Effect of Aging Treatment on the Corrosion Resistance Properties of 7N01 Extrusion Aluminum Alloy
Abstract
:1. Introduction
2. Experiment
2.1. Materials
2.2. Hardness Measurements
2.3. Electrical Conductivity Measurements
2.4. Potentiodynamic Polarization Tests
3. Results and Discussion
3.1. Hardness and Electrical Conductivity
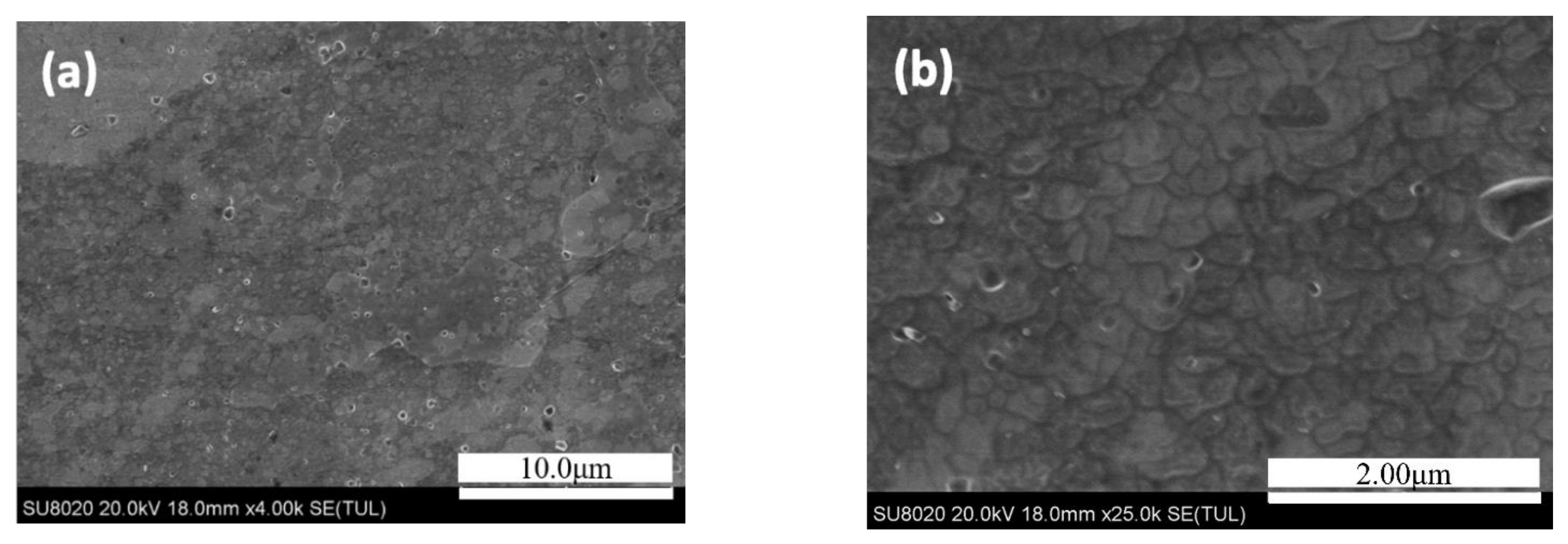
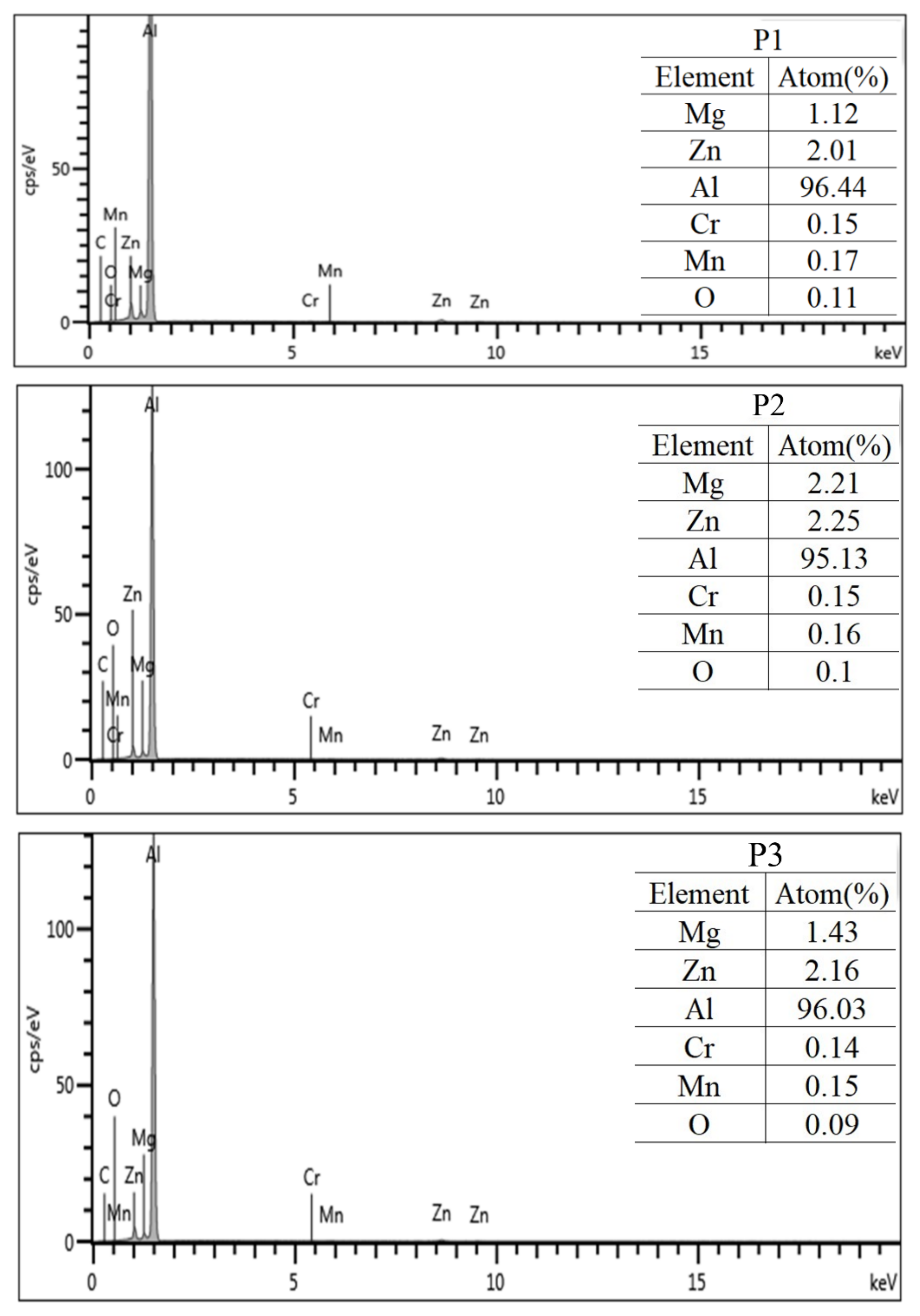
3.2. Microstructure of the 7N01 Extrusion Aluminum Alloy After Non-Isothermal Aging
3.3. Corrosion Resistance
3.3.1. Polarization Curve
