2. Characteristics of TCEs and Their Impact on the Environment
Germanium is an element that is often used in industry because of its semi-metallic nature as well as its semiconductor properties. The alloy of this metal, along with small admixtures of arsenic, gallium, indium, antimony, or phosphorus, is used to build transistors, which are essential components in electronic devices. Germanium and its GeO
2 oxide are transparent to infrared radiation; therefore, they are used as lenses and windows in optical instruments for the appropriate spectral range, and are used to detect thermal objects (this accounts for 30% of Ge applications). About 20% of produced germanium is used in optical materials (optical fibers) and in catalysts for the production of polyesters (polyethylene terephthalates, PET), as well as for synthetic textile fibers and photographic film [
5,
6].
The applications of tellurium are very diverse, but the quantities consumed are minimal, much smaller than many precious metals and semi-metals used in electronics. Initially, tellurium was used with lead as an alloying addition to steel. It was also used in catalysts, alloys with copper and lead (addition of tellurium to non-ferrous metals improves physical properties and resistance to chemical corrosion), for vulcanization of rubber, and in photocopiers and thermo-electronic equipment. Currently, tellurium is mainly used in the production of cadmium telluride (CdTe) thin-film solar cells, which is its major application (40% of global consumption), followed by thermo-electrics (30%). The cadmium present in cells would be toxic if released, however, this is impossible during normal operation of the cells. However, there is a risk of the release of harmful compounds during milling or other processes related to waste management [
7]. In the case of tellurium, a majority of it is obtained as a by-product of non-ferrous metal-refining processes.
Thallium is used in semiconductor materials, photocells, infrared measuring devices, or in glass lenses, prisms, and windows for optical fibers; it is also used as a catalyst in organic synthesis. This element is important for the production of glasses with a high density and refractive index, optical lenses, imitation jewelry, electrochemical equipment, and corrosion-resistant alloys. Today, approximately 70% of thallium production is used in electronic devices [
5]. Although thallium is a toxic element, it is used industrially and for the production of pesticides. In some countries, it has contributed to contamination—primarily of soil, water, and plants, and at subsequent levels of animal food chains [
8]. The production capacities of a thallium recovery installation in zinc smelters are used to a small extent, so the production of thallium can easily be increased as required [
5]. Components, such as Ge, Te, and Tl, in e-waste include printed circuit boards ( PCBs), cathode ray tubes (CRTs), liquid crystal display (LCD) screens, light-emitting diode (LED) lights, batteries, circuit boards, and solar and photovoltaic cells (PV) [
9]. Each of the above-mentioned wastes has a heterogeneous material composition (organic materials, metals, glass fiber, and ceramic) and the management of recycling these precious metals and hazardous metals requires sophisticated technologies and a multidisciplinary approach [
10].
Each year, approximately 20–50 million tons of electrical and electronic equipment waste (e-waste) are produced globally, and this amount is estimated to increase by 3–5% annually [
11]. Metals in PCBs consist of a large number of base metals; rare metals, such as Ta, Ga, and other precious metals. Hazardous metals, such as Cr, Pb, Be, Hg, Cd, Zn, and Ni are also present [
10]. However, when considering the possibility of recycling these metals, millions of tons of waste of electronic equipment are in circulation and should be taken into account. Recycling and unitary processes of disassembly, separation, grinding, and milling can be the source of uncontrolled emissions of metals into water, soil, and air.
Even with low TCE contents in electrical equipment, the problem becomes critical due to a large amount of produced e-wastes. These amounts also become so large that recovery of these metals from waste becomes feasible and profitable.
In addition, application of these processes cause the migration of TCE metals to nearby environments and/or exposes workers to the harmful effects of metals through inhalation, skin contact, or ingestion [
11]. In particular, thallium is a highly toxic metal, listed by the European Water Framework Directive [
1] and the United States Environmental Protection Agency (USEPA 2015) as a priority pollutant, of which penetration into surface environments, and its dispersion in soils, sediments, and waters, can occur relatively easily due to the high volatility and solubility of thallium compounds [
12]. The characteristics of germanium, tellurium, and thallium, along with the influence of these metals on living organisms, are presented in
Table 1.
