Hybrid Ultra-Low-Radioactive Material for Protecting Dark Matter Detector from Background Neutrons
Abstract
:1. Introduction
2. Theoretical Issue
3. Materials and Methods
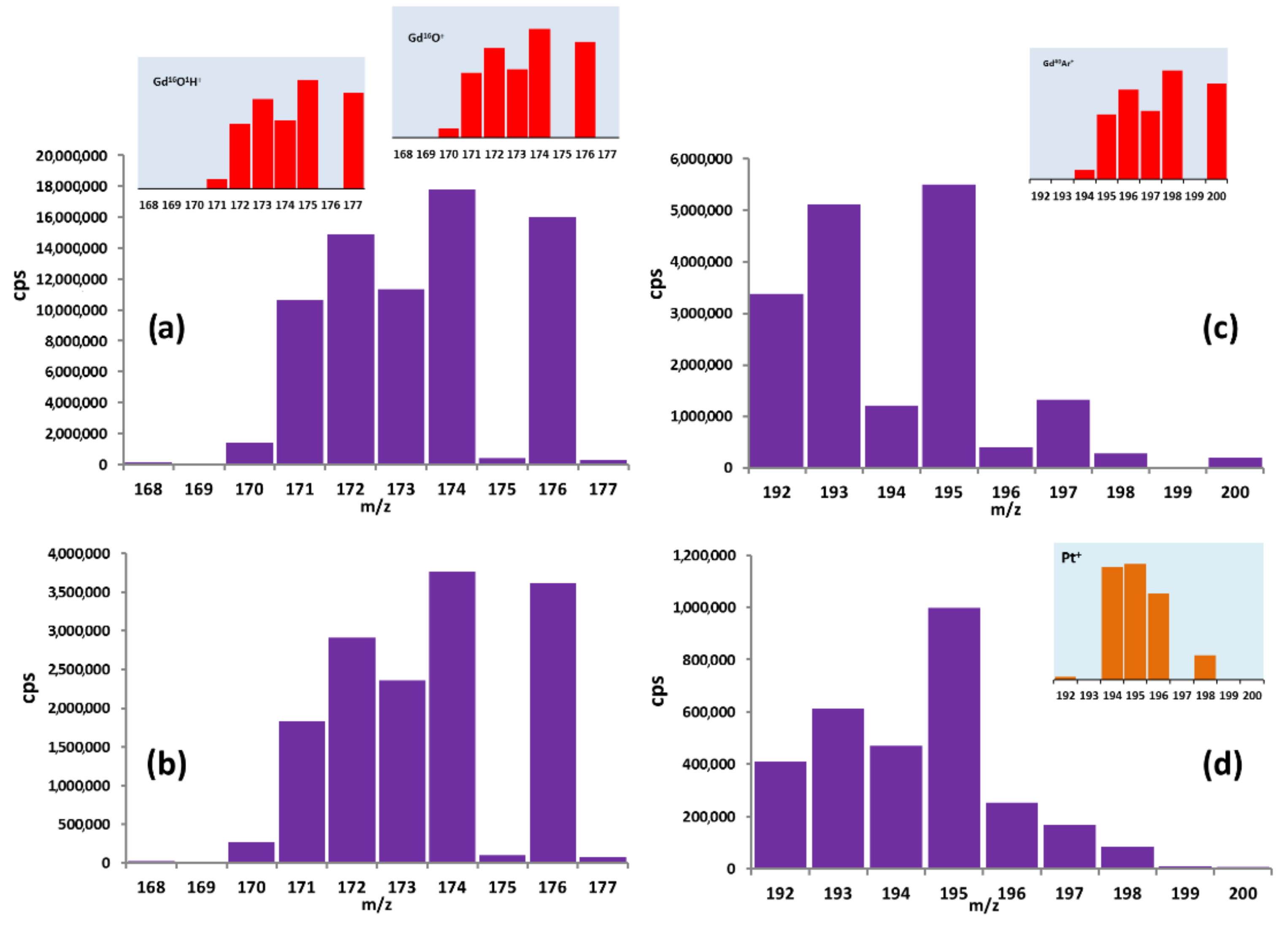
3.1. Impurity Determination by ICP-MS
3.2. Analysis of Gd Concentration Distribution
3.3. Mechanical Test of Polymer and Hybrid Materials
4. Results and Discussion
4.1. Analysis of Commercial Gd-Based Preparations
4.2. Purification of GdCl3 Preparations
- The chlorination process of the starting gadolinium chloride,
- Thermal annealing in vacuum.
