Identification of Volatile and Semi-Volatile Compounds in Polymeric Coatings Used in Metal Cans by GC-MS and SPME
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Description and FTIR Characterization
2.2. Sample Treatment
2.2.1. Solvent Extraction Procedure
2.2.2. SPME Procedure
2.3. Reagents and Analytical Standards
2.4. GC-MS Conditions for Solvent Extraction Samples
2.5. GC-MS Conditions for SPME Analysis
3. Results and Discussion
3.1. Solvent Selection for Can Extraction
3.2. Optimization of SPME Method
Selection of the Type of Fiber
3.3. Can Coatings Analysis via GC after a Solvent Extraction
3.4. Can Coatings Analysis via SPME
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- EFSA CEF Panel (EFSA Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids). Note for guidance for the preparation of an application for the safety assessment of a substance to be used in plastic food contact materials. EFSA J. 2008, 6. [Google Scholar] [CrossRef] [Green Version]
- Marsh, K.; Bugusu, B. Food packaging—Roles, materials, and environmental issues. J. Food Sci. 2007, 72, R39–R55. [Google Scholar] [CrossRef]
- Zhang, N.; Scarsella, J.B.; Hartman, T.G. Identification and quantitation studies of migrants from BPA alternative food-contact metal can coatings. Polymers 2020, 12, 2846. [Google Scholar] [CrossRef]
- Kremser, K.; Gerl, P.; Pellis, A.; Guebitz, G.M. A new bioleaching strategy for the selective recovery of aluminum from multi-layer beverage cans. Waste Manag. 2021, 120, 16–24. [Google Scholar] [CrossRef]
- Edlund, U.; Albertsson, A.-C. Polyesters based on diacid monomers. Adv. Drug Deliv. Rev. 2003, 55, 585–609. [Google Scholar] [CrossRef]
- Pietropaolo, E.; Albenga, R.; Gosetti, F.; Toson, V.; Koster, S.; Marin-Kuan, M.; Veyrand, J.; Patin, A.; Schilter, B.; Pistone, A.; et al. Synthesis, identification and quantification of oligomers from polyester coatings for metal packaging. J. Chromatogr. A 2018, 1578, 15–27. [Google Scholar] [CrossRef]
- Oprea, S.; Vlad, S.; Stanciu, A.; Macoveanu, M. Epoxy urethane acrylate. Eur. Polym. J. 2000, 36, 373–378. [Google Scholar] [CrossRef]
- Munguía-López, E.M.; Gerardo-Lugo, S.; Peralta, E.; Bolumen, S.; Soto-Valdez, H. Migration of bisphenol A (BPA) from can coatings into a fatty-food simulant and tuna fish. Food Addit. Contam. 2005, 22, 892–898. [Google Scholar] [CrossRef]
- Paseiro Losada, P.; Paz Abuín, S.; Vázquez Odériz, L.; Simal Lozano, J.; Simal Gándara, J. Determination of residual free monomers (m-xylylenediamine and bisphenol A diglycidyl ether) in the finished product. J. Chromatogr. A 1991, 585, 75–81. [Google Scholar]
- Losada, P.P.; Mahia, P.L.; Odériz, L.V.; Lozano, J.S.; Gándara, J.S. Sensitive and rapid reversed-phase liquid chromatography–fluorescence method for determining bisphenol A diglycidyl ether in aqueous–based food simulants. J. Assoc. Off. Anal. Chem. 1991, 74, 925–928. [Google Scholar] [CrossRef]
- Schaefer, A.; Simat, T.J. Migration from can coatings: Part 3. Synthesis, identification and quantification of migrating epoxy-based substances below 1000 Da. Food Addit. Contam. 2004, 21, 390–405. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Commission Regulation (EU) No 10/2011 on plastic materials and articles intended to come into contact with food. Off. J. Eur. Union 2011, 12, 1–89. [Google Scholar]
- Geueke, B. FPF Dossier: Can Coatings; Food Packaging Forum: Zurich, Switzerland, 2016. [Google Scholar]
- Paseiro-Cerrato, R.; Devries, J.; Begley, T.H. Evaluation of short-term and long-term migration testing from can coatings into food simulants: Epoxy and acrylic–phenolic coatings. J. Agric. Food Chem. 2017, 65, 2594–2602. [Google Scholar] [CrossRef]
- Bradley, E.L.; Driffield, M.; Harmer, N.; Oldring, P.K.T.; Castle, L. Identification of potential migrants in epoxy phenolic can coatings. Int. J. Polym. Anal. Charact. 2008, 13, 200–223. [Google Scholar] [CrossRef]
- Omer, E.; Bichon, E.; Hutinet, S.; Royer, A.-L.; Monteau, F.; Germon, H.; Hill, P.; Remaud, G.; Dervilly-Pinel, G.; Cariou, R.; et al. Toward the characterisation of non-intentionally added substances migrating from polyester-polyurethane lacquers by comprehensive gas chromatography-mass spectrometry technologies. J. Chromatogr. A 2019, 1601, 327–334. [Google Scholar] [CrossRef]
- Lestido-Cardama, A.; Loureiro, P.V.; Sendón, R.; Losada, P.P.; de Quirós, A.R.B. Application of chromatographic analysis for detecting components from polymeric can coatings and further determination in beverage samples. J. Chromatogr. A 2021, 1638, 461886. [Google Scholar] [CrossRef]
- Toxtree v2.6.13. Available online: http://toxtree.sourceforge.net/download.html (accessed on 10 May 2021).
