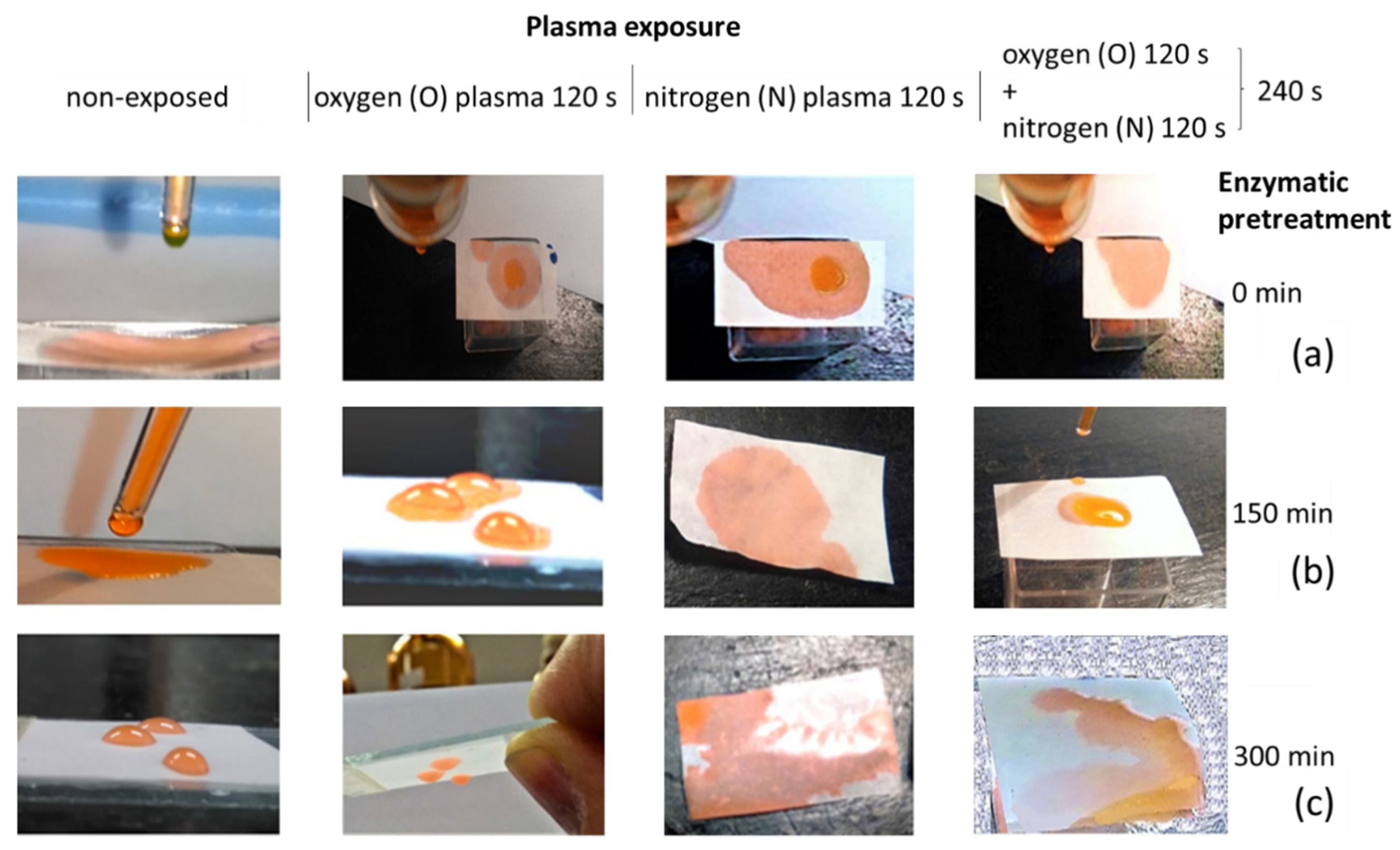
3.7. DoD Printing on DBD Plasma-Exposed Films
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Klemm, D.; Heublein, B.; Fink, H.P.; Bohn, A. Cellulose: Fascinating biopolymer and sustainable raw material. Angew. Chem. Int. Ed. 2005, 44, 3358–3393. [Google Scholar] [CrossRef]
- De Oliveira, E.M.; Sanchez, S.A.; Bettega, M.H.F.; Natalense, A.P.P.; Lima, M.A.P.; Varella, M.T.D.N. Shape resonance spectra of lignin subunits. Phys. Rev. A 2012, 86, 1–4. [Google Scholar] [CrossRef]
- Isogai, A.; Saito, T.; Fukuzumia, H. TEMPO-oxidized cellulose nanofibers. Nanoscale 2011, 3, 71–85. [Google Scholar] [CrossRef] [PubMed]
- Pääkkönen, T.; Dimic-Misic, K.; Orelma, H.; Pönni, R.; Vuorinen, T.; Maloney, T. Effect of xylan in hardwood pulp on the reaction rate of TEMPO-mediated oxidation and the rheology of the final nanofibrillated cellulose gel. Cellulose 2016, 23, 277–293. [Google Scholar] [CrossRef]
- Schenker, M.; Schoelkopf, J.; Mangin, P.; Gane, P. Rheological investigation of complex micro nanofibrillated cellulose (MNFC) suspensions: Discussion of flow curves and gel stability. Tappi J. 2016, 15, 405–416. [Google Scholar] [CrossRef]
- Imani, M.; Ghasemian, A.; Dehghani-Firouzabadi, M.R.; Afra, E.; Borghei, M.; Johansson, L.S.; Rojas, O.J. Coupling nanofibril lateral size and residual lignin to tailor the properties of lignocellulose films. Adv. Mater. Interfaces 2019, 6, 1900770. [Google Scholar] [CrossRef]
- Fukuzumi, H.; Saito, T.; Iwata, T.; Kumamoto, Y.; Isogai, A. Transparent and high gas barrier films of cellulose nanofibers prepared by TEMPO-mediated oxidation. Biomacromolecules 2009, 10, 162–165. [Google Scholar] [CrossRef]
- Syverud, K.; Stenius, P. Strength and barrier properties of MFC films. Cellulose 2009, 16, 75–85. [Google Scholar] [CrossRef]
- Aulin, C.; Gällstedt, M.; Lindström, T. Oxygen and oil barrier properties of microfibrillated cellulose films and coatings. Cellulose 2010, 17, 559–574. [Google Scholar] [CrossRef]
- Hult, E.L.; Iotti, M.; Lenes, M. Efficient approach to high barrier packaging using microfibrillar cellulose and shellac. Cellulose 2010, 17, 575–586. [Google Scholar] [CrossRef]
- Plackett, D.; Anturi, H.; Hedenqvist, M.; Ankerfors, M.; Gällstedt, M.; Lindström, T.; Siró, I. Physical properties and morphology of films prepared from microfibrillated cellulose and microfibrillated cellulose in combination with amylopectin. J. Appl. Polym. Sci. 2010, 117, 3601–3609. [Google Scholar] [CrossRef]
- Chinga-Carrasco, G.; Syverud, K. On the structure and oxygen transmission rate of biodegradable cellulose nanobarriers. Nanoscale Res. Lett. 2012, 7, 1–6. [Google Scholar] [CrossRef]