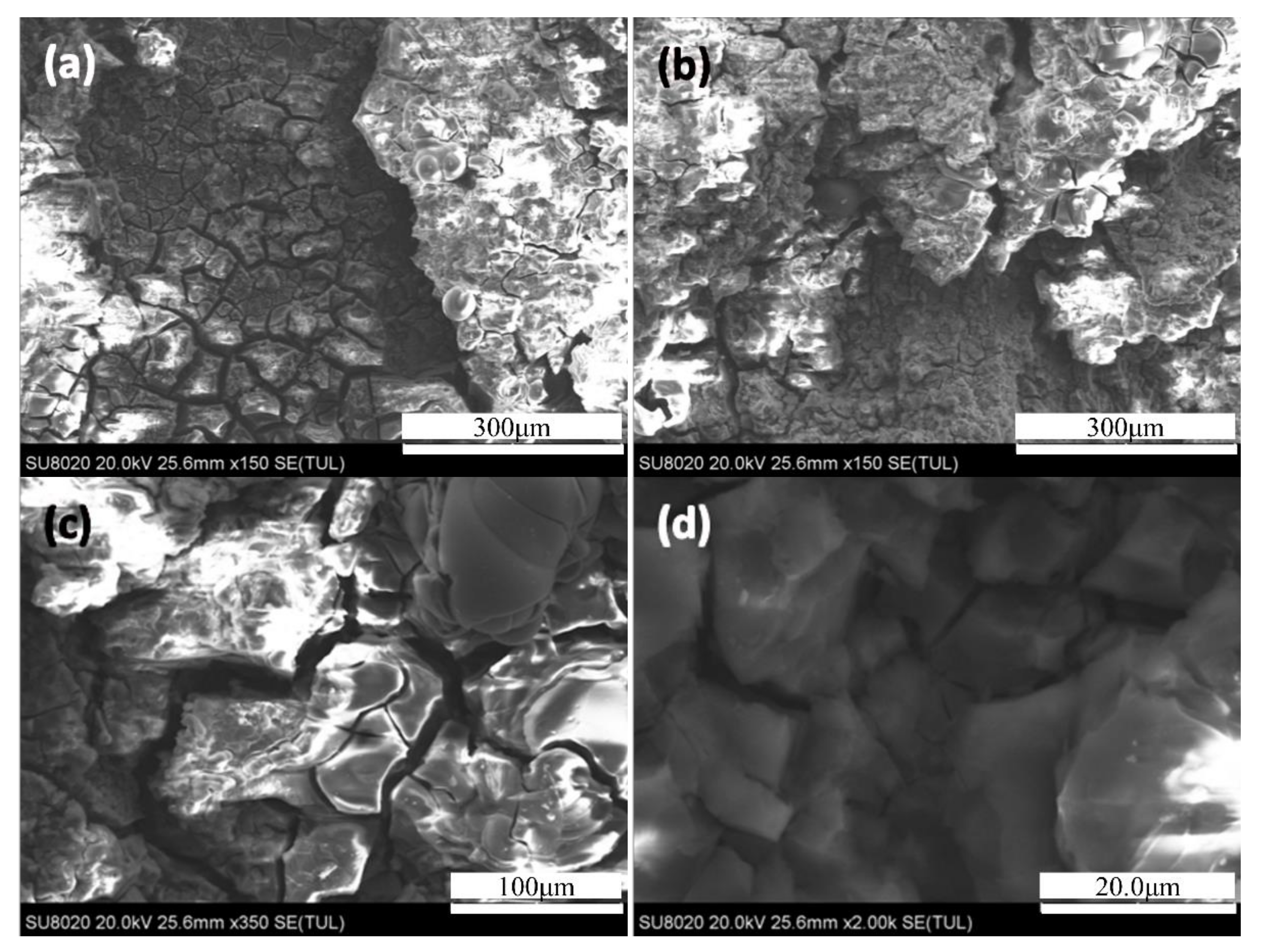
3.3.2. Exfoliation Corrosion Internal Analysis of Aging
4. Conclusions
- (1)
- As the 7N01 extrusion aluminum alloy is subject to non-isothermal aging, the hardness of the alloy can reach 136.9 HV1 and the electrical conductivity can reach 35.8%IACS, which is close to the hardness of single-peak aging and the conductivity of two-stage aging, respectively.
- (2)
- The steady phase η and metastable phase η′ are precipitated in the grain boundary of 7N01 aluminum alloy after non-isothermal aging, and their distribution is discontinuous. The precipitates are mainly composed of fine grains of the metastable phase η′ and a small amount of Mg-rich and Zn-rich atomic layer (GP).
- (3)
- According to the dynamic potential of the anode polarization curves, it can be concluded that the corrosion rate of non-isothermal aging samples is the lowest. Compared with single-peak aging, the corrosion current density of non-isothermal aging is reduced by 15.5%, and that of two-stage aging is reduced by 28.9%. So, the corrosion resistance is better.