- Guidance document. Guidance on the use of the threshold of toxicological concern approach in food safety assessment. EFSA J. 2019, 17, e05708. [Google Scholar]
- Regulation (EC) of the European Parliament and of the Council of 16 December 2008 No 1334/2008 on flavourings and certain food ingredients with flavouring properties for use in and on foods and amending Council Regulation (EEC) No 1601/91, Regulations (EC) No 2232/96 and (EC) No 110/2008 and Di-rective 2000/13/EC. Off. J. Eur. Union 2008, 354, 34–207.
- Canellas, E.; Vera, P.; Nerín, C. Ion mobility quadrupole time-of-flight mass spectrometry for the identification of non-intentionally added substances in UV varnishes applied on food contact materials. A safety by design study. Talanta 2019, 205, 120103. [Google Scholar] [CrossRef] [PubMed]
- Beardslee, T.; Picataggio, S. Bio-based adipic acid from renewable oils. Lipid Technol. 2012, 24, 223–225. [Google Scholar] [CrossRef]
- Mirhosseini, H.; Salmah, Y.; Hamid, N.; Tan, C.P. Solid-phase microextraction for headspace analysis of key volatile compounds in orange beverage emulsion. Food Chem. 2007, 105, 1659–1670. [Google Scholar] [CrossRef]
- Machiels, D. Evaluation of two commercial solid-phase microextraction fibres for the analysis of target aroma compounds in cooked beef meat. Talanta 2003, 61, 529–537. [Google Scholar] [CrossRef]
- Park, S.-Y.; Yoon, Y.-M.; Schilling, M.W.; Chin, K.-B. Evaluation of volatile compounds isolated from pork loin (Longissimus dorsi) as affected by fiber type of Solid-Phase Microextraction (SPME), preheating and storage time. Food Sci. Anim. Resour. 2009, 29, 579–589. [Google Scholar] [CrossRef] [Green Version]
- Dragone, G.; Mussatto, S.I.; Oliveira, J.M.; Teixeira, J.A. Characterisation of volatile compounds in an alcoholic beverage produced by whey fermentation. Food Chem. 2009, 112, 929–935. [Google Scholar] [CrossRef] [Green Version]
- Mastelic, J.; Jerkovic, I. Gas chromatography–Mass spectrometry analysis of free and glycoconjugated aroma compounds of seasonally collected Satureja montana L. Food Chem. 2003, 80, 135–140. [Google Scholar] [CrossRef]
- Iturbide-Zuñiga, A.S.; Colinas-León, M.T.B.; Lozoya-Saldaña, H.; Medina-Moreno, S.A.; Ayala-Arreola, J. Evaluación In Vitro de extractos del genero Lilium para el control de Fusarium oxysporum. Rev. Mex. Fitopatol. Mex. J. Phytopathol. 2017, 35, 611–622. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.-L.; Sung, H.-H. The toxic effect of phthalate esters on immune responses of giant freshwater prawn (Macrobrachium rosenbergii) via oral treatment. Aquat. Toxicol. 2005, 74, 160–171. [Google Scholar] [CrossRef]