- Rodionova, G.; Saito, T.; Lenes, M.; Eriksen, Ø.; Gregersen, Ø.; Fukuzumi, H.; Isogai, A. Mechanical and oxygen barrier properties of films prepared from fibrillated dispersions of TEMPO-oxidized Norway spruce and Eucalyptus pulps. Cellulose 2012, 19, 705–711. [Google Scholar] [CrossRef]
- Rodionova, G.; Eriksen, Ø.; Gregersen, Ø. TEMPO-oxidized cellulose nanofiber films: Effect of surface morphology on water resistance. Cellulose 2012, 19, 1115–1123. [Google Scholar] [CrossRef]
- Shimizu, M.; Saito, T.; Isogai, A. Water-resistant and high oxygen-barrier nanocellulose films with interfibrillar cross-linkages formed through multivalent metal ions. J. Membr. Sci. J. Membr. Sci. 2016, 500, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Hashmi, S.G.; Özkan, M.; Halme, J.; Dimic-Misic, K.; Zakeeruddin, S.M.; Paltakari, J.; Grätzel, M.; Lund, P.D. High performance dye-sensitized solar cells with inkjet printed ionic liquid electrolyte. Nano Energy 2015, 17, 206–215. [Google Scholar] [CrossRef]
- Tenhunen, T.-M.; Pöhler, T.; Kokko, A.; Orelma, H.; Gane, P.; Schenker, M.; Tammelin, T. Enhancing the stability of aqueous dispersions and foams comprising cellulose nanofibrils (CNF) with CaCO3 particles. Nanomaterials 2018, 8, 651. [Google Scholar] [CrossRef] [Green Version]
- Osorio, D.A.; Seifried, B.; Moquin, P.; Grandfield, K.; Cranston, E.D. Morphology of cross-linked cellulose nanocrystal aerogels: Cryo-templating versus pressurized gas expansion processing. J. Mater. Sci. 2018, 53, 9842–9860. [Google Scholar] [CrossRef]
- Denes, F.; Young, R.A. Improvement in surface properties of lignocellulosics using cold-plasma treatment. In Science and Technology of Polymers and Advanced Materials; Springer: Boston, MA, USA, 1998; pp. 763–779. [Google Scholar]
- Desmet, T.; Morent, R.; de Geyter, N.; Leys, C.; Schacht, E.; Dubruel, P. Non-thermal plasma technology as a versatile strategy for polymeric biomaterials surface modification: A review. Biomacromolecules 2009, 10, 2351–2378. [Google Scholar] [CrossRef] [Green Version]
- Valtakari, D.; Stepien, M.; Haapanen, J.; Teisala, H.; Tuominen, M.; Kuusipalo, J.; Saarinen, J.J. Planar fluidic channels on TiO2 nanoparticle coated paperboard. Nord. Pulp. Pap. Res. J. 2016, 31, 232–238. [Google Scholar] [CrossRef] [Green Version]
- Mukhopadhyay, S.; Fangueiro, R. Physical modification of natural fibers and thermoplastic films for composites—A review. J. Thermoplast. Compos. Mater. 2009, 22, 135–162. [Google Scholar] [CrossRef]
- Parvinzadeh Gashti, M.; Pournaserani, A.; Ehsani, H. Surface oxidation of cellulose by ozone-gas in a vacuum cylinder to improve the functionality of fluoromonomer. Vacuum 2013, 91, 7–13. [Google Scholar] [CrossRef]