- (4)
- In the exfoliation corrosion test, the corrosion degree and the weight loss of the samples treated by the non-isothermal aging process are both lower than those of the two-stage aging and single peak aging. The weight loss of non-isothermal aging compared to the single-peak aging sample was reduced by 93.3%, 59.9%, and 43.1% after aging for six, 12, and 24 h, respectively. Compared with two-stage aging, the weight loss was reduced by 89.1%, 39.2%, and 25.7% after aging for six, 12, and 24 h, respectively. This shows that non-isothermal aging demonstrates the best corrosion performance.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lin, L.; Liu, Z.; Bai, S.; Ying, P.; Wang, X. Effects of germanium on quench sensitivity in Al-Zn-Mg-Zr alloy. Mater. Des. 2015, 86, 679–685. [Google Scholar] [CrossRef]
- Zhu, Q.; Cao, L.; Wu, X.; Zou, Y.; Couper, M.J. Effect of Ag on age-hardening response of Al-Zn-Mg-Cu alloys. Mater. Sci. Eng. A-Struct. Mater. Prop. Microstruct. Process. 2019, 754, 265–268. [Google Scholar] [CrossRef]
- Li, Y.; Chen, L.; Tang, J.; Zhao, G.; Zhang, C. Effects of asymmetric feeder on microstructure and mechanical properties of high strength Al-Zn-Mg alloy by hot extrusion. J. Alloy. Compd. 2018, 749, 293–304. [Google Scholar] [CrossRef]
- Liu, W.; Liu, W.; Bao, A. Preparation of Micro-arc Oxidation Ceramic Coating on Al Alloy Surface Used on Railway Vehicles. Hot Work. Technol. 2012, 41, 140–141. [Google Scholar]
- Zhao, Y.; Yang, Z.; Domblesky, J.P.; Han, J.; Li, Z.; Liu, X. Investigation of through thickness microstructure and mechanical properties in friction stir welded 7N01 aluminum alloy plate. Mater. Sci. Eng. A-Struct. Mater. Prop. Microstruct. Process. 2019, 760, 316–327. [Google Scholar] [CrossRef]
- BibTeX. Failure Analysis of A7N01S-T5 Aluminum Alloy Buffer Beam for High-Speed Train. Adv. Mater. Res. 2013, 652–654, 1290–1294. [Google Scholar]
- Wang, X.M.; Chuan-Ping, M.A.; Gou, G.Q.; Yan, L.; Hui, C.; Heng-Kui, L.I. Failure Analysis of A7N01S-T5 Aluminum Alloy Crossbeam for High Speed Train. Corros. Prot. 2013, 34, 258–261. [Google Scholar]
- Xu, D.; Chen, K.; Hu, G.; Chen, S. Effects of Trace Rare Earth Ce on Microstructure and Corrosion Properties of Al-Zn-Mg Aluminum Alloy. Mater. Rev. 2020, 34, 8100–8105. [Google Scholar]
- Yu, X.; Zhao, Z.; Shi, D.; Dong, X.; Shi, X.; Li, C.; Zhao, J.; Dai, H. Enhancing the Corrosion Resistance and Mechanical Properties of a High-Alloying Al-Zn-Mg-Cu-Zr Alloy by Ce Addition and Aging Treatment. Metals 2020, 10, 1318. [Google Scholar] [CrossRef]
- Enjo, T.; Kuroda, T. Stress Corrosion Cracking in Welds of Al-Zn-Mg Series 7N01-T4 Alloy. Trans. Jwri 2013, 29, 617–622. [Google Scholar] [CrossRef] [Green Version]
- Zhang, K.; Chen, J.Q.; Ma, P.Z.; Zhang, X.H. Effect of welding thermal cycle on microstructural evolution of Al-Zn-Mg-Cu alloy. Mater. Sci. Eng. A-Struct. Mater. Prop. Microstruct. Process. 2018, 717, 85–94. [Google Scholar] [CrossRef]
- Cao, X.; Zhang, Y.; Li, J.; Hui, C. Composition design of 7XXX aluminum alloys optimizing stress corrosion cracking resistance using machine learning. Mater. Res. Express 2020, 7. [Google Scholar] [CrossRef]
- Li, B.; Wang, X.; Chen, H.; Hu, J.; Huang, C.; Gou, G. Influence of heat treatment on the strength and fracture toughness of 7N01 aluminum alloy. J. Alloy. Compd. 2016, 678, 160–166. [Google Scholar] [CrossRef]