- Parkerton, T.; Konkel, W.J. Application of quantitative structure-activity relationships for assessing the aquatic toxicity of phthalate esters. Ecotoxicol. Environ. Saf. 2000, 45, 61–78. [Google Scholar] [CrossRef]
- Lorz, P.M.; Towae, F.K.; Enke, W.; Jäckh, R.; Bhargava, N.; Hillesheim, W. Phthalic acid and derivatives. In Ullmann’s Encyclopedia of Industrial Chemistry, 7th ed.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2007; Volume 27, pp. 131–180. [Google Scholar]
- Willy, B.; Neumann, M. Processing-Friendly Dianhydride Hardener for Epoxy Resin Systems Based on 5,5′-carbonylbis (iso-benzofuran-1,3-dione). U.S. Patent Application No. US20150175740A1 14/575,397, 25 June 2015. [Google Scholar]
- Zhang, Y.; Xia, Z.; Huang, H.; Chen, H. Thermal degradation of polyurethane based on IPDI. J. Anal. Appl. Pyrolysis 2009, 84, 89–94. [Google Scholar] [CrossRef]
- Rauert, C.; Kaserzon, S.; Veal, C.; Yeh, R.Y.; Mueller, J.F.; Thomas, K.V. The first environmental assessment of hexa(methoxymethyl)melamine and co-occurring cyclic amines in Australian waterways. Sci. Total Environ. 2020, 743, 140834. [Google Scholar] [CrossRef]
- Félix, J.S.; Isella, F.; Bosetti, O.; Nerín, C. Analytical tools for identification of non-intentionally added substances (NIAS) coming from polyurethane adhesives in multilayer packaging materials and their migration into food simulants. Anal. Bioanal. Chem. 2012, 403, 2869–2882. [Google Scholar] [CrossRef]
- Skjevrak, I.; Brede, C.; Steffensen, I.-L.; Mikalsen, A.; Alexander, J.; Fjeldal, P.; Herikstad, H. Non-targeted multi-component analytical surveillance of plastic food contact materials: Identification of substances not included in EU positive lists and their risk assessment. Food Addit. Contam. 2005, 22, 1012–1022. [Google Scholar] [CrossRef]
- Vera, P.; Canellas, E.; Nerín, C. Compounds responsible for off-odors in several samples composed by polypropylene, polyethylene, paper and cardboard used as food packaging materials. Food Chem. 2020, 309, 125792. [Google Scholar] [CrossRef]
- Wiedmer, C.; Velasco-Schön, C.; Buettner, A. Characterization of off-odours and potentially harmful substances in a fancy dress accessory handbag for children. Sci. Rep. 2017, 7, 1807. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tyapkova, O.; Czerny, M.; Buettner, A. Characterisation of flavour compounds formed by γ-irradiation of polypropylene. Polym. Degrad. Stab. 2009, 94, 757–769. [Google Scholar] [CrossRef]