The Ge content varies in various US soils, from <0.1 to 2.1 mg kg
−1, and from <1 to 95 mg kg
−1 in the topsoils of Sweden. The occurrence of Te on Earth is about 1 µg kg
−1, and its content in rocks ranges from 1 to 5 µg kg
−1. Tl content ranges from 0.01 to 2.8 mg kg
−1 and is increased in organic soils [
12]. As mentioned earlier, the indicated critical metals, due to their low content in e-waste, are poorly described in the literature. It is difficult to compare the above values with their content in secondary raw materials, as there is no comprehensive information on TCE content per ton of waste.
PCBs are diverse and complex in terms of type, size, shape, components, and composition. In addition, as technology progresses, PCB compositions are continuously changing, making it more difficult to obtain stable material compositions. The presence of plastics, ceramics, and metals in PCBs, in a complex manner, leads to great difficulty in liberation and separation of each fraction. Metals in PCBs consist of a large number of base and rare metals, as well ashazardous metals (such as Ge, Te, Tl) [
13,
14,
15].
At present, there are no literature reports on what the levels of these metals in particular types of e-waste are, as well as no detailed information on recovery processes of the development of Ge, Tl, and Te. The situation is similar in the case of individual WEEE types, e.g., LEDs, as rare earths are also not currently recycled from these devices [
16]. The main obstacle in carrying out processes for recycling germanium, tellurium, and thallium are the small amounts of TCE materials that are dispersed in shredded waste. However, due to their toxicity and effect on human health, they require at least proper landfill storage.
Therefore, the shredding of LED backlit displays could have the same effect as that of displays containing mercury-based backlighting systems, i.e., they could lead to hazardous contamination by recyclable fractions. Current EU WEEE legislation does not yet address specific treatments for LED-based products, mainly because the massive use of LEDs in EEE only began recently and there is still little research regarding their potential toxicity and their proper EoL treatment (end-of-life) [
17].
The life span of currently produced solar modules is 25–30 years, and, after that, they will require proper management. If they are deposited in landfills, the metals they contain can potentially be released into the environment. PV modules can be stored in ordinary landfills, as long as the contained CdTe does not leach out. In other cases, when the concentration of metals exceeds the limit values (the modules have the ability to release, among others, cadmium), it is necessary to subject them to the recycling processrd or depositing them in a hazardous-waste landfill [
18,
19]. PV recycling processes begin with the physical separation of individual elements; the modules are then crushed, and the metals are removed in subsequent stages of chemical dissolution, mechanical separation, as well as precipitation or electrolytic deposition. Finally, glass and the metal fractions are recovered (e.g., 80–96% Te, Se, and Pb). Other metals (e.g., Cd, Te, Sn, Ni, Al, and Cu) are contained in sludge, which is then subjected to further recycling processes [
7].
Mechanical processing of e-waste, shredded into pieces using hammer mills, is an integrated part of its recycling process. Mechanical process and physical separation techniques (screening, magnetic, eddy current, and density separation techniques) allow to separate non-metals fraction (polymers, ceramics, and glass) from metals and concentrate them in one fraction [
20,
21,
22]. This approach allows maximum material recovery, increases the efficiency of their processing and recovery. The size of the material particle, obtained in the milling process, is one of the most important parameters affecting metal dissolution efficiency during hydrometallurgical e-waste processing [
21,
22]. However, the individual processes of crushing and milling of e-waste create conditions in which metals may be released if grinding of the products is performed. In addition, the activities of collecting and transporting the fragmented fractions can lead to the dispersion and migration of pollutants in the form of dust containing these metals and other organic compounds into water and soil environments [
23]. The process of releasing metals from waste and their migration to the environment is affected by physico-chemical environmental factors. This process is favored by lowering the pH of the environment in which the waste is collected/stored, temperature changes, oxidation and reduction conditions, as well as the processes of material fragmentation and increases in organometallic organic complexity [
24,
25,
26].
Recycling of TCE, including gallium, germanium, indium, or rare earth elements (REEs), is difficult due to their low concentration, dispersion in electronic components (a multitude of products with different concentrations), and material heterogeneity, where metals are combined with alloys, ceramics, glass, composites or tightly packed in material structures [
27,
28]. Due to these factors, elements may already be lost at the stage of WEEE shredding, which tends to accumulate the fractions of dust and ferrous metals [
29].