- Li, S.; Liu, Z.; Mao, F.; Wang, X.; Hu, X.; Shi, L.; Dong, H.; Shangguan, L. Mechanical and Corrosion Properties of 7N01 Aluminum Alloy After Non-isothermal Heat Treatment under Different Quenching Conditions. Int. J. Electrochem. Sci. 2021, 16. [Google Scholar] [CrossRef]
- Li, S.; Dong, H.; Li, P.; Chen, S. Effect of repetitious non-isothermal heat treatment on corrosion behavior of Al-Zn-Mg alloy. Corros. Sci. 2018, 131, 278–289. [Google Scholar] [CrossRef]
- Liu, W.; Qiu, Q.; Zhou, F.; Chen, Y. Effects of Aging Treatments on the Dynamic Mechanical Properties and Microstructure Evolution of 7N01 Aluminum Alloy at High Temperature. Rare Met. Mater. Eng. 2018, 47, 311–316. [Google Scholar]
- Li, J.; Feng, D.; Xia, W.; Guo, W.; Wang, G. The Non-Isothermal Double Ageing Behaviour of 7055 Aluminum Alloy. Acta Metall. Sin. 2020, 56, 1495–1506. [Google Scholar] [CrossRef]
- Wang, W.; Pan, Q.; Wang, X.; Sun, Y.; Ye, J.; Lin, G.; Liu, S.; Huang, Z.; Xiang, S.; Wang, X.; et al. Non-isothermal aging: A heat treatment method that simultaneously improves the mechanical properties and corrosion resistance of ultra-high strength Al-Zn-Mg-Cu alloy. J. Alloy. Compd. 2020, 845. [Google Scholar] [CrossRef]
- Huang, L.; He, L.; Chen, S.; Chen, K.; Li, J.; Li, S.; Liu, W. Effects of non-isothermal aging on microstructure, mechanical properties and corrosion resistance of 2A14 aluminum alloy. J. Alloy. Compd. 2020, 842. [Google Scholar] [CrossRef]
- Li, J.; Feng, D.; Xia, W.; Lin, G.; Zhang, X.; Ren, M. Effect of Non-Isothermal Ageing on Microstructure and Properties of 7B50 Aluminum Alloy. Acta Metall. Sin. 2020, 56, 1255–1264. [Google Scholar] [CrossRef]
- Hou, Y.; Liu, C.; Dai, H.; Chen, Y.; Xu, Z.; Peng, Y. Effects of Sc addition and heat treatment on microstructure and mechanical properties of 7xxx Al alloy after friction stir processing. Trans. Mater. Heat Treat. 2020, 41, 32–38. [Google Scholar]
- Li, S.; Dong, H.; Shi, L.; Wang, X.; Liu, Z.; Shangguan, L.; Tian, Y. The Effects of Heat Straightening Temperature on the Microstructure and Properties of 7N01 Aluminum Alloy. Materials 2019, 12, 2949. [Google Scholar] [CrossRef] [Green Version]
- Zhang, T.H.; Wang, Y.; Fang, X.F.; Liang, P.; Zhao, Y.; Li, Y.H.; Liu, X.M.; Iop. Effect of water-cooling treatment times on properties of friction stir welded joints of 7N01-T4 aluminum alloy. In International Conference on Mechanical Engineering and Applied Composite Materials; Iop Publishing Ltd.: Bristol, UK, 2018; Volume 307. [Google Scholar]
- Staley, J.T. Method and Process of Non-Isothermal Aging for Aluminum Alloys; WO: Durham, NC, USA, 2007. [Google Scholar]
- Xiao, W. Study on Corrosion Resistance of Nonisothermal Aged 7050 Aluminum Alloy; Harbin Institute of Technology: Harbin, China, 2012. [Google Scholar]
- ASTM G34-01(2007). Standard Test Method for Exfoliation Corrosion Susceptibility in 2XXX and 7XXX Series Aluminum Alloys (EXCO Test); ASTM International: West Conshohocken, PA, USA, 2007. [Google Scholar]
- Lendvai, J.; Honyek, G.; Kovács, I. Dissolution of second phases in an Al-Zn-Mg alloy investigated by calorimetric method. Scr. Metall. 1979, 13, 593–594. [Google Scholar] [CrossRef]





| Element | Si | Fe | Cu | Mn | Mg | Cr | Ti | Zr | Zn | Al |
|---|---|---|---|---|---|---|---|---|---|---|
| 7N01 | 0.055 | 0.153 | 0.021 | 0.33 | 1.40 | 0.22 | 0.088 | 0.13 | 4.45 | Bal. |