- Czerny, M. Odors in Paper and Cardboard Packaging; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 2017; pp. 41–42. [Google Scholar]
- Vera, P.; Canellas, E.; Nerín, C. Migration of odorous compounds from adhesives used in market samples of food packaging materials by chromatography olfactometry and mass spectrometry (GC–O–MS). Food Chem. 2014, 145, 237–244. [Google Scholar] [CrossRef]
- Pai Panandiker, K.A.; Danick, C. Coating Powders for Protective Films Based on Epsilon-Caprolactam Blocked Isocyanates. U.S. Patent Application No. 4395529A, 26 July 1983. [Google Scholar]
- Program, N.T. Carcinogenesis Bioassay of Caprolactam (CAS No. 105-60-2) in F344 Rats and B6C3F1 Mice (Feed Study). Natl. Toxicol. Program Tech. Rep. Ser 1982, 214, 1–129. [Google Scholar]
- Schettler, T. Human exposure to phthalates via consumer products. Int. J. Androl. 2006, 29, 134–139. [Google Scholar] [CrossRef]
- Magami, S.M. Functional can coatings Part 2: Composition, attributes, applications and performance. Surf. Coat. Int. 2013, 96, 148–155. [Google Scholar]
- Cheng, L.; Shi, W. Synthesis and photoinitiating behavior of benzophenone-based polymeric photoinitiators used for UV curing coatings. Prog. Org. Coat. 2011, 71, 355–361. [Google Scholar] [CrossRef]
- Zepka, L.Q.; Garruti, D.S.; Sampaio, K.L.; Mercadante, A.Z.; Da Silva, M.A.A. Aroma compounds derived from the thermal degradation of carotenoids in a cashew apple juice model. Food Res. Int. 2014, 56, 108–114. [Google Scholar] [CrossRef] [Green Version]
- Sawpan, M.A. Polyurethanes from vegetable oils and applications: A review. J. Polym. Res. 2018, 25, 184. [Google Scholar] [CrossRef]
- Chiou, B.-S.; Schoen, P.E. Effects of crosslinking on thermal and mechanical properties of polyurethanes. J. Appl. Polym. Sci. 2001, 83, 212–223. [Google Scholar] [CrossRef]
- Guart, A.; Wagner, M.; Mezquida, A.; Lacorte, S.; Oehlmann, J.; Borrell, A. Migration of plasticisers from Tritan™ and polycarbonate bottles and toxicological evaluation. Food Chem. 2013, 141, 373–380. [Google Scholar] [CrossRef] [PubMed]
- Cardama, A.L.; Sendón, R.; Bustos, J.; Santillana, M.I.; Losada, P.P.; De Quirós, A.R.B. GC-MS screening for the identification of potential migrants present in polymeric coatings of food cans. Polymers 2019, 11, 2086. [Google Scholar] [CrossRef] [Green Version]

| Code | Beverage | Polymeric Coating | Thickness (µm) |
|---|---|---|---|
| BC01 | Traditional Beer | Lat. Ext.: PU | Lateral: 114.5 Lid: 313.0 |
| Lat. Int.: Phx | |||
| Lid Int.: Phx | |||
| Lid Ext.: Phx | |||
