Extraction of Al from Coarse Al–Si Alloy by The Selective Liquation Method
Abstract
:1. Introduction
2. Materials and Methods
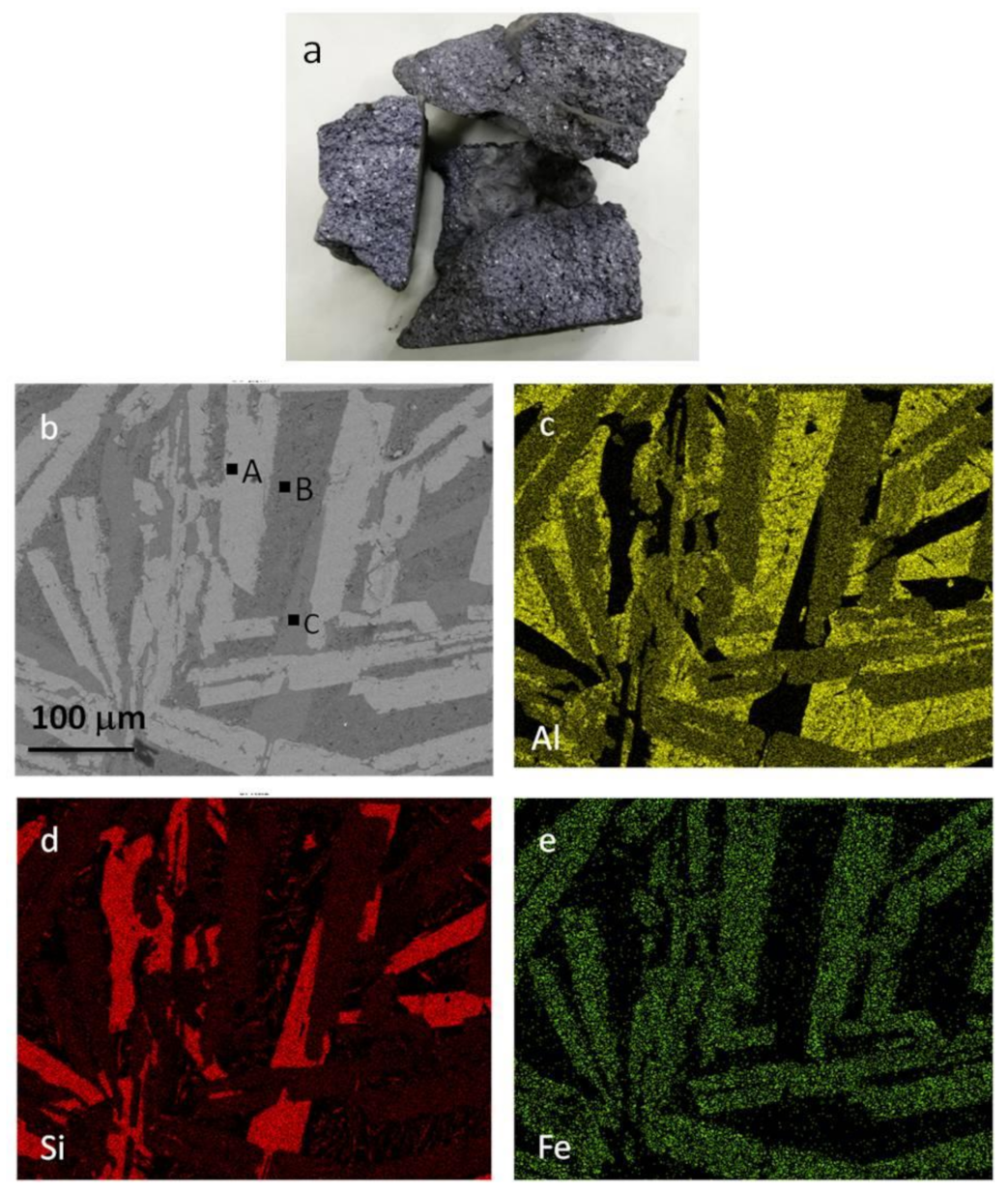
2.1. Materials
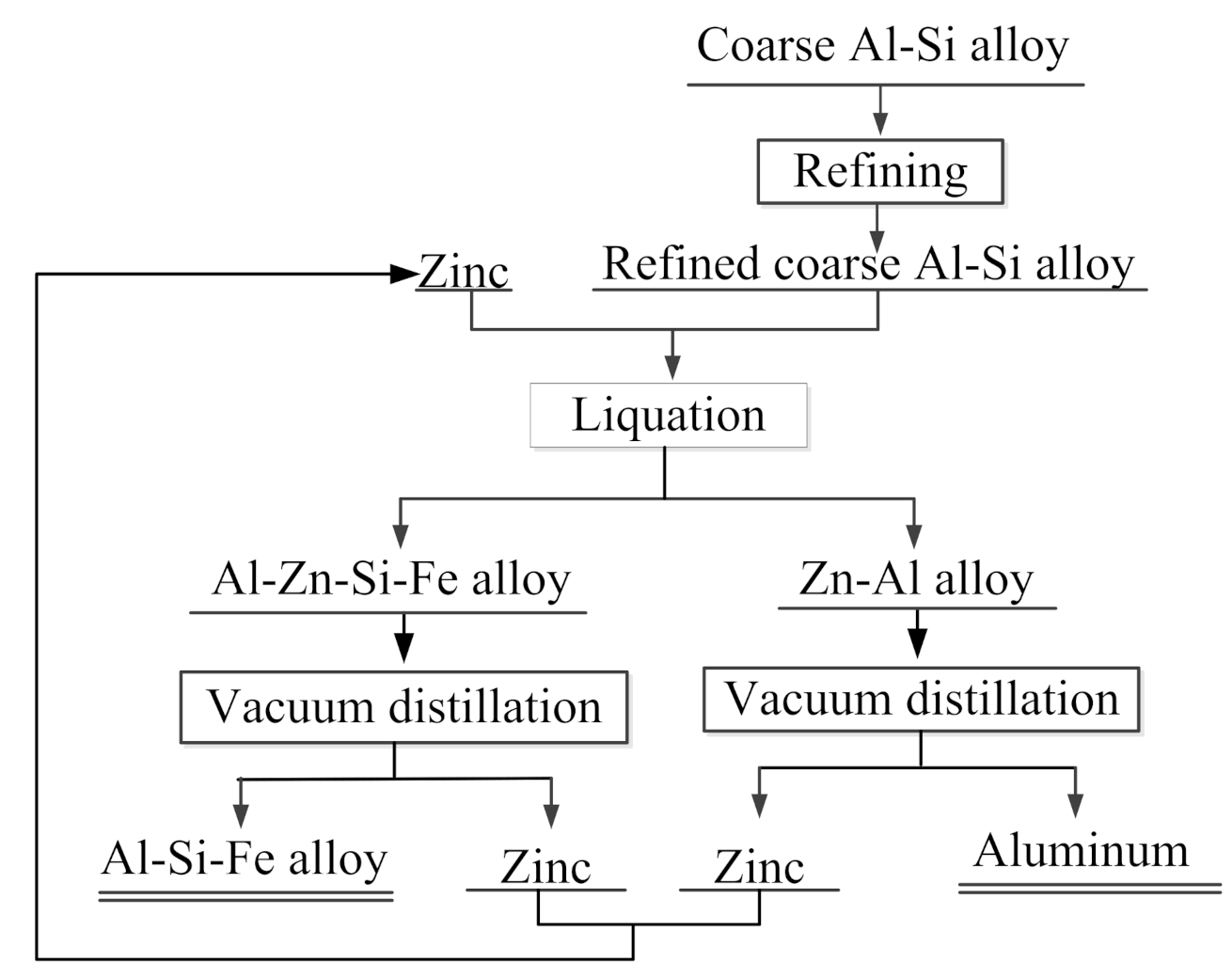
2.2. Experimental Process
3. Results
3.1. Selective Liquation Process
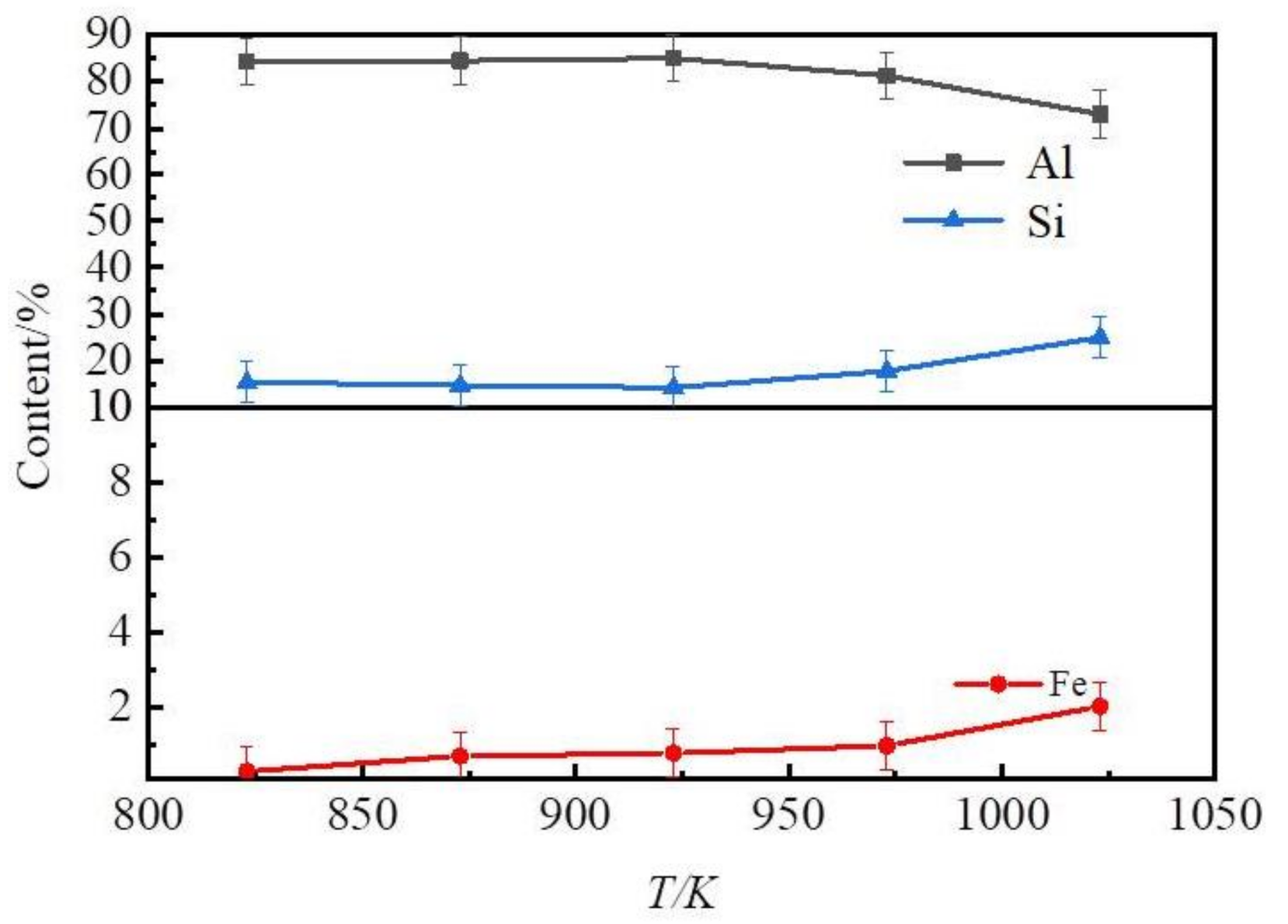
3.1.1. The Influence of Liquation Temperature
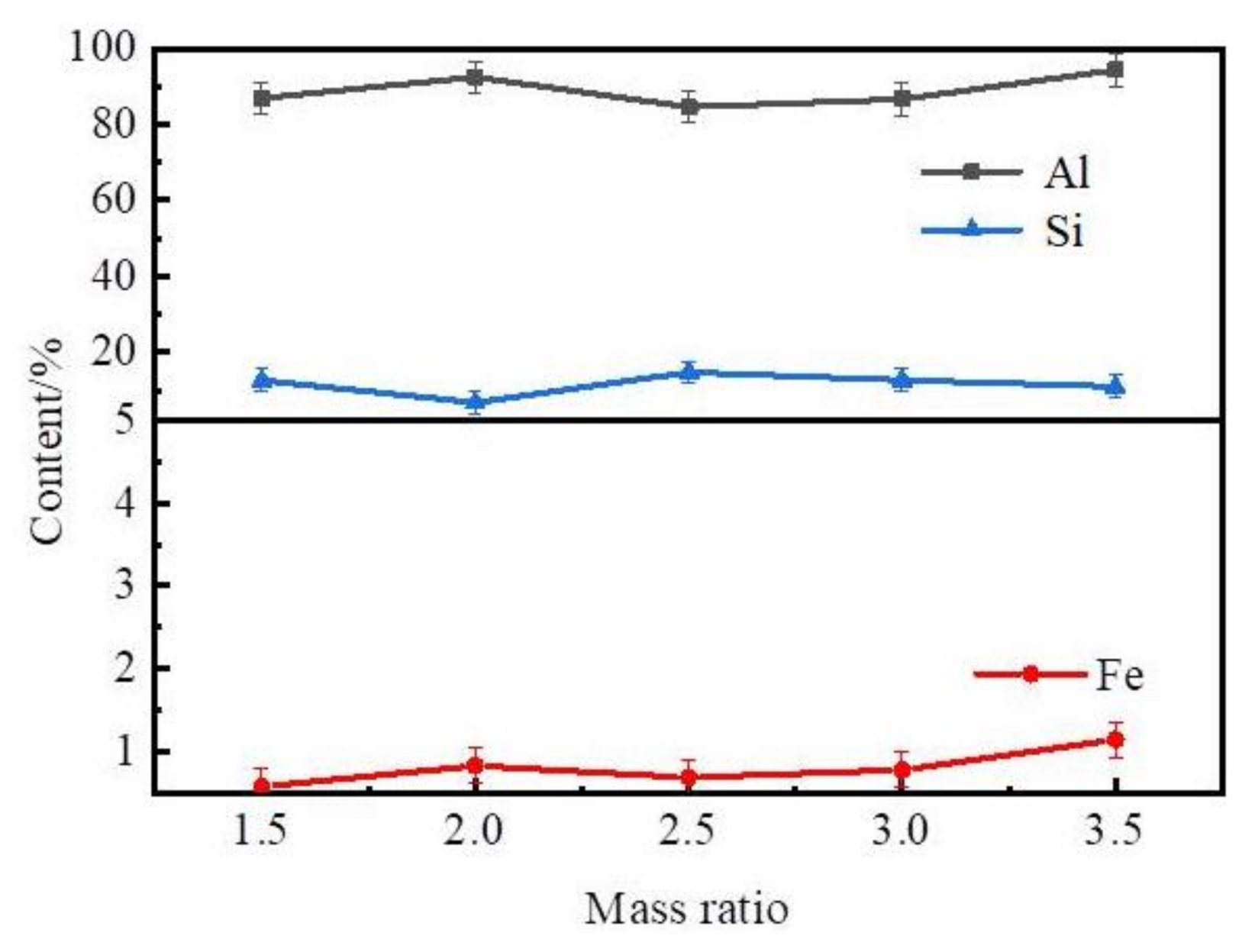
3.1.2. The Influence of the Mass Ratio of Zinc to Coarse Al–Si Alloy
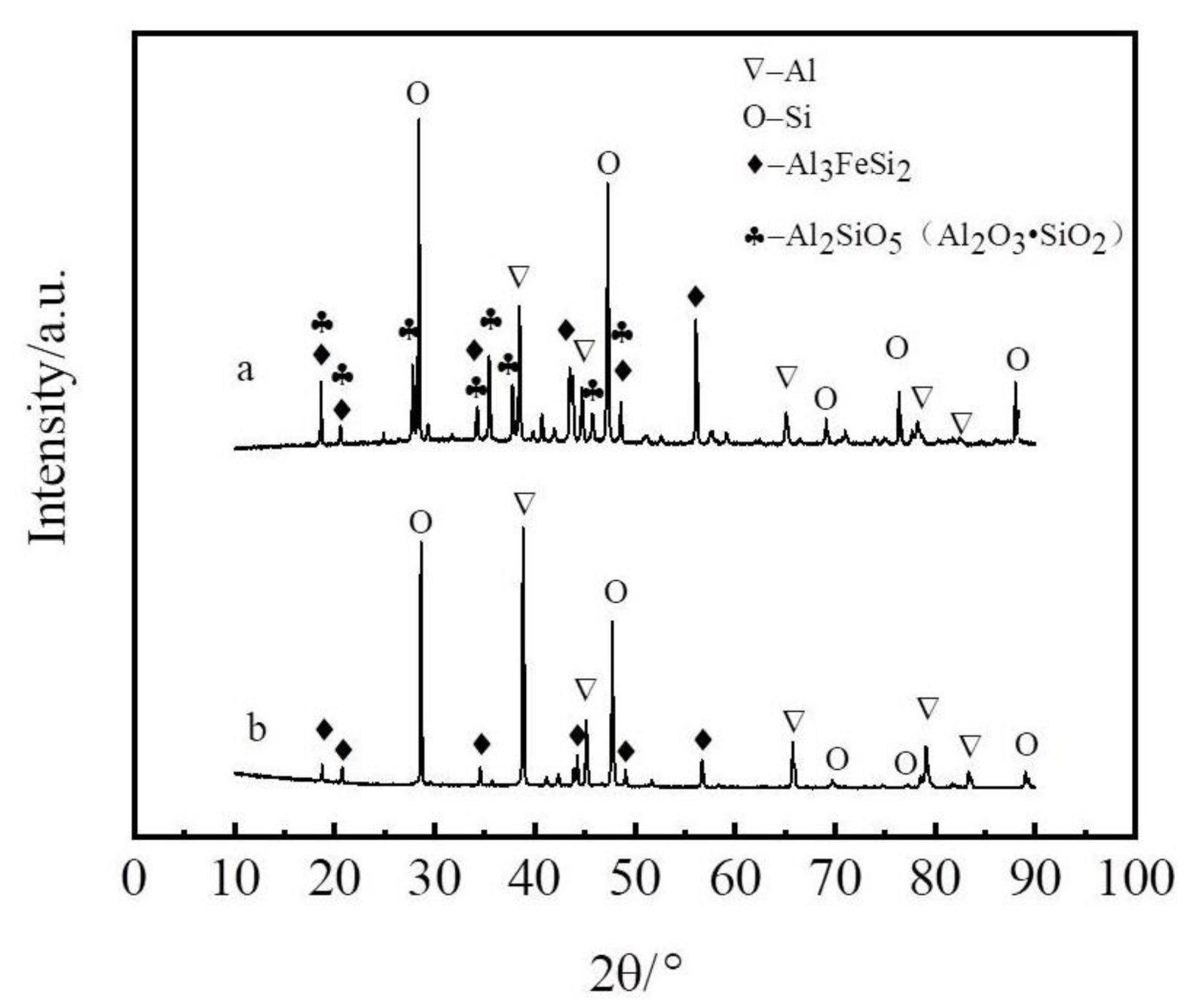
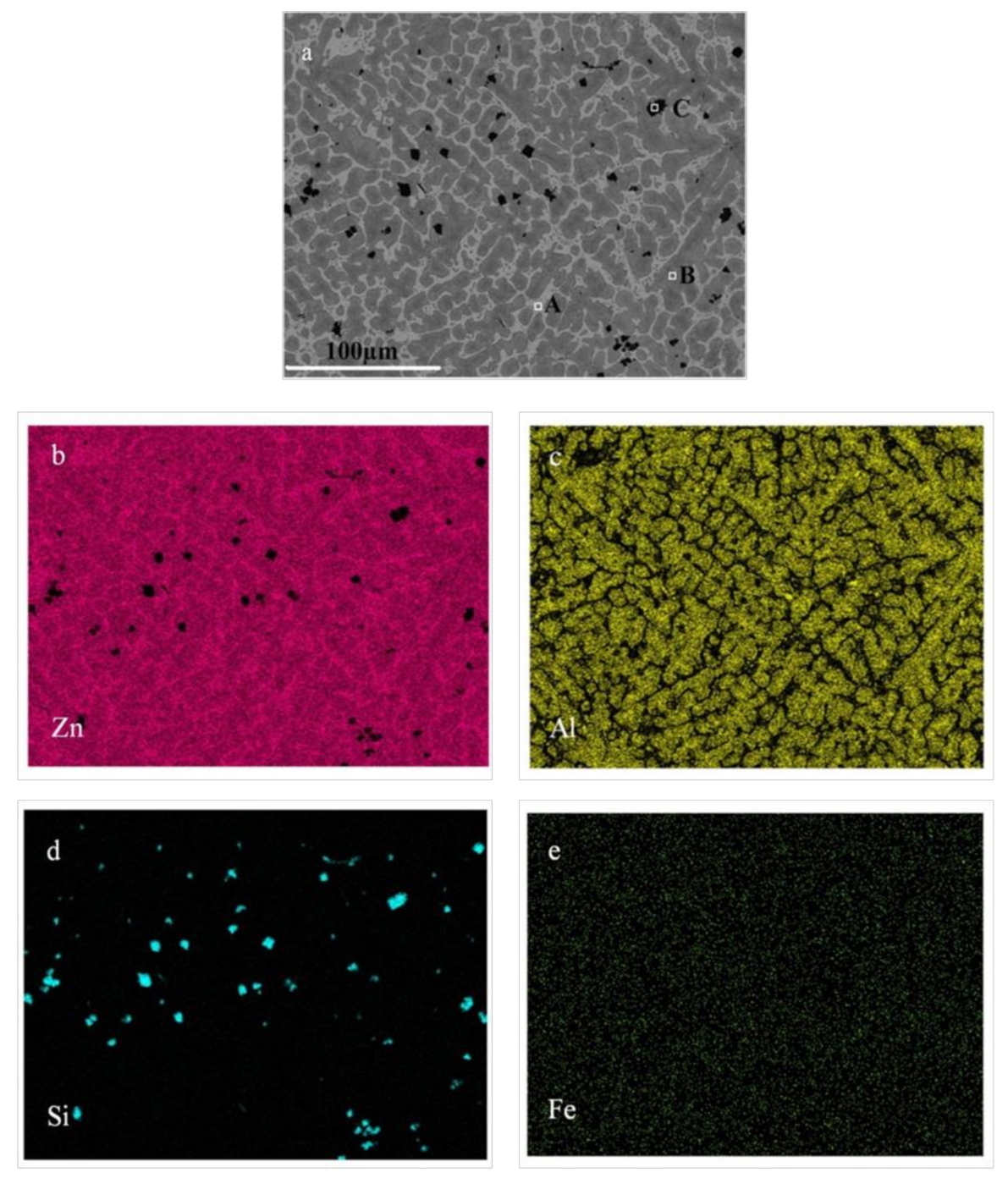
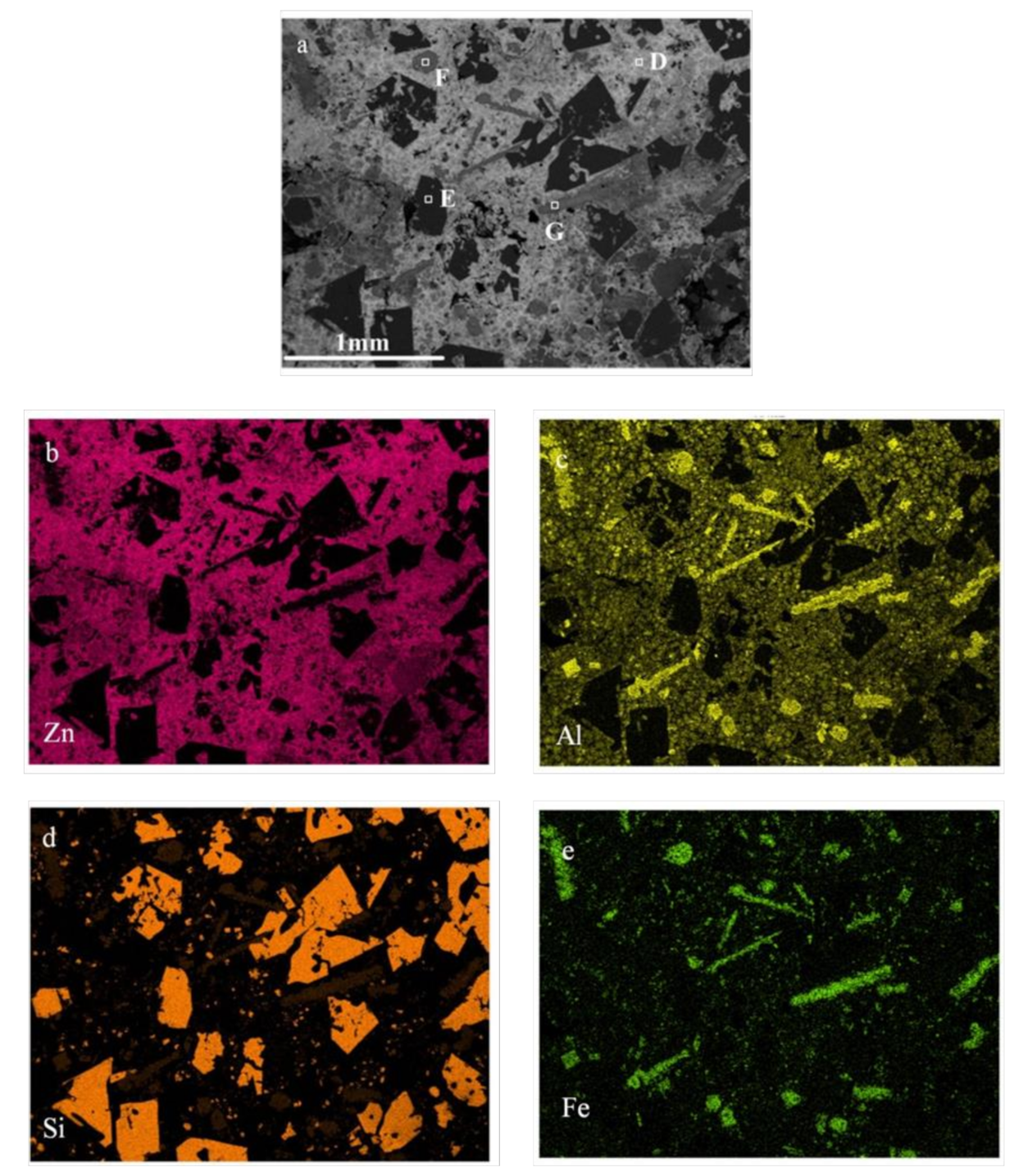
3.2. Principle of Selective Liquation Process with Zinc
3.3. Zinc Separated by a Distillation Process