| Aging Process | Processing Steps |
|---|---|
| Single-peak aging | Aging at 120 °C for 24 h, then water quenched was followed at room temperature. |
| Two-stage aging | Aging at 105 °C for 8 h first, then increasing aging temperature to 135 °C and keeping for 12 h, water quenched was followed finally at room temperature. |
| Stage(h) | Variable Temperature Range (°C) | Heating Rate(°C/h) |
|---|---|---|
| 0→4.5 | 60→150 | 20 |
| 4.5→8.5 | 150→170 | 5 |
| 8.5→9.25 | 170→185 | 20 |
| 9.25→10 | 185→170 | 20 |
| 10→14 | 170→150 | 5 |
| 14→18.5 | 150→60 | 20 |
| Code | Classification | Descriptions of the Various Classifications |
|---|---|---|
| N | No appreciable attack | Surface may be discolored or etched, but no evidence of pitting or exfoliation. |
| P | Pitting | Discrete pits, sometimes with a tendency for undermining and slight lifting of metal at the pit edges. |
| EA through ED | Exfoliation | Descriptions are showed in Table 5. |
| Code | Descriptions of the Various Classifications |
|---|---|
| EA | (superficial) tiny blisters, thin slivers, flakes, or powder, with only slight separation of metal. |
| EB | (moderate) notable layering and penetration into the metal. |
| EC | (severe) penetration to a considerable depth into the metal. |
| ED | (very severe) similar to EC except for much greater penetration and loss of metal. |
| Aging Process | Hardness (HV1) | Electrical Conductivity (%IACS) |
|---|---|---|
| Single-peak aging | 139.1 ± 1.7 | 31.4 |
| Two-stage aging | 127.8 ± 1.5 | 37.0 |
| Non-isothermal aging | 136.9 ± 2.1 | 35.8 |
| Aging Process | Critical Passivation Potential (V) | Critical Passivation Current Density (A /cm2) | Corrosion Potential (V) | Corrosion Current Density (A/cm2) |
|---|---|---|---|---|
| Single-peak aging | −1.194 | 5.10261 × 10−5 | −907 × 10−3 | 1.75272 × 10−5 |
| Two-stage aging | −1.211 | 6.37800 × 10−5 | −859 × 10−3 | 2.08200 × 10−5 |
| Non-isothermal aging | −1.212 | 3.98307 × 10−5 | −860 × 10−3 | 1.48128 × 10−5 |
| Heat Treatment Classification | Exfoliation Corrosion Rating of Different Time Periods | ||
|---|---|---|---|
| 6 h | 12 h | 24 h | |
| Single-peak aging | EA | EB | EC |
| Two-stage aging | PC | EA | EB |
| Non-isothermal aging | N | PB | PC |
| Heat treatment classification | The Weight Loss of The Different Time Periods(g) | ||
|---|---|---|---|
| 6 h | 12 h | 24 h | |
| Single-peak aging | 0.1052 ± 0.0023 | 0.1728 ± 0.0026 | 0.2455 ± 0.0033 |
| Two-stage aging | 0.0651 ± 0.0011 | 0.1139 ± 0.0014 | 0.1882 ± 0.0028 |
| Non-isothermal aging | 0.0071 ± 0.0016 | 0.0693 ± 0.0012 | 0.1398 ± 0.0020 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Qin, W.; Yu, S.; La, J.; Fu, Y.; Li, J.; Yang, W.; Zhan, Y. Effect of Aging Treatment on the Corrosion Resistance Properties of 7N01 Extrusion Aluminum Alloy. Materials 2021, 14, 3615. https://doi.org/10.3390/ma14133615
Li Y, Qin W, Yu S, La J, Fu Y, Li J, Yang W, Zhan Y. Effect of Aging Treatment on the Corrosion Resistance Properties of 7N01 Extrusion Aluminum Alloy. Materials. 2021; 14(13):3615. https://doi.org/10.3390/ma14133615
Chicago/Turabian StyleLi, Yitai, Weiou Qin, Shuyuan Yu, Jun La, Yaokun Fu, Jidong Li, Wenchao Yang, and Yongzhong Zhan. 2021. "Effect of Aging Treatment on the Corrosion Resistance Properties of 7N01 Extrusion Aluminum Alloy" Materials 14, no. 13: 3615. https://doi.org/10.3390/ma14133615