| BC02 | Vodka mixed drink | Lat. Ext.: PU | Lateral: 109.0 Lid: 218.0 |
| Lat. Int.: Phx | |||
| Lid Int.: Epx | |||
| Lid Ext.: Epx | |||
| BC03 | Mixed lemon flavour | Lat. Ext.: PU | Lateral: 104.0 Lid: 218.0 |
| Lat. Int.: Phx | |||
| Lid Int.: Phx | |||
| Lid Ext.: Phx | |||
| BC04 | Energy Drink Zero | Lat. Ext.: PU | Lateral: 112.0 Lid: 264.0 |
| Lat. Int.: Phx | |||
| Lid Int.: Epx | |||
| Lid Ext.: Epx | |||
| BC05 | Star Wars Space Punch | Lat. Ext.: PP | Lateral: 114.0 Lid: 331.0 |
| Lat. Int.: Acrylic | |||
| Lid Int.: Polyester | |||
| Lid Ext.: Phx | |||
| BC06 | Green cola | Lat. Ext.: PU | Lateral: 115.0 Lid: 234.0 |
| Lat. Int.: Phx | |||
| Lid Int.: Phx | |||
| Lid Ext.: Phx | |||
| BC07 | Tonic original | Lat. Ext.: PU | Lateral: 111.0 Lid: 258.0 |
| Lat. Int.: Phx | |||
| Lid Int.: Epx | |||
| Lid Ext.: Epx | |||
| BC08 | Tonic water original | Lat. Ext.: PU | Lateral: 113.0 Lid: 230.0 |
| Lat. Int.: Phx | |||
| Lid Int.: Epx | |||
| Lid Ext.: Epx | |||
| BC09 | Premium tonic water | Lat. Ext.: PU | Lateral: 123.0 Lid: 226.0 |
| Lat. Int.: Acrylic | |||
| Lid Int.: Polyester | |||
| Lid Ext.: Phx | |||
| BC10 | Natural mineral water drink | Lat. Ext.: PU | Lateral: 103.0 Lid: 248.0 |
| Lat. Int.: Phx | |||
| Lid Int.: Epx | |||
| Lid Ext.: Epx |
| Tr/min | Compound | CAS | Fl No. | MeOH | ACN | Hex | Hex: EtOH (3:1 v/v) |
|---|---|---|---|---|---|---|---|
| 10.56 | Benzoic acid methyl ester | 93-58-3 | 09.725 | x | |||
| 11.01 | Caprylic acid methyl ester | 111-11-5 | 09.117 | x | |||
| 12.97 | Adipic acid methyl ester | 627-93-0 | x | ||||
| 17.17 | Lauric acid methyl ester | 111-82-0 | 09.101 | x | |||
| 17.55 | Unknown compound (m/z 129) | x | |||||
| 18.07 | Diethyl phthalate * | 84-66-2 | x | x | x | ||
| 19.92 | Ester | x | |||||
| 20.25 | Unknown compound (m/z 56) | x | |||||
| 21.71 | Thiophene | x | |||||
| 25.07 | Unknown compound (m/z 151) | x | |||||
| 25.56 | Adipate structure | x | x | x | x | ||
| 25.65 | Adipate structure | x | x | x | x | ||
| 30.43 | Unknown compound | x | x | x | x |
| Tr/min | Compound | CAS | Fl No. | SI | RSI | Sample(s) | TC |
|---|---|---|---|---|---|---|---|
| 10.56 | Benzoic acid methyl ester | 93-58-3 | 09.725 | 745 | 857 | BC04 | I |
| 11.01 | Caprylic acid methyl ester | 111-11-5 | 09.117 | 701 | 789 | BC04 | I |
| 11.37 | 2-Oxepanone | 502-44-3 | 729 | 862 | BC06, BC07 | I | |
| 12.34 | α-Terpineol * | 98-55-5 | 02.014 | 902 | 936 | 9 | III |
| 12.97 | Adipic acid methyl ester | 627-93-0 | 794 | 867 | BC01, BC03, BC04, BC06–BC08 | I | |