4.3. Analysis of Commercial Polymers
4.4. Hybrid Material Fabrication
4.5. Mechanical Test of Hybrid Material
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A
References
- Empl, A.; Jasim, R.; Hungerford, E.; Mosteiro, P. Study of Cosmogenic Neutron Backgrounds at LNGS. arXiv 2012, arXiv:1210.2708. [Google Scholar]
- Westerdale, S.; Meyers, P. Radiogenic neutron yield calculations for low-background experiments. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrometers Detect. Assoc. Equip. 2017, 875, 57–64. [Google Scholar] [CrossRef] [Green Version]
- Agnes, P.; Agostino, L.; Albuquerque, I.; Alexander, T.; Alton, A.; Arisaka, K.; Back, H.; Baldin, B.; Biery, K.; Bonfini, G.; et al. The veto system of the DarkSide-50 experiment. J. Instrum. 2016, 11, P03016. [Google Scholar] [CrossRef]
- Kikoin, I.K. Tables of Physical Values; Handbook, Atomizdat: Moscow, Russia, 1976; 1008p. [Google Scholar]
- Poehlmann, D.M.; Barker, D.; Chagani, H.; Cushman, P.; Heuermann, G.; Medved, A.; Rogers, H.E.; Schmitz, R. Characterization of Gadolinium-Loaded Plastic Scintillator for Use as a Neutron Veto. arXiv 2018, arXiv:1812.11267v1. [Google Scholar]
- Dumazert, J.; Coulon, R.; LeComte, Q.; Bertrand, G.H.V.; Hamel, M. Gadolinium for neutron detection in current nuclear instrumentation research: A review. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrom. Detect. Assoc. Equip. 2018, 882, 53–68. [Google Scholar] [CrossRef]
- Hagiwara, K.; Yano, T.; Tanaka, T.; Reen, M.S.; Das, P.K.; Lorenz, S.; Ou, I.; Sudo, T.; Yamada, Y.; Mori, T.; et al. Gamma-ray spectrum from thermal neutron capture on gadolinium-157. Prog. Theor. Exp. Phys. 2019, 023D01. [Google Scholar] [CrossRef]
- Aalseth, C.E.; Acerbi, F.; Agnes, P.; Albuquerque, I.F.M.; Alexander, T.; Alici, A.; Alton, A.K.; Antonioli, P.; Arcelli, S.; Ardito, R.; et al. DarkSide-20k: A 20 tonne two-phase LAr TPC for direct dark matter detection at LNGS. Eur. Phys. J. Plus 2018, 133, 131. [Google Scholar] [CrossRef]
- NeuCBOT (Neutron Calculator Based On TALYS). Available online: https://github.com/shawest/neucbot (accessed on 1 July 2021).
- TALYS-Based Evaluated Nuclear Data Library. Available online: https://tendl.web.psi.ch/tendl_2019/talys.html (accessed on 1 July 2021).
- Koning, A.; Rochman, D.; Sublet, J.-C.; Dzysiuk, N.; Fleming, M.; van der Marck, S. TENDL: Complete Nuclear Data Library for Innovative Nuclear Science and Technology. Nucl. Data Sheets 2019, 155, 1–55. [Google Scholar] [CrossRef]
- Pruszkowski, E.; Life, P.E. Total Quant Analysis of Teas and Wines by ICP-MS, Perkin Elmer Life and Analytical Sciences, Field Application Report, ICP Mass Spectrometry, USA. 2004. Available online: https://ru.scribd.com/document/102447386/TotalQuant-Analysis-of-Teas-and-Wines-by-ICP-MS (accessed on 1 July 2021).
- Berglund, M.; Wieser, M.E. Isotopic compositions of the elements 2009 (IUPAC Technical Report). Pure Appl. Chem. 2011, 83, 397–410. [Google Scholar] [CrossRef] [Green Version]
- Wilbur, S.M.; Sugiyama, N.; McCurdy, E. Optimizing Performance for a Collision/Reaction Cell ICP-MS System Operating in Helium Collision Mode. Spectrosc. Appl. ICP ICP-MS Suppl. 2010, 25, 11. Available online: http://www.spectroscopyonline.com/optimizing-performance-collisionreaction-cell-icp-ms-system-operating-helium-collision-mode (accessed on 30 June 2021).
- Zhernokleeva, K.V. Analysis of Rare-Earth Metals and Their Oxides by Atomic-Emission and Mass-Spectrometry Techniques with Inductively Coupled Plasma; Federal State Research and Develpment Institute of Rare Metal Industry “Giredmet”: Russia, Moscow, 2011. [Google Scholar]
- Young, H.A.; Reiber, H.G. Vapor Phase Chlorination of Uranium Oxides; University of California MDDC-1729 Unites States Atomic Comission Energy Technical Information Division, Oak Ridge Directed Operations: Oak Ridge, TN, USA, 1947. [Google Scholar]
- Van Dyke, R.E.; Evers, E.C. Preparation of Uranium Hexachloride. U.S. Patent 2,725,279, 29 November 1955. Available online: https://pdfpiw.uspto.gov/.piw?Docid=02725279&homeurl=http%3A%2F%2Fpatft1.uspto.gov%2Fnetacgi%2Fnph-Parser%3FSect1%3DPTO1%2526Sect2%3DHITOFF%2526d%3DPALL%2526p%3D1%2526u%3D%25252Fnetahtml%25252FPTO%25252Fsrchnum.htm%2526r%3D1%2526f%3DG%2526l%3D50%2526s1%3D2725279.PN.%2526OS%3DPN%2F2725279%2526RS%3DPN%2F2725279&PageNum=&Rtype=&SectionNum=&idkey=NONE&Input=View+first+page (accessed on 1 July 2021).