- Mohanty, A.K.; Misra, M.; Drzal, L.T. Surface modifications of natural fibers and performance of the resulting biocomposites: An overview. Compos. Interfaces 2001, 8, 313–343. [Google Scholar] [CrossRef]
- Balu, B.; Breedveld, V.; Hess, D.W. Fabrication of “roll-off” and “sticky” superhydrophobic cellulose surfaces via plasma processing. Langmuir 2008, 2, 4785–4790. [Google Scholar] [CrossRef]
- Chan Tang, T.W.; Bosisio, R.G. Enhanced wettability of cellulose strips treated in a microwave plasma. J. Tech. Assoc. Pulp Pap. Ind. 1980, 63, 111–113. [Google Scholar]
- Relvas, C.; Castro, G.; Rana, S.; Fangueiro, R. Characterization of physical, mechanical and chemical properties of quiscal fibres: The influence of atmospheric DBD plasma treatment. Plasma Chem. Plasma Process. 2015, 35, 863–878. [Google Scholar] [CrossRef]
- Carlsson, C.G.; Ström, G. Reduction and oxidation of cellulose surfaces by means of cold plasma. Langmuir 1991, 7, 2492–2497. [Google Scholar] [CrossRef]
- Willberg-Keyriläinen, P.; Vartiainen, J.; Pelto, J.; Ropponen, J. Hydrophobization and smoothing of cellulose nanofibril films by cellulose ester coatings. Carbohydr. Polym. 2017, 170, 160–165. [Google Scholar] [CrossRef]
- Westerlind, B.; Larsson, A.; Rigdahl, M. Determination of the degree of adhesion in plasma-treated polyethylene/paper laminates. Int. J. Adhes. Adhes. 1987, 7, 141–146. [Google Scholar] [CrossRef]
- Kolářová, K.; Vosmanská, V.; Rimpelová, S.; Švorčík, V. Effect of plasma treatment on cellulose fiber. Cellulose 2013, 20, 953–961. [Google Scholar] [CrossRef]
- Benoit, M.; Rodrigues, A.; Zhang, Q.; Fourré, E.; de Oliveira Vigier, K.; Tatibouët, J.M.; Jérôme, F. Depolymerization of cellulose assisted by a non-thermal atmospheric plasma. Angew. Chem. Int. Ed. 2011, 50, 8964–8967. [Google Scholar] [CrossRef] [PubMed]
- Saarinen, J.J.; Valtakari, D.; Haapanen, J.; Salminen, T.; Mäkelä, J.M.; Uozumi, J. Surface-enhanced Raman scattering active substrates by liquid flame spray deposited and inkjet printed silver nanoparticles. Opt. Rev. 2014, 21, 339–344. [Google Scholar] [CrossRef]
- Jérôme, F.; Chatel, G.; de Oliveira Vigier, K. Depolymerization of cellulose to processable glucans by non-thermal technologies. Green Chem. 2016, 18, 3903–3913. [Google Scholar] [CrossRef]
- Amorim, J.; Oliveira, C.; Souza-Corrêa, J.A.; Ridenti, M.A. Treatment of sugar cane bagasse lignin employing atmospheric pressure microplasma jet in argon. Plasma Process Polym. 2013, 10, 670–678. [Google Scholar] [CrossRef]
- Gupta, D.; Siddhan, P.; and Banerjee, A. Basic dyeable polyester: A new approach using a VUV excimer lamp. Color. Technol. 2007, 123, 248–251. [Google Scholar] [CrossRef]