| 14.2 | Isobenzofuran-1,3-dione | 85-44-9 | 841 | 922 | BC02, BC05, BC07, BC10 | III | |
| 15.58 | Unknown diol | BC05 | |||||
| 16.93 | (+)-Ledene | 21747-46-6 | 893 | 927 | BC09 | I | |
| 17.17 | Lauric acid methyl ester | 111-82-0 | 09.101 | 855 | 878 | BC01–BC10 | I |
| 17.55 | Ester structure (m/z 129) | BC01–BC10 | |||||
| 18.08 | Diethyl phthalate * | 84-66-2 | 929 | 938 | BC01–BC05, BC09, BC10 | I | |
| 18.37 | Unknown compound (m/z 107, 163) | BC09 | |||||
| 19.28 | Dodecalactone | 2305-05-7 | 10.019 | 855 | 894 | BC05 | II |
| 19.92 | Ester structure (m/z 129) | BC01–BC10 | |||||
| 20.29 | Unknown compound (m/z 56, 111) | BC08 | |||||
| 20.72 | Butyl octyl phthalate | 84-78-6 | 714 | 758 | BC02 | I | |
| 20.99 | Ketone structure | BC05, BC07 | |||||
| 21.53 | Unknown compound m/z (45, 109) | BC05 | |||||
| 21.73 | 2-Isobutyl-5-propylthiophene | 4861-63-6 | BC01–BC06, BC08–BC10 | III | |||
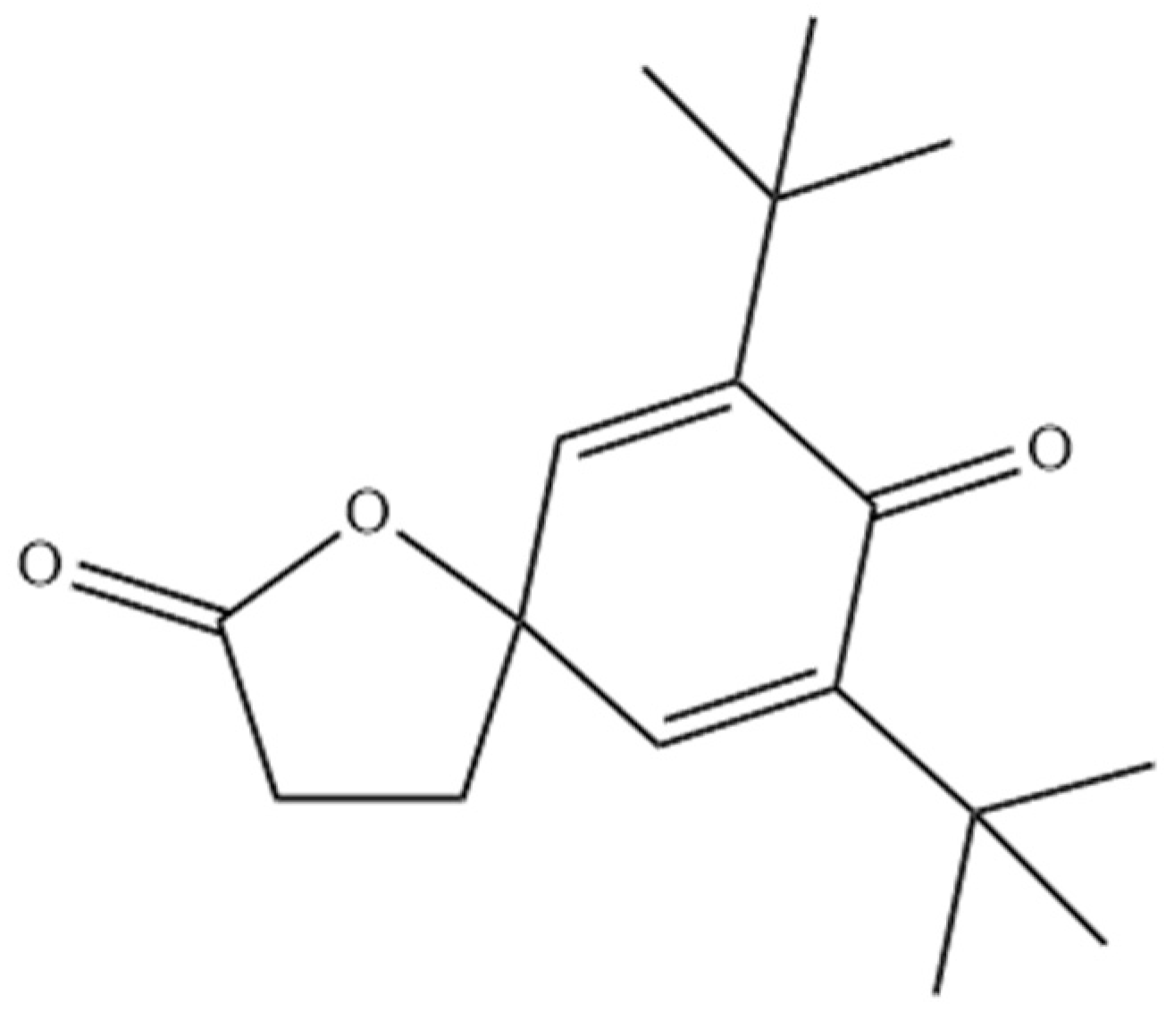
| 21.99 | 7,9-Di-tert-butyl-1-oxaspiro[4,5]deca-6,9-diene-2,8-dione | 82304-66-3 | BC05 | III | |||
| 22.33 and 23.3 | Unknown compound (Phthalate structure m/z: 149) | BC02, BC05, BC07 | |||||
| 23.81 | Unknown compound (m/z 151) | BC06–BC08, BC10 | |||||
| 25.08 | Unknown compound (m/z 151) | BC01, BC02, BC04, BC08–BC10 | |||||
| 25.56 | Unknown compound (m/z 129, 111) | BC01–BC10 | |||||
| 25.65 | Unknown compound (m/z 129, 111) | BC01–BC010 | |||||
| 26.53 | Unknown compound (m/z 163) | BC08, BC10 | |||||
| 27.19 | α-Methyl-δ-oxo-2-phenyl-1,3-dioxolane-2-heptanenitrile | 58422-90-5 | 782 | 940 | BC02, BC05, BC07, BC09, BC10 | III | |
| 27.42 | Hexa(methoxymethyl)melamine | 68002-20-0 | 857 | 874 | BC01- BC03, BC05, BC08, BC10 | III | |
| 27.87 | Unknown compound (m/z 143, 111) | BC08 | |||||
| 28.4 | Unknown compound (Phthalate structure m/z 149) | BC02, BC05, BC07, BC09, BC10 | |||||
| 29.96 | Unknown compound (m/z 301) | BC02, BC05, BC09, BC10 | |||||
| 30.46 | Unknown compound (m/z 69, 81) | BC08 | |||||
| 32.45 | Unknown compound (m/z 345) | BC02, 5 BC0, BC09, BC10 | |||||
| 35.36 | Unknown compound (Phthalate structure m/z: 149) | BC02, BC05, BC010 | |||||
| 35.95 | Unknown compound (m/z 389) | BC02, BC05, BC09 |
| Tr/min | Compound | CAS | Fl No. | SI | RSI | Sample(s) | TC |
|---|---|---|---|---|---|---|---|
| 9.61 | Propylene glycol | 57-55-6 | 571 | 818 | BC04 | I | |
| 13.72 | 2-Butoxyethanol * | 111-76-2 | 02.242 | 866 | 925 | BC01–BC10 | I |
| 15.37 | α-Terpinene | 99-86-5 | 01.019 | 586 | 754 | BC09 | I |
| 15.61 | Benzaldehyde | 100-52-7 | 05.013 | 631 | 865 | BC01, BC05 | I |