- Gaede, D. Chlorination and Selective Vaporization of Rare Earth Elements. Master’s Thesis, University of Montana, Missoula, MT, USA, 2016. [Google Scholar]
- Carbaugh, D.J.; Wright, J.T.; Parthiban, R.; Rahman, F. Photolithography with polymethyl methacrylate (PMMA). Semicond. Sci. Technol. 2015, 31, 025010. [Google Scholar] [CrossRef]
- Nijenhuis, D. Their Correlation with Chemical Structure; Their Numerical Estimation and Prediction from Additive Group Contributions, 4th ed.; Elsevier Science: New York, NY, USA, 2009. [Google Scholar]
- Holden, G. Elastomers, thermoplastic, Encyclopedia of polymer science and technology. In Thermoplastic Elastomers, 3rd ed.; Wiley & Sons: Hoboken, NJ, USA, 2004; pp. 63–88. [Google Scholar]
- Binnemans, K. Rare-earth beta-diketonates. In Lanthanides/Actinides: Chemistry; Elsevier BV: Amsterdam, The Netherlands, 2005; pp. 107–272. [Google Scholar]
- Pretsch, E.; Bühlmann, P.; Badertscher, M. Structure Determination of Organic Compounds; Springer Science and Business Media LLC: Berlin, Germany, 2020. [Google Scholar]








| Material | |||||
|---|---|---|---|---|---|
| PMMA | 13.18 | 14.02 | 2.11 | 8.47 | 1.16 |
| PMMA + Gd2O3 (1.5 wt% Gd) | 13.10 | 13.94 | 2.09 | 8.42 | 1.15 |
| PMMA + Gd(C5H7O2)3 (1.5 wt% Gd) | 13.12 | 13.96 | 2.10 | 8.44 | 1.15 |
| PMMA + GdF3 (1.5 wt% Gd, ≈ 0.5 wt% F) | 18.41 | 19.41 | 2.50 | 11.74 | 1.51 |
| Nebulizer type | Concentric (Meinhard), PFA |
| Spray chamber | Scott double-pass chamber, PFA |
| Argon flow rate, L/min | |
| through the nebulizer | 0.96 |
| Plasma-forming | 15 |
| Auxiliary | 1.2 |
| Generator power, W | 1450 |
| Collision gas (He) flow rate, L/min | 4.6 |
| Number of scan cycles | 8 |
| Element | Isotope (Natural Abundance, %) [13] | Interferences | Element | Isotope (Natural Abundance, %) [13] | Interferences |
|---|---|---|---|---|---|
| Mg | 24 (78.99) | 12C12C+ | Fe | 56 (91.72) | 40Ar16O+ |
| Al | 27 (100) | 12C15N+ 12C14N1H+ | Co | 59 (100) | 40Ar18O1H+ |
| K | 39 (93.25) | 38Ar1H+ | Ni | 60 (26.23) | 40Ar18O1H1H+ |
| Ca | 40 (96.941) | 40Ar+ | V | 51 (99.750) | 38Ar12C1H+ |
| Mn | 55 (100) | 40Ar14N1H+ | Cr | 52 (83.789) | 40Ar12C+ |
| Se | 76 (9.36) 77 (7.63) 78 (23.78) 80 (49.61) | 152Gd++ 154Gd++ 156Gd++ 160Gd++ | Yb | 171 (14.3) 172 (21.3) 173 (16.12) 174 (31.8) | 155Gd16O+ 154Gd16O1H+ 156Gd16O+ 155Gd16O1H+ 157Gd16O+ 156Gd16O1H+ 158Gd16O+ 157Gd16O1H+ |
| Dy | 156 (0.06) 158 (0.10) 160 (2.34) 161 (18.91) | 156Gd+ 158Gd+ 160Gd+ 160Gd1H+ | Tb | 159 (100) | 155Gd16O+ 154Gd16O1H+ 156Gd16O+ 155Gd16O1H+ 157Gd16O+ 156Gd16O1H+ 158Gd16O+ 157Gd16O1H+ |
| Sm | 152 (26.75) 154 (22.75) | 152Gd+ 154Gd+ | Tm | 169 (100) | 158Gd1H+ |
| Hf | 174 (0.16) 176 (5.26) 178 (27.28) | 158Gd16O+ 160Gd16O+ 162Gd16O+ | Lu | 175 (97.41) 176 (2.59) | 159Gd16O+ 160Gd16O+ |
| Isotope | Natural Abundance, % | Isotope | Natural Abundance, % |
|---|---|---|---|