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Barry, T.S.; Saidou, T.; Birinci, M.; Erdemoglu, M. Thermal and mechanical activation in acid leaching processes of non-bauxite ores available for alumina production A Review. Min. Metall. Explor. 2019, 36, 557–569. [Google Scholar] [CrossRef]
- Mymrin, V.; Molinetti, A.; Alekseev, K.; Avanci, M.A.; Klitzke, W.; Silva, D.A.; Ferraz, F.A.; Larozinski, N.A.; Catai, R.E. Characterization of construction materials on the base of mortar waste, activated by aluminum anodization sludge and lime production waste. Constr. Build. Mater. 2019, 212, 202–212. [Google Scholar] [CrossRef]
- Vigneshwaran, S.; Uthayakumar, M.; Arumugaprabu, V. Development and sustainability of industrial waste-based red mud hybrid composites. J. Clean. Prod. 2019, 230, 862–868. [Google Scholar] [CrossRef]
- Wang, Y.W.; Peng, J.P.; Di, Y.Z. Separation and recycling of spent carbon cathode blocks in the aluminum industry by the vacuum distillation process. JOM 2018, 70, 1877–1883. [Google Scholar]
- Xu, X.H.; Song, J.; Li, Y.; Wu, J.F.; Liu, X.; Zhang, C. The microstructure and properties of ceramic tiles from solid wastes of Bayer red muds. Constr. Build. Mater. 2019, 212, 266–274. [Google Scholar] [CrossRef]
- Zhu, X.B.; Li, W.; Zhao, H.; Zhang, C.X. Selective Dealkalization of Red Mud Using Calcium Oxide with Pressure Leaching. JOM 2019, 70, 2800–2806. [Google Scholar] [CrossRef]
- Sørlie, M.; Øye, H.A. Cathodes in Aluminum Electrolysis, 3rd ed.; Aluminum-Verlag Marketing & Kommunikation GmbH: Dusseldorf, Germany, 2010. [Google Scholar]