| 15.85 | 2,2-Dimethyl-1,3-Propanediol * | 126-30-7 | 821 | 892 | BC01, BC02, BC03, BC04, BC08 | I | |
| 16.11 | Octanal * | 124-13-0 | 05.009 | 505 | 701 | BC04 | I |
| 16.28 | 1,2,3,4-Tetramethyl benzene | 488-23-3 | 849 | 879 | BC09 | I | |
| 16.43 | Limonene * | 5989-27-5 | 01.045 | 916 | 924 | BC02, BC03, BC05–BC08 | I |
| 16.5 | p-Cymene | 99-87-6 | 01.002 | 909 | 926 | BC02, BC03, BC06, BC09 | I |
| 16.69 | 1-Hexanol-2-ethyl | 104-76-7 | 02.082 | 809 | 913 | BC05, BC06, BC08 | I |
| 17.05 | g-Terpinene | 99-85-4 | 01.020 | 882 | 894 | BC02, BC09 | I |
| 17.68 | Terpinolene | 586-62-9 | 01.005 | 795 | 864 | BC02 | I |
| 18.07 | Benzene structure | BC02, BC05, BC07, BC09 | |||||
| 18.45 | Nonanal * | 124-19-6 | 05.025 | 701 | 857 | BC01–BC04, BC06 | I |
| 19.02 | Unknown compound (m/z 79, 121; cyclohexenol structure) | BC05 | |||||
| 19.35 and 20.51 | Unknown compound (m/z 134; cyclohexanol structure) | BC05 | |||||
| 19.39 | Benzenemethanol | 60-12-8 | 02.019 | 842 | 896 | BC01, BC03 | I |
| 19.92 | Ethyl octanoate | 106-32-1 | 09.111 | 913 | 938 | BC01–BC03 | I |
| 20.35 | Octanoic acid | 124-07-2 | 08.010 | 763 | 928 | BC01, BC02 | I |
| 20.61 | Decanal | 112-31-2 | 05.010 | 853 | 924 | BC02, BC04, BC06–BC08 | I |
| 20.74 | Unknown compound (m/z 70, 119; ester structure) | BC05 | |||||
| 20.92 | Benzoic acid * | 65-85-0 | 08.021 | 917 | 931 | BC04 | I |
| 21.43 | Unknown compound (m/z 109, 71; cyclohexanol structure) | BC05 | |||||
| 21.5 | Unknown compound (m/z 135, 79, 107) | BC05, BC07 | |||||
| 21.67 | 2-Phenoxyethanol * | 122-99-6 | 696 | 965 | BC04 | I | |
| 21.68 | 2-Phenethyl acetate | 103-45-7 | 09.031 | 822 | 911 | BC01 | I |
| 21.76 | Carvone | 99-49-0 | 07.012 | 860 | 914 | BC05, BC07, BC09 | II |
| 22.15 | Nonanoic acid | 112-05-0 | 08.029 | 806 | 915 | BC01, BC04 | I |
| 22.36 | Unknown compound (m/z 73) | BC03 | |||||
| 22.47 | Undecanal | 112-44-7 | 05.034 | 633 | 824 | BC04 | I |
| 22.98 | 2-Azepanone * | 105-60-2 | 16.052 | 883 | 889 | BC01, BC03–BC10 | III |
| 23.57 | Ethyl-decanoate | 110-38-3 | 09.059 | 902 | 935 | BC01–BC03, BC05 | I |
| 23.92 | Decanoic acid | 334-48-5 | 08.011 | 671 | 775 | BC04 | I |
| 24.25 | Dodecanal | 112-54-9 | 05.011 | 606 | 872 | BC02, BC06, BC0 7 | I |
| 24.68 | 2-Methylaminobenzoic acid | 85-91-6 | 09.781 | 676 | 933 | BC04 | I |
| 25.05 | 6,10-Dimethyl-5,9-undecadien-2-one | 3796-70-1 | 07.123 | 688 | 773 | BC02 | I |
| 25.39 | Benzaldehyde-4-hydroxy-3-methoxy * | 121-33-5 | 05.018 | 830 | 904 | BC04 | I |
| 25.51 | Napthalene structure | 792 | 833 | BC09 | |||