- Tu, X.; Young, R.A.; Denes, F. Improvement of bonding between cellulose and polypropylene by plasma treatment. Cellulose 1994, 1, 87–106. [Google Scholar] [CrossRef]
- Hoyaux, M.F. Plasma physics and its applications. Contemp. Phys. 1996, 7, 241–260. [Google Scholar] [CrossRef]
- Tabar, I.B.; Zhang, X.; Youngblood, J.P.; Mosier, N.S. Production of cellulose nanofibers using phenolic enhanced surface oxidation. Carbohydr. Polym. 2017, 174, 120–127. [Google Scholar] [CrossRef]
- Shenton, M.J.; Lovell-Hoare, M.C.; Stevens, G.C. Adhesion enhancement of polymer surfaces by atmospheric plasma treatment. J. Phys. D Appl. Phys. 2001, 34, 2754. [Google Scholar] [CrossRef]
- Flynn, C.N.; Byrne, C.P.; Meenan, B.J. Surface modification of cellulose via atmospheric pressure plasma processing in air and ammonia–nitrogen gas. Surf. Coat. Technol. 2013, 233, 108–118. [Google Scholar] [CrossRef]
- Chaturvedi, V.; Verma, P. An overview of key pretreatment processes employed for bioconversion of lignocellulosic biomass into biofuels and value-added products. Biotechnology 2013, 3, 415–431. [Google Scholar] [CrossRef] [Green Version]
- Kostić, M.; Radić, N.; Obradović, B.M.; Dimitrijević, S.; Kuraica, M.M.; Škundrić, P. Silver-loaded cotton/polyester fabric modified by dielectric barrier discharge treatment. Plasma Process Polym. 2009, 6, 58–67. [Google Scholar] [CrossRef]
- Prysiazhnyi, V.; Kramar, A.; Dojcinovic, B.; Zekic, A.; Obradovic, B.M.; Kuraica, M.M.; Kostic, M. Silver incorporation on viscose and cotton fibers after air, nitrogen and oxygen DBD plasma pretreatment. Cellulose 2013, 20, 315–325. [Google Scholar] [CrossRef]
- Mihailović, D.; Šaponjić, Z.; Radoičić, M.; Lazović, S.; Baily, C.J.; Radetić, M. Functionalization of cotton fabrics with corona/air RF plasma and colloidal TiO2 nanoparticles. Cellulose 2011, 18, 811–825. [Google Scholar] [CrossRef]
- Chu, P.K.; Chen, J.Y.; Wang, L.P.; Huang, N. Plasma-surface modification of biomaterials. Mater. Sci. Eng. R Rep. 2002, 36, 143–206. [Google Scholar] [CrossRef] [Green Version]
- Vanneste, J.; Ennaert, T.; Vanhulsel, A.; Sels, B. Unconventional Pretreatment of Lignocellulose with Low-Temperature Plasma. ChemSusChem 2017, 10, 14–31. [Google Scholar] [CrossRef] [PubMed]
- Pertile, R.A.; Andrade, F.K.; Alves, J.C.; Gama, M. Surface modification of bacterial cellulose by nitrogen-containing plasma for improved interaction with cells. Carbohydr. Polym. 2010, 82, 692–698. [Google Scholar] [CrossRef]
- Benerito, R.R.; Ward, T.L.; Soignet, D.M.; Hinojosa, O. Modifications of cotton cellulose surfaces by use of radiofrequency cold plasmas and characterization of surface changes by ESCA. Text. Res. J. 1981, 51, 224–232. [Google Scholar] [CrossRef]