- Shi, Z.N.; Li, W. Study on reclaiming spent pot lin-ing of aluminium electrolysis cells by alkaline leaching and acid leaching. Rare Met. 2009, 28, 728–731. [Google Scholar]
- Yang, W.J.; Liu, X.W.; Liu, J.Z.; Wang, Z.H.; Zhou, J.H.; Cen, K.F. Thermodynamics analysis of carbothermal-chlorination reduction in aluminum production. Appl. Therm. Eng. 2017, 111, 876–883. [Google Scholar] [CrossRef]
- Foley, E.; Herwig, G.L. Production of Aluminum and Aluminum Alloys from Aluminum Chloride. U.S. Patent 3,464,900, 2 September 1969. [Google Scholar]
- Zhao, L. Calcination of aluminum chloride hexahydrate (ACH) for alumina production: Implications for alumina extraction from aluminum rich fly ash (ARFA). Arch. Metall. Mater. 2018, 63, 235–240. [Google Scholar]
- Guo, Y.X.; Li, J.; Yan, K.Z.; Cao, L.Q.; Cheng, F.Q. A prospective process for alumina extraction via the co-treatment of coal fly ash and bauxite red mud: Investigation of the process. Hydrometallugy 2019, 186, 98–104. [Google Scholar] [CrossRef]
- Chen, X.M.; Yang, B.; Tao, D.P.; Dai, Y.N. Formation Mechanism of [AlCl] n during Production of Aluminum by AlCl Disproportionation. Acta Phys. Chim. Sin. 2019, 26, 415–421. [Google Scholar]
- Lu, H.M.; Han, H.Q. Producing aluminum-silicon alloys from high-aluminum bearing coal fly ash by carbothermal reduction method. In Proceedings of the 2007 TMS Annual Meeting: Technical Sessions on Light Metal, Orlando, FL, USA, 25 February–1 March 2007. [Google Scholar]
- You, J.; Wang, Y.W.; Feng, N.X. Preparation of casting alloy ZL101 with coarse aluminum-silicon alloy. Trans. Nonferr. Met. Soc. China 2018, 18, 116–120. [Google Scholar] [CrossRef]