| 25.54 | 2, 6-Di-tert-butyl-1,4-benzoquinone (2,6-DTBQ) * | 719-22-2 | 729 | 804 | BC06 | II | |
| 25.77 | 1,3-Diacetylbenzene | 6781-42-6 | 818 | 875 | BC01 | I | |
| 26.34 | 2,4-Ditertbutylphenol | 97-76-4 | BC03, BC06–BC08, BC10 | I | |||
| 26.40 | Decalactone-g | 706-14-9 | 10.017 | 887 | 912 | BC05, BC09 | II |
| 26.49 | Unknown compound (m/z 43, 163,120; phenol structure) | BC01 | |||||
| 26.78 | Dodecanoate-ethyl | 106-33-2 | 09.099 | 879 | 935 | BC01 | I |
| 27.39 | Unknown compound (m/z 129, 111; ester structure) | BC03 | |||||
| 27.75 | Diethyl phthalate * | 84-66-2 | 922 | 930 | BC01–BC10 | I | |
| 28.03 | Undecalactone-g | 104-67-6 | 10.002 | 755 | 849 | BC05, BC07 | II |
| 28.28 | Unknown compound (m/z 213,109) | BC05 | |||||
| 28.32 | Alcohol | BC01, BC04 | |||||
| 28.60 | Diphenylmethanone * | 119-61-9 | 07.032 | 717 | 941 | BC01- BC03 | III |
| 29.56 | Lactone structure | BC05 | |||||
| 29.69 | Unknown compound (m/z 81, 99; methanone structure) | BC01, BC07 | |||||
| 30.07 | Tetradecanoate | 110-27-0 | 09.105 | 619 | 848 | BC01, BC04 | I |
| 30.65 | Unknown compound (m/z 219,191) | BC08 | |||||
| 30.85 | Unknown compound (m/z 69) | BC08 | |||||
| 31.85 | Phthalate structure (m/z 149) | BC04, BC08 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vázquez-Loureiro, P.; Lestido-Cardama, A.; Sendón, R.; López-Hernández, J.; Paseiro-Losada, P.; Rodríguez-Bernaldo de Quirós, A. Identification of Volatile and Semi-Volatile Compounds in Polymeric Coatings Used in Metal Cans by GC-MS and SPME. Materials 2021, 14, 3704. https://doi.org/10.3390/ma14133704
Vázquez-Loureiro P, Lestido-Cardama A, Sendón R, López-Hernández J, Paseiro-Losada P, Rodríguez-Bernaldo de Quirós A. Identification of Volatile and Semi-Volatile Compounds in Polymeric Coatings Used in Metal Cans by GC-MS and SPME. Materials. 2021; 14(13):3704. https://doi.org/10.3390/ma14133704
Chicago/Turabian StyleVázquez-Loureiro, Patricia, Antía Lestido-Cardama, Raquel Sendón, Julia López-Hernández, Perfecto Paseiro-Losada, and Ana Rodríguez-Bernaldo de Quirós. 2021. "Identification of Volatile and Semi-Volatile Compounds in Polymeric Coatings Used in Metal Cans by GC-MS and SPME" Materials 14, no. 13: 3704. https://doi.org/10.3390/ma14133704
APA StyleVázquez-Loureiro, P., Lestido-Cardama, A., Sendón, R., López-Hernández, J., Paseiro-Losada, P., & Rodríguez-Bernaldo de Quirós, A. (2021). Identification of Volatile and Semi-Volatile Compounds in Polymeric Coatings Used in Metal Cans by GC-MS and SPME. Materials, 14(13), 3704. https://doi.org/10.3390/ma14133704