- Vesel, A.; Mozetic, M.; Hladnik, A.; Dolenc, J.; Zule, J.; Milosevic, S.; Krstulovic, N.; Klanjšek-Gunde, M.; Hauptmann, N. Modification of ink-jet paper by oxygen-plasma treatment. J. Phys. D Appl. Phys. 2007, 40, 3689. [Google Scholar] [CrossRef]
- Maqsood, H.S.; Bashir, U.; Wiener, J.; Puchalski, M.; Sztajnowski, S.; Militky, J. Ozone treatment of jute fibers. Cellulose 2017, 24, 1543–1553. [Google Scholar] [CrossRef]
- Lemeune, S.; Barbe, J.M.; Trichet, A.; Guilard, R. Degradation of cellulose models during an ozone treatment. Ozonation of glucose and cellobiose with oxygen or nitrogen as carrier gas at different pH. Ozone Sci Eng. 2000, 22, 447–460. [Google Scholar] [CrossRef]
- Cogo, E.; Albet, J.; Malmary, G.; Coste, C.; Molinier, J. Effect of reaction medium on ozone mass transfer and applications to pulp bleaching. Chem. Eng. Technol. 1999, 73, 23–28. [Google Scholar] [CrossRef]
- Belkind, A.; Freilich, A.; Scholl, R. Using pulsed direct current power for reactive sputtering of Al2O3. J. Vac. Sci. Technol. 1999, 17, 1934–1940. [Google Scholar] [CrossRef]
- Eid, K.F.; Panth, M.; Sommers, A.D. The physics of water droplets on surfaces: Exploring the effects of roughness and surface chemistry. Eur. J. Phys. 2018, 39, 025804. [Google Scholar] [CrossRef]
- Gao, N.; Yan, Y. Modeling superhydrophobic contact angles and wetting transition. J. Bionic Eng. 2009, 6, 335–340. [Google Scholar] [CrossRef]
- Van der Wielen, L.C.; Östenson, M.; Gatenholm, P.; Ragauskas, A.J. Surface modification of cellulosic fibers using dielectric-barrier discharge. Carbohydr. Polym. 2006, 65, 179–184. [Google Scholar] [CrossRef]
- Dimic-Misic, K.; Kostić, M.; Obradović, B.; Kramar, A.; Jovanović, S.; Stepanenko, D.; Mitrovic-Dankulov, M.; Lazovic, S.; Maloney, T.; Gane, P. Nitrogen plasma surface treatment for improving polar ink adhesion on micro/nanofibrillated cellulose films. Cellulose 2019, 26, 3845–3857. [Google Scholar] [CrossRef] [Green Version]
- Sun, S.; Qiu, Y. Influence of moisture on wettability and sizing properties of raw cotton yarns treated with He/O2 atmospheric pressure plasma jet. Surf. Coat. Technol. 2012, 206, 2281–2286. [Google Scholar] [CrossRef]
- Liu, C.; Cui, N.; Brown, N.M.; Meenan, B.J. Effects of DBD plasma operating parameters on the polymer surface modification. Surf. Coat. Technol. 2004, 185, 311–320. [Google Scholar] [CrossRef]
- Schultz, J.; Tsutsumi, K.; Donnet, J.B. Surface properties of high-energy solids: II. Determination of the nondispersive component of the surface free energy of mica and its energy of adhesion to polar liquids. J. Colloid Interface Sci. 1977, 59, 277–282. [Google Scholar] [CrossRef]