- Feng, Y.B.; Yang, B.; Dai, Y.N. Thermodynamics on formation of C, Al4C3 and Al2O3 in AlCl disproportionation process in vacuum to produce aluminum. Trans. Nonferr. Met. Soc. China 2014, 24, 3366–3371. [Google Scholar] [CrossRef]
- Chen, X.M.; Yang, B.; Tao, D.P.; Dai, Y.N. Theory Study of AlCl Disproportionation Reaction Mechanism on Al (110) Surface. Metall. Mater. Trans. B Process. Metall. Mater. Process. Sci. 2010, 41, 137–145. [Google Scholar] [CrossRef]
- Tang, H.Q.; Zeng, J.M.; Li, H.L.; Si, J.Y. Phase Diagram of Zn-Al binary system. Spec. Cast. Nonferr. Alloy. 2006, 6, 387–389. [Google Scholar]
- Li, G.L.; Jiang, Z.X. Explanation of phase structure of Zn-Al binary alloy. Steel Wire Prod. 2002, 28, 54–55. [Google Scholar]
- Liu, Y.Y.; Yang, B. Study on Separation of Al—Zn Alloy by vacuum distillation. Jiangsu Metall. 2008, 36, 32–34. [Google Scholar]
- Utigard, T.A. The properties and uses of fluxes in molten aluminum processing. JOM 1998, 50, 38–43. [Google Scholar] [CrossRef]
- Tang, H.Q.; Zeng, J.M.; Li, H.L.; Si, J.Y. Study on binary phase diagram of Zn-Al system. Spec. Cast. Nonferr. Alloy 2006, 26, 387–388. [Google Scholar]



| Element | Al | Si | Fe | Ca | Ti | Mg | Zn | Cu | Other Impurities |
|---|---|---|---|---|---|---|---|---|---|
| Coarse alloy | 58.50 | 25.48 | 4.36 | 2.06 | 0.72 | 0.01 | 0.01 | 0.01 | <12.35 |
| Refining coarse alloy | 60.50 | 31.35 | 5.31 | 1.87 | 0.94 | 0.01 | 0.01 | 0.01 | - |
| Element | Al | Si | Fe | Zn | Ti | Other Impurities |
|---|---|---|---|---|---|---|
| Content | 84.13 | 14.59 | 0.58 | 0.10 | <0.1 | <0.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, B.; Wang, Y.; Gao, B. Extraction of Al from Coarse Al–Si Alloy by The Selective Liquation Method. Materials 2021, 14, 3680. https://doi.org/10.3390/ma14133680
Li B, Wang Y, Gao B. Extraction of Al from Coarse Al–Si Alloy by The Selective Liquation Method. Materials. 2021; 14(13):3680. https://doi.org/10.3390/ma14133680
Chicago/Turabian StyleLi, Bo, Yaowu Wang, and Bingliang Gao. 2021. "Extraction of Al from Coarse Al–Si Alloy by The Selective Liquation Method" Materials 14, no. 13: 3680. https://doi.org/10.3390/ma14133680
APA StyleLi, B., Wang, Y., & Gao, B. (2021). Extraction of Al from Coarse Al–Si Alloy by The Selective Liquation Method. Materials, 14(13), 3680. https://doi.org/10.3390/ma14133680