Worldwide, about 30% of the total germanium consumed is produced from recycled materials, electronic devices, and optical fibers. During the manufacturing of most optical devices, more than 60% of germanium is routinely recycled as new scrap [
6]. The basic procedure of obtaining germanium from wastes is leaching using hot sulfuric acid. Among the techniques of separating Ge from other elements (contained in leachate), precipitation, adsorption, solvent extraction, adsorption on chelating, ion-exchange extraction, and vacuum reduction metallurgical process are used [
28]. For traditional metallurgical and chemical uses, there were little or no old scrap from which to extract secondary tellurium because these uses of tellurium were highly dispersive. A very small amount of tellurium has been recovered from scrapped selenium–tellurium photoreceptors employed in older plain-paper copiers. Tellurium recycling from CdTe solar cells is still slight, as most CdTe solar cells are relatively new and have not yet reached the end of their use [
6].
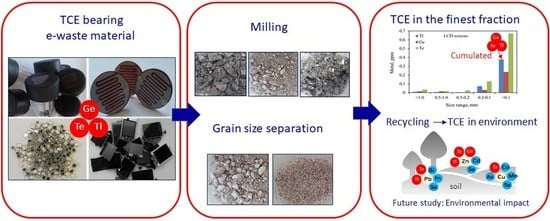
In the article, elements and electronic subassemblies that are carriers of Ge, Te, and Tl, were subjected to disassembly and mechanical treatment (cutting, grinding), and then quantitative analyses were made. Due to the low concentrations of Ge, Te, and Tl in the e-waste stream, materials for testing were selected according to their content. Grinding of the selected metal-bearing electronic elements enabled the release of Ge, Te, and Tl from one non-complicated component matrix (e.g., glass, ceramics only) ensuring accurate determination of the concentration of these metals in a given fraction without major losses. Currently, knowledge on the presence of Ge, Te, and Tl in the electronic scrap circulation is negligible.
This research aimed to determine the distribution of Ge, Te, and Tl in the groundmass of waste in individual fractions and to determine the possible tendency to accumulate/concentrate metals in a particular fraction. In addition to the analyses of Ge, Te, and Tl contents, this article also draws attention to other metals (Ba, Co, Mn, Cu, Ni, and Zn) occurring in the matrices of electronic components, especially harmful elements (Cd, Cr, and Pb). The distribution of metals in the ground fractions of e-waste is crucial knowledge in the analysis of migration issues of pollutants from e-waste collection and recycling plants in the form of dust to the environment. To better understand the relationship between TCE migration to the environment and their mobility in soils in e-waste recycling areas, correlation studies will be discussed in future works.
Our research results evidence the impact of an electrowaste processing plant on an increase of germanium and tellurium concentration in soils in the area directly surrounding the WEEE plant. The direction and extent of emission of dusts (created during mechanical disassembly and shredding) depended on the strength and dominant wind direction. Our results showed also that the WEEE plant had little impact on the increase in thallium concentrations in the topsoil surrounding the plant. The studies showed that the slight increase in the thallium content in the topsoil did not have a geogenic basis, but was only due to the influence of human activity.
3. Materials and Methods
3.1. Sample Collection and Preparation
For quantitative and qualitative composition tests, elements of electronic waste and electronic components that were carriers of Ge, Te, and Tl were selected; outdoor solar lamps, solar cell, LED TV screen matrix, LCD screens of used mobile phones, as well as uniform electronic elements, such as photoresistors, photodiodes, and phototransistors. Except for TV screen matrixes and LCD screens, all elements were new, and were purchased from an electrical and electronic parts warehouse. LCD screens and the Samsung TV screen matrix came from used electronic equipment, obtained from a local e-waste market. These elements are examples of typical electronic waste that wind up at collection and processing places. Specifications on the e-waste samples used in this study are presented in
Table 2.