- Hansson, P.M.; Skedung, L.; Claesson, P.M.; Swerin, A.; Schoelkopf, J.; Gane, P.A.C.; Rutland, M.W.; Thormann, E. Robust hydrophobic surfaces displaying different surface roughness scales while maintaining the same wettability. Langmuir 2011, 27, 8153–8159. [Google Scholar] [CrossRef]
- Hoeng, F.; Denneulin, A.; Bras, J. Use of nanocellulose in printed electronics: A review. Nanoscale 2016, 8, 13131–13154. [Google Scholar] [CrossRef] [PubMed]
- Bule, M.V.; Gao, A.H.; Hiscox, W.; Chen, S. Structural modification of lignin and characterization of pretreated wheat straw by ozonation. J. Agric. Food Chem. 2013, 61, 3916–3925. [Google Scholar] [CrossRef]
- Panaitescu, D.; Vizireanu, S.; Nicolae, C.; Frone, A.; Casarica, A.; Carpen, L.; Dinescu, G. Treatment of nanocellulose by submerged liquid plasma for surface functionalization. Nanomaterials 2018, 8, 467. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maloney, T.C. Network swelling of TEMPO-oxidized nanocellulose. Holzforschung 2015, 69, 207–213. [Google Scholar] [CrossRef]
- Mohtaschemi, M.; Dimic-Misic, K.; Puisto, A.; Korhonen, M.; Maloney, T.C.; Paltakari, J.; Alava, M.J. Rheological characterization of fibrillated cellulose suspensions via bucket vane viscometer. Cellulose 2014, 21, 1305–1312. [Google Scholar] [CrossRef] [Green Version]
- Rantanen, J.; Dimic-Misic, K.; Kuusisto, J.; Maloney, T.C. The effect of micro and nanofibrillated cellulose water uptake on high filler content composite paper properties and furnish dewatering. Cellulose 2015, 22, 4003–4015. [Google Scholar] [CrossRef]
- Dimic-Misic, K.; Puisto, A.; Gane, P.; Nieminen, K.; Alava, M.; Paltakari, J.; Maloney, T.C. The role of MFC/NFC swelling in the rheological behavior and dewatering of high consistency furnishes. Cellulose 2013, 20, 2847–2861. [Google Scholar] [CrossRef]
- Girifalco, L.A.; Good, R.J. A theory for the estimation of surface and interfacial energies. I. Derivation and application to interfacial tension. J. Phys. Chem. 1957, 61, 904–909. [Google Scholar] [CrossRef]
- Alheshibri, M.H.; Rogers, N.G.; Sommers, A.D.; Eid, K.F. Spontaneous movement of water droplets on patterned Cu and Al surfaces with wedge-shaped gradients. Appl. Phys. Lett. 2013, 102, 174103. [Google Scholar] [CrossRef] [Green Version]
- Johansson, L.S.; Campbell, J.M. Reproducible XPS on biopolymers: Cellulose studies. Surf. Interface Anal. 2004, 36, 1018–1022. [Google Scholar] [CrossRef]
- Möller, M.; Leyland, N.; Copeland, G.; Cassidy, M. Self-powered electrochromic display as an example for integrated modules in printed electronics applications. EPJ Appl. Phys. 2010, 5, 33205. [Google Scholar] [CrossRef] [Green Version]