| 82Se | 82 | 178Hf | 178 |
| 147Sm | 147 | 188Os | 188 |
| 151Eu | 151 | 194Pt | 194 |
| 163Dy | 163 |
| Compound | ΔH0298, kJ/mole | Tm, K | Tboil., K |
|---|---|---|---|
| ThCl4 | −1189 | 1043 | 1193 |
| UCl3 | −891,2 | 1108 | 1657 |
| UCl4 | −1051 | 863 | 1071 |
| UCl5 | −1094 | Unstable disproportionate | - |
| UCl6 | −1133 | 450.5 | 823 |
| Sample ID | Step | T, K | Time, h | Atmosphere | Concentration, ppb | |
|---|---|---|---|---|---|---|
| Th | U | |||||
| Gd-01 | mix (NH4Cl:GdCl3 = 10:1) | 593 | 3 | HCl, NH3 | 2.45 ± 0.08 | 0.58 ± 0.01 |
| Vacuum annealing | 873 | 6 | 10−5 Torr | |||
| Gd-02 | Atmosphere NH4Cl | 613 | 5 | HCl, NH3 | 3.23 ± 0.12 | 1.21 ± 0.09 |
| Vacuum annealing | 873 | 6 | 10−5 Torr | |||
| Gd-03 | Chlorination | 423 | 3 | HCl | 5.26 ± 0.35 | 1.09 ± 0.02 |
| Vacuum annealing | 873 | 6 | 10−5 Torr | |||
| Gd-04 | Chlorination | 573 | 5 | HCl | 4.30 ± 0.09 | 0.30 ± 0.01 |
| Vacuum annealing | 873 | 9 | 10−5 Torr | |||
| Gd-05 | Chlorination | 773 | 6 | HCl | 0.08 ± 0.01 | 0.09 ± 0.01 |
| Vacuum annealing | 903 | 12 | 10−5 Torr | |||
| Gd-06 | Chlorination | 773 | 9 | HCl | 0.07 ± 0.01 | 0.06 ± 0.01 |
| Vacuum annealing | 903 | 20 | 10−5 Torr | |||
| ID Sample | T K | Time, h | Annealing Condition | CTh, ppb | CU, ppb |
|---|---|---|---|---|---|
| GA-00 | 353 | 10 | air | 0.14 ± 0.02 | 1.60 ± 0.03 |
| GA-01 | 353 | 5 | 10−5 Torr (dynamic) | 0.08 ± 0.01 | 0.40 ± 0.01 |
| GA-02 | 353→423 | 1→3 | 10−5 Torr (dynamic) | <0.016 | <0.011 |
| GA-03 | 353→423→513 | 1→3→3 | 10−5 Torr (dynamic) | <0.016 | <0.011 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zykova, M.; Grishechkin, M.; Khomyakov, A.; Mozhevitina, E.; Avetisov, R.; Surikova, N.; Gromov, M.; Chepurnov, A.; Nikulin, I.; Avetissov, I. Hybrid Ultra-Low-Radioactive Material for Protecting Dark Matter Detector from Background Neutrons. Materials 2021, 14, 3757. https://doi.org/10.3390/ma14133757
Zykova M, Grishechkin M, Khomyakov A, Mozhevitina E, Avetisov R, Surikova N, Gromov M, Chepurnov A, Nikulin I, Avetissov I. Hybrid Ultra-Low-Radioactive Material for Protecting Dark Matter Detector from Background Neutrons. Materials. 2021; 14(13):3757. https://doi.org/10.3390/ma14133757
Chicago/Turabian StyleZykova, Marina, Mikhail Grishechkin, Andrew Khomyakov, Elena Mozhevitina, Roman Avetisov, Nadezda Surikova, Maxim Gromov, Alexander Chepurnov, Ivan Nikulin, and Igor Avetissov. 2021. "Hybrid Ultra-Low-Radioactive Material for Protecting Dark Matter Detector from Background Neutrons" Materials 14, no. 13: 3757. https://doi.org/10.3390/ma14133757
APA StyleZykova, M., Grishechkin, M., Khomyakov, A., Mozhevitina, E., Avetisov, R., Surikova, N., Gromov, M., Chepurnov, A., Nikulin, I., & Avetissov, I. (2021). Hybrid Ultra-Low-Radioactive Material for Protecting Dark Matter Detector from Background Neutrons. Materials, 14(13), 3757. https://doi.org/10.3390/ma14133757