Elements and sub-assemblies were subjected to deep, manual disassembly, removing all elements that were material ballast and/or covering carriers of tested metals. Plastic housings, covers (solar lamps), polymetric frames protecting the LCD/LED screen structure (mobile and TV screens), rubber or silicone layers (solar panels), and connection pins, (photoresistors, photodiodes) were removed. Only the Ge, Te, and Tl metals carriers were intended for further analyses. The separated material was crushed and cut into smaller size fractions (1 × 1 cm2), if needed, and then ground using a knife mill (Chemland, model FW135, Stargard Szczecinski, Poland) to provide an effective liberation of the metals. The milling time was 2 min or more, depending on the type of ground material. Samples consisting mainly of silica were rapidly disintegrated, while ceramic type materials required a longer disintegration time. Magnetic separation was used for the recovery of ferromagnetic metals from residues in ground material (e.g., photoresistors). To determine the degree of metal dispersion in the ground materials, and to obtain the knowledge necessary to determine the degree of release of Te, Ge, and Tl, depending on the grain size of the ground waste, the materials were subjected to grain classification. Samples were sieved in an electromagnetic sieve shaker (Sieve shaker LPzE-2e, Multiserw-Morek, Brzeznica, Poland) equipped with sieves of standard sizes: 1.0, 0.5, 0.2, and 0.1 mm.
The adopted grain range of ground elements (>1.0–<0.1 mm) corresponded to the possible size of dust particles formed during mechanical processes of e-waste scrap treatment. The distribution of individual grain fractions in ground materials is presented in
Table 3.
The results presented above allow the conclusion that the smallest fraction, below 0.1 mm, also has the smallest share in the total fraction distribution. On the other hand, in the case of larger fractions, the distribution was unevenly distributed between individual fractions. The exceptions were photoresistors and LCD screens, where the thickest fraction >1.0 was a small percentage of the total. The relationship between the metal concentration vs. size and their distributions is described in more detail in
Section 4.3.
3.2. Digestion Method of Electronic Components
The analysis of the content of elements in e-waste material is difficult due to the heterogeneity of the individual components of this waste. There is still no single standardized method and different digestion protocols have been adopted for sample preparation. The lack of a standardized method of analysis has caused discrepancies in the results of various authors and makes it difficult to compare the efficiency of recovery processes [
32,
33].
The digestion process carried out in an open vessel under reflux conditions, or in a closed vessel assisted by microwave radiation, involving the use of HNO
3, HCl, HBF
4, HF H
2O
2, and their mixtures, and are described in many publications [
19,
34,
35,
36,
37,
38]. The efficiency of a digestion protocol for solid waste is dependent on the waste matrix, the chemical form of the metals in the waste matrix, and the acids used in the digestion process [
33]. The authors of [
32] tested 11 WEEE digestion protocols using various combinations of inorganic acids. It was shown that dissolution of basic metals. Such as Cu, Fe, Ni, Zn, Pb, Al, and the noble metals, Pd, Au, and Ag, from an e-waste matrix with a mixture of aqua regia and HF was found to be the most effective combination for maximum metal extraction. However, using other possible acid combinations, the authors achieved an equally high transition efficiency for metals such as Zn, Pd, Cu, and Ag for solutions, or even better transition for Fe and Sn metals.It isdifficult to standardize the digestion protocol due to the heterogeneity of material in e-waste [
39].
There is no available certified reference material for electronic waste or its individual components, such as PCBs or LCD. However, first attempts have been made to develop reference materials (RM) for PCBs, indicating the leaching procedure in diluted aqua regia (AR 50% v/v) and microwave radiation as adequate for determining Ag, Au, Cr, Fe, Sb, Sn, and Zn [
33]. Both the results for RM and the results of the analyses of the tested digestions of e-waste protocols refer only to basic and noble metals; there is no analysis for critical metals, such as Ge, Te, and Tl. Lack of certified reference materials (CRMs) for electronic waste, combined with the complex composition of WEEE and the difficulties of sample leaching, has made analyzing this material challenging [
33]. As the methodology for the analysis of Cu and other basic metals used by the authors has already been repeatedly verified, the validation of the digestion method has been limited only to metals from the TCE group (Ge, Te and Tl).