- Dimic-Misic, K.; Karakoc, A.; Özkan, M.; Ghufran, H.S.; Maloney, T.; Paltakari, J. Flow characteristics of ink-jet inks used for functional printing. J. Appl. Eng. Sci. 2015, 13, 207–212. [Google Scholar] [CrossRef]
- Hubbe, M.A.; Tayeb, P.; Joyce, M.; Tyagi, P.; Kehoe, M.; Dimic-Misic, K.; Pal, L. Rheology of nanocellulose-rich aqueous suspensions: A review. BioResources 2017, 12, 9556–9661. [Google Scholar] [CrossRef]
- Wakida, T.; Takeda, K.; Tanaka, I.; Takagishi, T. Free radicals in cellulose fibers treated with low temperature plasma. Text. Res. J. 1989, 59, 49–53. [Google Scholar] [CrossRef]
- Jun, W.; Fengcai, Z.; Bingqiang, C. The solubility of natural cellulose after DBD plasma treatment. Plasma Sci. Technol. 2008, 10, 743. [Google Scholar] [CrossRef]
- Zhu, H.; Narakathu, B.B.; Fang, Z.; Aijazi, A.T.; Joyce, M.; Atashbar, M.; Hu, L. A gravure printed antenna on shape-stable transparent nanopaper. Nanoscale 2014, 6, 9110–9115. [Google Scholar] [CrossRef] [PubMed]
- Yinhua, Z.; Fuentes-Hernandez, C.; Khan, T.M.; Liu, J.C.; Hsu, J.; Shim, J.W.; Dindar, A.; Youngblood, J.P.; Moon, R.J.; Kippelen, B. Recyclable organic solar cells on cellulose nanocrystal substrates. Sci. Rep. 2013, 3, 1536. [Google Scholar] [CrossRef]




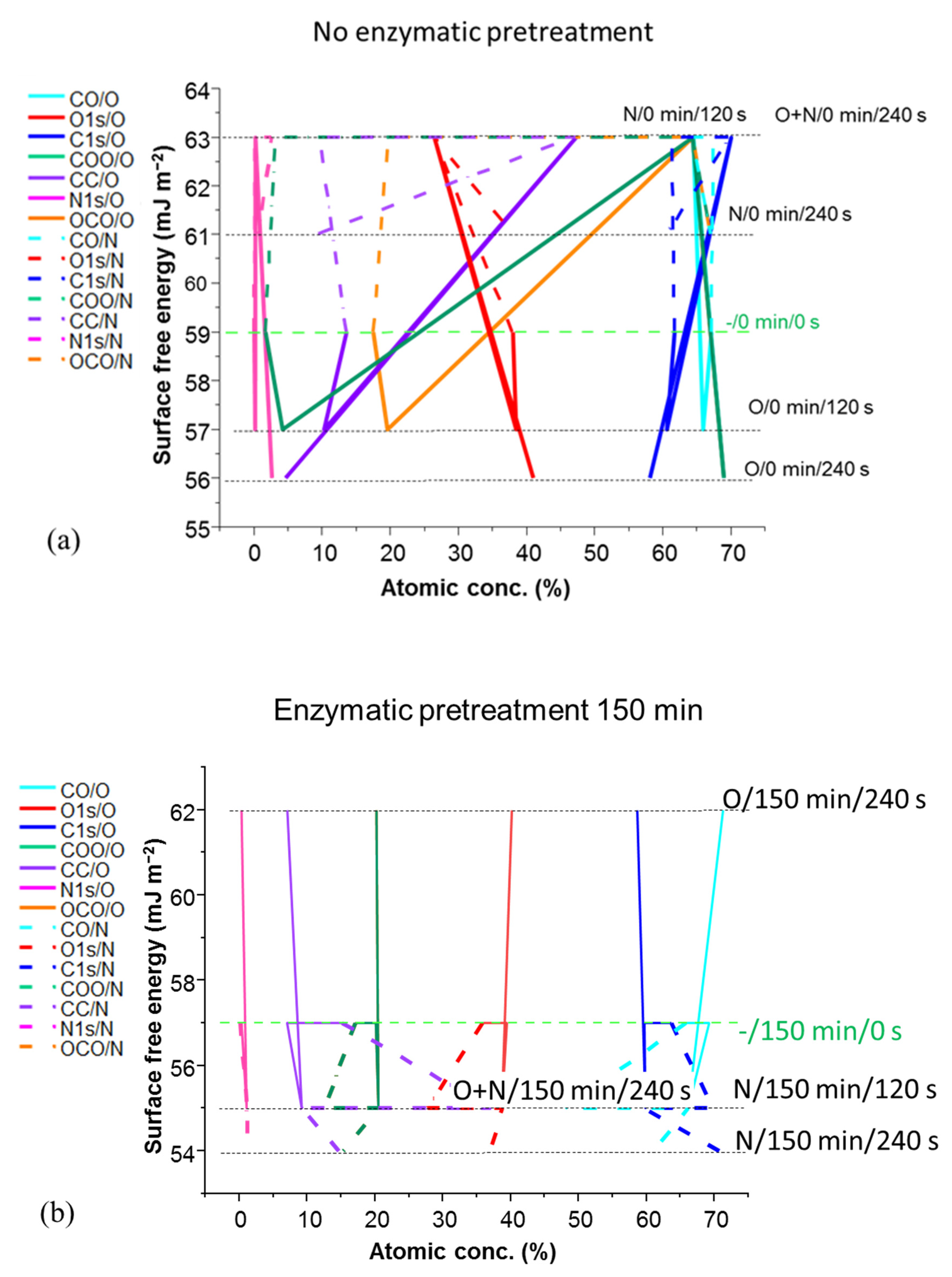
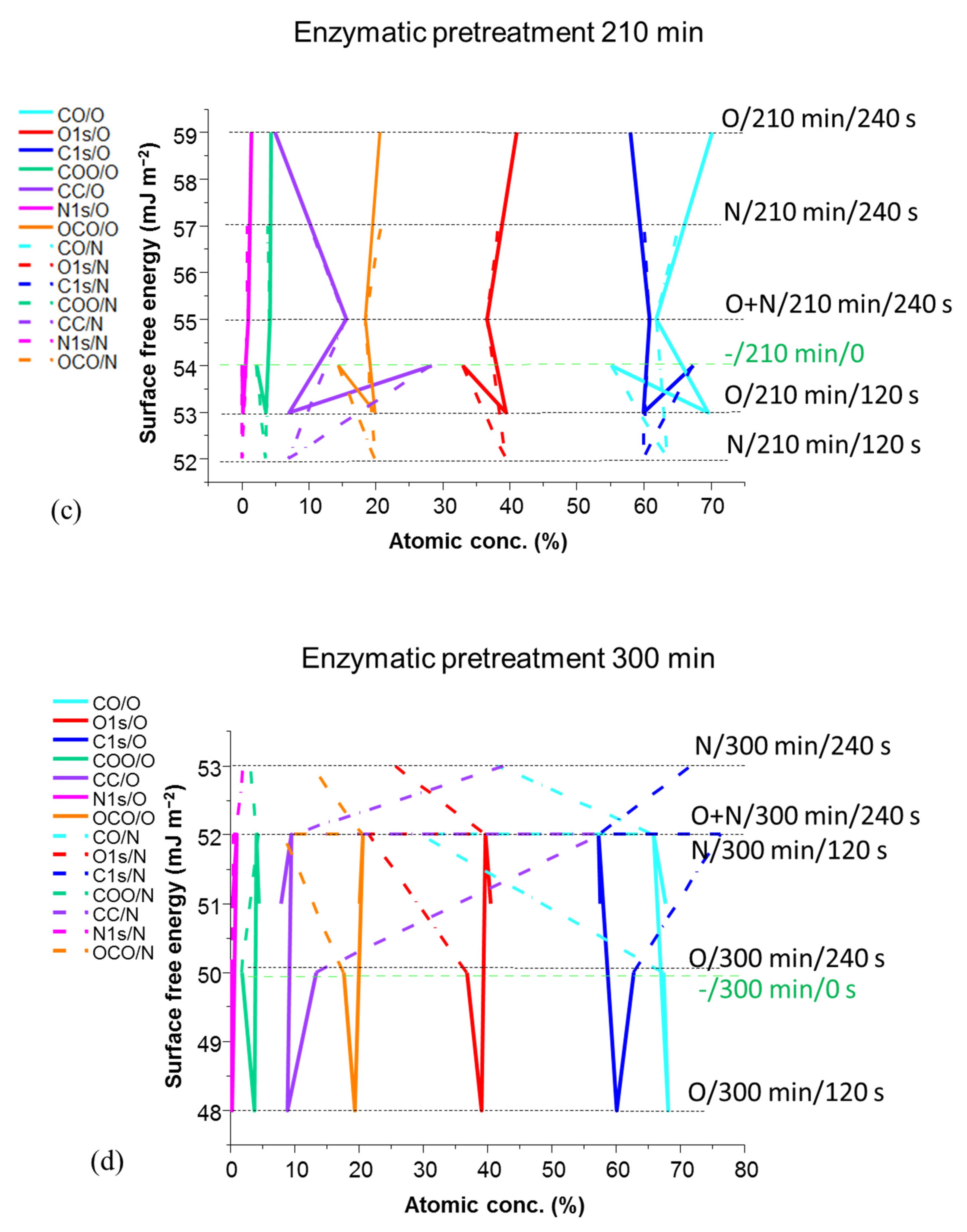
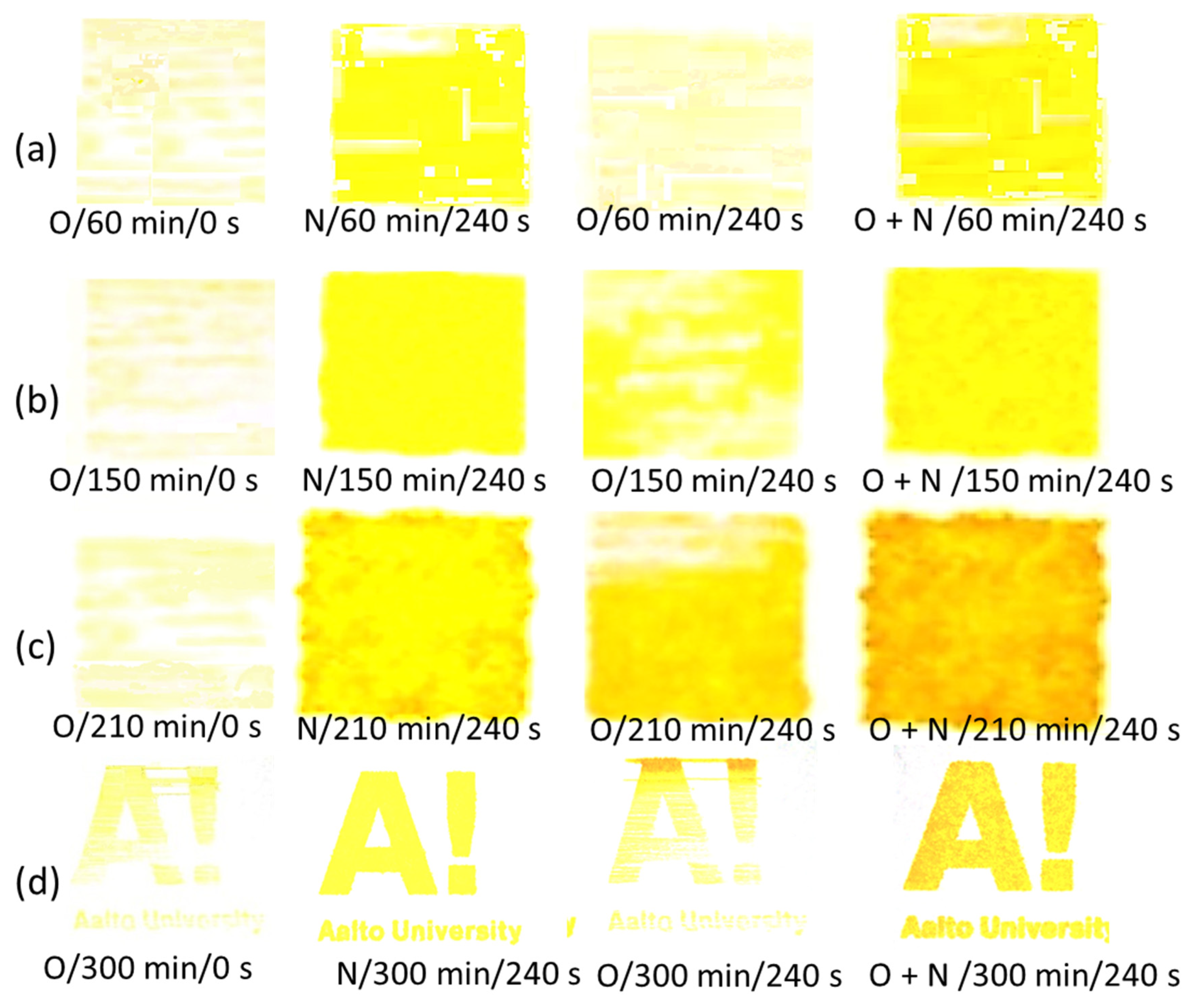

| Experimental Sequencing of Enzymatic Pretreatment and Plasma Exposure: Plasma Treatment Type/MNFC Enzymatic Pretreatment Time/Plasma Exposure Interval | ||||||
|---|---|---|---|---|---|---|
| MNFC Enzymatic Pretreatment Time (min) | Gas Type Introduced to DBD Plasma Chamber and the Sample Exposure Time (s) | |||||
| No Plasma (-) | Nitrogen | Oxygen | Oxygen (120 s) + Nitrogen (120 s) | |||
| (no enzymatic pretreatment) 0 | 0 | 120 | 240 | 120 | 240 | 240 |
| -/0 min/0 s | N/0 min/120 s | N/0 min/240 s | O/0 min/120 s | O/0 min/240 s | O+N/0 min/240 s | |
| 60 | -/60 min/0 s | N/60 min/120 s | N/60 min/240 s | O/60 min/120 s | O/60 min/240 s | O+N/60 min/240 s |
| 150 | -/150 min/0 s | N/150 min/120 s | N/150 min/240 s | O/150 min/120 s | O/150 min/240 s | O+N/150 min/240 s |
| 210 | -/210 min/0 s | N/210 min/120 s | N/210 min/240 s | O/210 min/120 s | O/210 min/240 s | O+N/210 min/240 s |
| 300 | -/300 min/0 s | N/300 min/120 s | N/300 min/240 s | O/300 min/120 s | O/300 min/240 s | O+N/300 min/240 s |
| MNFC Suspension Properties | ||||||||
|---|---|---|---|---|---|---|---|---|