Due to the lack of available CRM for Ge, Te, and Tl in WEEE, CRM NCSDC 73322 (China National Analysis Center for Tron and Steel, Beijing, China) soil was used to verify the adopted methodology. A total of 0.5 g of soil sample was digested in hot aqua regia for 3 h. The results presented in
Table 4 confirm that the applied method was appropriate for thallium and tellurium analysis and the results of their digestion were consistent with the levels presented for the certified reference material used in the tests. Germanium can form volatile chloride that is not fully retained by aqua regia [
40].
In the digestion process of electronic components, mixtures of HCl and HNO
3 acids (aqua regia) were used. Aqua regia, as a common medium for digestion of multi-material samples, has been widely used for e-waste [
36,
41,
42]. The ground elements of e-waste were sieved and sorted into five groups: (i) particle sizes larger than 1 mm; (ii) particles sizes between 1 and 0.5 mm; (iii) particles sizes between 0.5 and 0.2 mm; (iv) particles sizes between 0.2 and 0.1 mm; and (v) particles sizes less than 0.1 mm. To determine the metal content of the fractionated material, 0.5 g of each sample was digested using 0.03 L of HCl (35% m/v) and 0.01 L of HNO
3 (69% m/v) acids. The digestion process was carried out in open systems by heating on a hot plate (95°C ± 5 °C) for 3 h, using freshly prepared aqua regia. All reagents were of analytical grade (APM Poland S.A.-POCH ™ brand from Avantor ™, Gliwice, Poland). The mixture was then filtered through filter paper into a 0.05 L standard volumetric flask.
3.3. Determination of the Total Element Contents
The total content of Ge, Te, and Tl, as well as other metals and metalloids, were determined using an ICP-MS and ICP-OES spectrometer. The Elan 6100 DRC-e ICP-MS spectrometer (Perkin Elmer, Waltham, MA, USA) was used for quantitative analyses of total Ge, Tl, Te, Cd, Ba, Co, and Mn, while metals, such as Cr, Cu, Ni, Pb, Sr, and Zn were quantified using an Avio 200 ICP-OES (Perkin Elmer, Waltham, MA, USA) spectrometer.
The ICP-MS apparatus was equipped with a standard ICP quartz torch, cross-flow nebulizer, and nickel cones. Samples and standards were delivered with a peristaltic pump. The spectrometer was optimized daily with a 10-µg/L solution (Mg, Cu, Rh, Cd, In, Ba, Ce, Pb, and U) in 1% HNO3 Elan 6100 Setup/ Stab/Masscal solution (Perkin Elmer, Waltham, MA, USA). The concentrations of metals were measured with an internal 103Rh standard.
Germanium determination using ICP-MS spectroscopy is difficult due to numerous spectral interferences:
70Ge (
35Cl
17O
18O
+,
36Ar
34S
+,
38Ar
32S
+,
70Zn
+),
72Ge (
36Ar
2+,
56Fe
16O
+,
40Ar
32S
+,
40Ar
16O
2+,
55Mn
16OH
+),
74Ge (
40Ar
34S
+,
36Ar
38Ar
+,
40Ar
36S
+,
37Cl
2+,
74Se
+) [
39].
To eliminate these polyatomic interferences, DRC technology with CH4 as the reaction gas was used. The flow rate of the reaction gas and the value of the rejection parameter (Rpq) were the key parameters for DRC operation. The optimized CH4 flow rate was selected to be 0.4 mL/min and when the Rpq value was 0.65, the signal of 74Ge tended to be stable. Under the optimized conditions, the analytical performance of the proposed method was evaluated. Standard solutions with Ge concentrations from 1 to 25 µg/L were analyzed to construct a calibration curve with a correlation coefficient of 0.9998. Limits of detection (LOD) was determined as a three-time value of standard deviation for the blank sample, which was an acidified water sample, used to prepare calibration solution and the dilution of the real samples. The limits of quantification (LOQ) were expressed as three times the value of the limit of detection. The limits of detection and quantitation were 0.05 µg/L and 0.3 µg/L, respectively.
Direct determination of tellurium in environmental samples by using coupled plasma mass spectrometry (ICP-MS) is often complicated by its low abundance, poor analytical sensitivity, and the presence of xenon interference. Therefore
125Te,
126Te,
128Te,
130Te isotopes were measured using correction equations (−0.003404 ×
129Xe) for
126Te, (–0.072617 ×
129Xe) for
128Te, (−0.009437 ×
137Ba − 0.154312 ×
129Xe) for
130Te. The best results were obtained with the
126Te isotope, as was done in the work of Filella and Rodushkin [
40]. Standard solutions with Te concentrations from 1 to 25 µg/L were analyzed to construct a calibration curve with a correlation coefficient of 0.9997. The limit of quantitation (LOQ, three times to the LOD) was 0.42 µg/L, while the LOD was 0.14 µg/L. Methodology for determination of the ionic Te(VI) and Te(IV) forms has been described in detail in the work by Grygoyc and Jabłonska-Czapla [
43].
Other elements, such as
114Cd,
130Ba,
59Co,
55Mn, and
205Tl, were measured using an ICP-MS spectrometer in standard mode. The research into the total Cr, Cu, Ni, Pb, Sr, and Zn contents was conducted using an Avio 200 inductively coupled plasma-optical emission spectrometer (ICP-OES, Perkin Elmer, Waltham, MA, USA) with an axial viewing configuration. The operating conditions of the instrument are shown in
Table 5. The ICP OES was equipped with a standard ICP quartz torch with a corundum injector tube, a cross-flow nebulizer, and a Scott’s spray chamber. The instrument performance was checked by measuring an optimization solution (Optima Family Multi-Element Standard, Perkin Elmer, Waltham, MA, USA). The calibration mixed standard solutions were prepared from the stock standard solution of 1000 μg/L. The following spectral lines have were for analyses: Cr—205.560 nm, Cu—327.339nm, Ni—221.648 nm, Pb—217.000 nm, Sr—407.771 nm, and Zn—213.857 nm.
3.4. Scanning Electron Microscopy (SEM-EDS) and X-Ray Diffraction (XRD)
Observations of the surface of the tested samples were made with a Hitachi S-4200 scanning electron microscope (Mannheim, Germany) using a secondary electron detectors (SE). Chemical composition tests were performed using an X-ray energy dispersion spectrometer (EDS) from Thermo Noran (System Seven, Mannheim, Germany) at an accelerating voltage for the electron beam of 15 keV. Results of EDS microanalyses of the chemical composition included also surface analyses.
The phase composition was analyzed by X-ray diffraction (XRD) using a Panalytical Empyrean diffractometer (Malvern, UK) equipped with PIXCel detector ( and a Johansson monochromator. Data collection was performed over a 2q range of 10–80° with a 0.02°/step using Ni-filtered and Cu radiation.
6. Conclusions
Ge, Te, and Tl are little known elements, the use of which, as components of electronics in new technologies, grows every year. It is necessary to comprehensively analyze the issues of WEEE material characterization in relation to elements, such as Ge, Te, and Tl, as well as an analysis of the methods of their treatment, recovery, the mechanisms of their release from WEEE, carried as dust to the environment, in addition to the environmental and human impacts. Our research is one of the first to pay attention to the problems of rare elements, and to identify the quantitative and qualitative composition of electronic elements containing Ge, Te, and Tl and their grain size distribution of the ground material.
The results of quantitative and qualitative research on electronic components and sub-assemblies containing Ge, Te, and Tl revealed that the contents of metals increase with decreasing particle size. Ge, Te, and Tl are concentrated in the finest fractions 0.2–0.1 mm and <0.1 mm of ground e-waste. In addition to these, other harmful elements, such as cadmium and chromium, were concentrated in the finest material fraction (<0.1 mm) as well. Toxic metals in the finest fractions might be easily released into the environment during mechanical processing of e-waste, which is particularly dangerous. The contents of other metals (Ba, Co, Cu, Mn, Ni, Sr, and Zn) in studied e-waste are diverse in sieve fractions. Due to the lack of CRM for Ge, Te, and Tl in WEEE, the correctness of the digestion method, using aqua regia, was verified against the CRM for soil. However, during the adopting of this methodology, the possible losses of Ge should be taken into account and the application of a different system and medium (Ge digestion) should be considered.
The obtained results will be used for further investigations on the anthropogenic migration of Ge, Te, and Tl to the environment in areas associated with e-waste recycling and to determine the mobility of speciation forms of metals in the soil.