| Enzymatic Pretreatment Time /min | Water Retention Value (WRV) /cm3g−1 | Dynamic Drainage Jar MNFC Content in Filtrate /w/w% | Dynamic Yield Point () /Pa | Static Yield Point () /Pa | Consistency Coefficient (k) /Pa.s | Complex Consistency Coefficient (k*) /Pa sn* | Shear Thinning Coefficient |(1 − n)| | Complex Shear Thinning Coefficient |(1 − n*)| |
| 0 | 1.25 | 95.8 | 334.12 | 478.16 | 431.23 | 482.46 | 0.82 | 0. 86 |
| 60 | 1.83 | 78.6 | 74.23 | 81.23 | 139.65 | 163.51 | 0.81 | 0.87 |
| 150 | 2.85 | 25.4 | 53.34 | 88.34 | 57.23 | 73.47 | 0.84 | 0.88 |
| 210 | 3.33 | 10.7 | 7.64 | 11.64 | 19.67 | 22.67 | 0.86 | 0.93 |
| 300 | 3.34 | 1.3 | 4.74 | 6.74 | 5.45 | 7.26 | 0.91 | 0.95 |
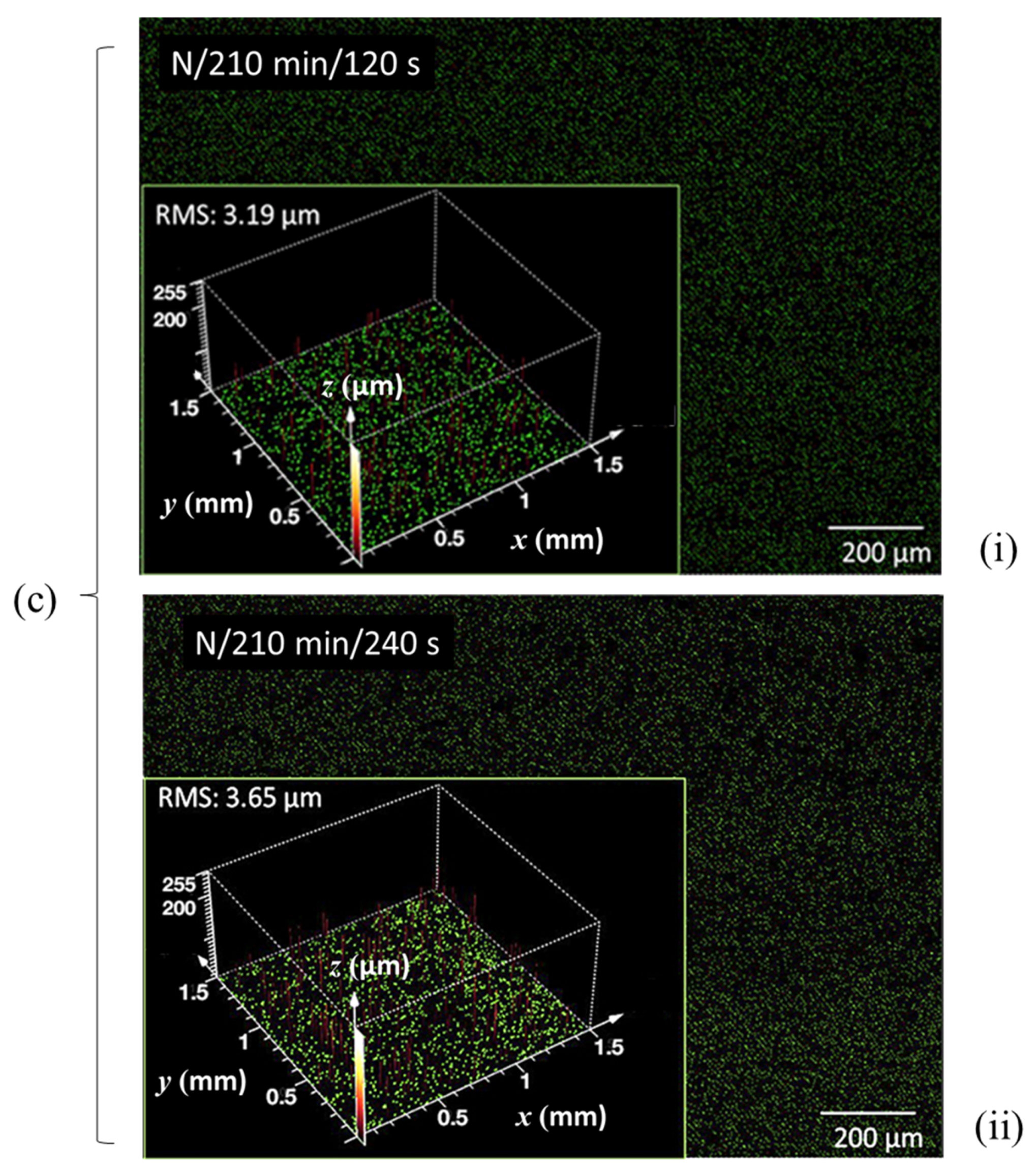
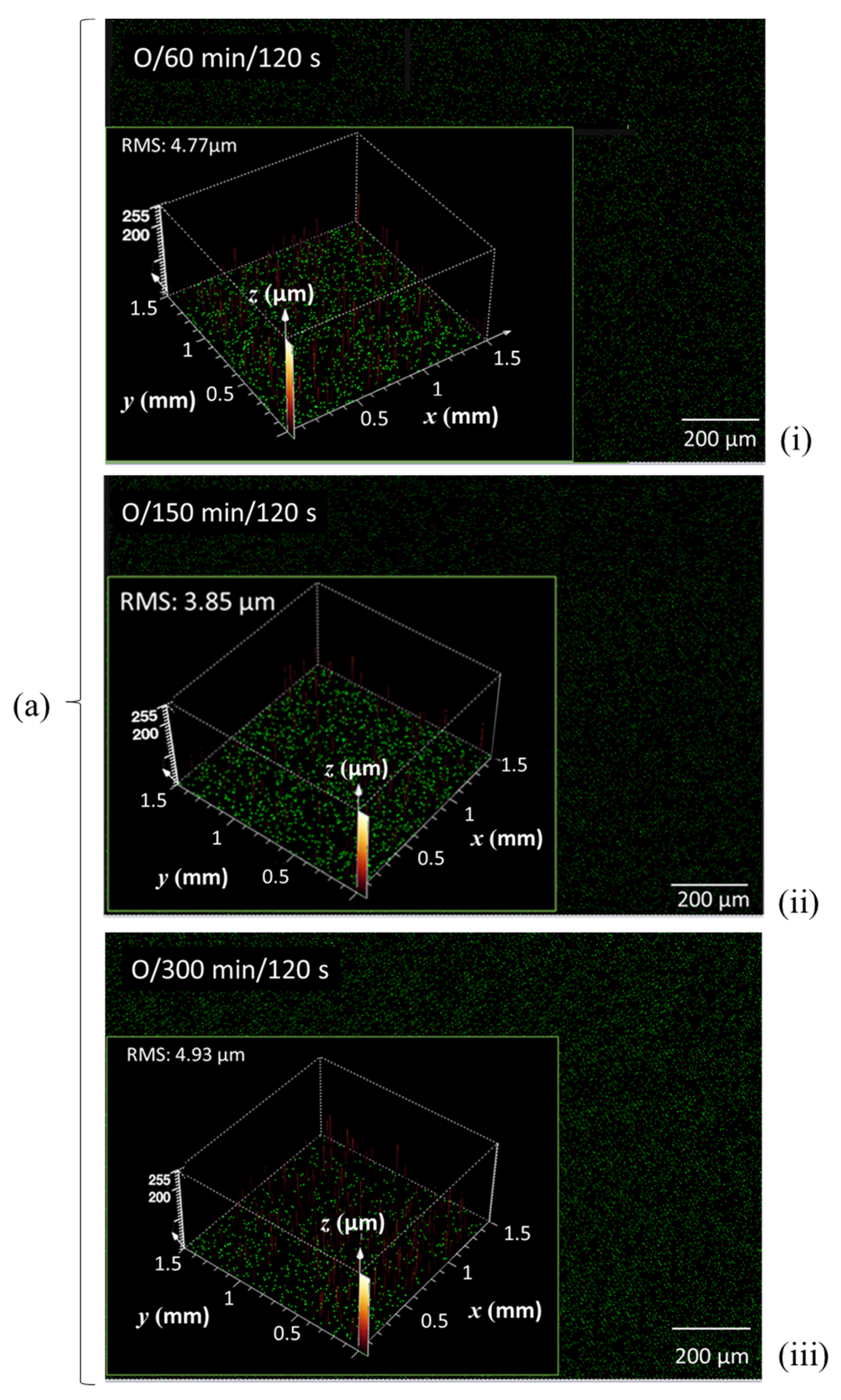
| CLSM Scan | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Enzymatic pretreatment time (min) | 0 | 60 | 210 | 0 | 60 | 150 | 300 | |||||||
| N plasma | O plasma | |||||||||||||
| Plasma exposure time (s) | 120 | 240 | 120 | 240 | 120 | 240 | 120 | 240 | 120 | 240 | 120 | 240 | 120 | 240 |
| RMS microrough-ness (μm) | 4.74 | 5.21 | 4.46 | 4.71 | 3.19 | 3.65 | - | 5.63 | 4.77 | - | 3.85 | 4.11 | 4.93 | - |
| Plasma | Plasma Exposure Time (s) | Duration of Enzymatic Pretreatment (min) | ||||
|---|---|---|---|---|---|---|
| 0 | 60 | 150 | 210 | 300 | ||
| Surface Free Energy (SFE) (mJ m−2) | ||||||
| No exposure | 0 | 59 | 59 | 57 | 54 | 50 |
| N (nitrogen mono-gas) | 120 | 63 | 57 | 55 | 52 | 52 |
| 240 | 61 | 63 | 59 | 57 | 53 | |
| O (oxygen mono-gas) | 120 | 57 | 57 | 57 | 53 | 48 |
| 240 | 56 | 61 | 62 | 59 | 51 | |
| O + N (sequential oxygen followed by nitrogen) |  | 63 | 61 | 55 | 54 | 52 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dimić-Mišić, K.; Kostić, M.; Obradović, B.; Kuraica, M.; Kramar, A.; Imani, M.; Gane, P. Iso- and Anisotropic Etching of Micro Nanofibrillated Cellulose Films by Sequential Oxygen and Nitrogen Gas Plasma Exposure for Tunable Wettability on Crystalline and Amorphous Regions. Materials 2021, 14, 3571. https://doi.org/10.3390/ma14133571
Dimić-Mišić K, Kostić M, Obradović B, Kuraica M, Kramar A, Imani M, Gane P. Iso- and Anisotropic Etching of Micro Nanofibrillated Cellulose Films by Sequential Oxygen and Nitrogen Gas Plasma Exposure for Tunable Wettability on Crystalline and Amorphous Regions. Materials. 2021; 14(13):3571. https://doi.org/10.3390/ma14133571
Chicago/Turabian StyleDimić-Mišić, Katarina, Mirjana Kostić, Bratislav Obradović, Milorad Kuraica, Ana Kramar, Monireh Imani, and Patrick Gane. 2021. "Iso- and Anisotropic Etching of Micro Nanofibrillated Cellulose Films by Sequential Oxygen and Nitrogen Gas Plasma Exposure for Tunable Wettability on Crystalline and Amorphous Regions" Materials 14, no. 13: 3571. https://doi.org/10.3390/ma14133571








