Experimental and Theoretical Study of Radiation Shielding Features of CaO-K2O-Na2O-P2O5 Glass Systems
Abstract
:1. Introduction
2. Materials and Methods
2.1. Methodology for Glasses Preparation
2.2. Radiation Attenuation Coefficient Determination
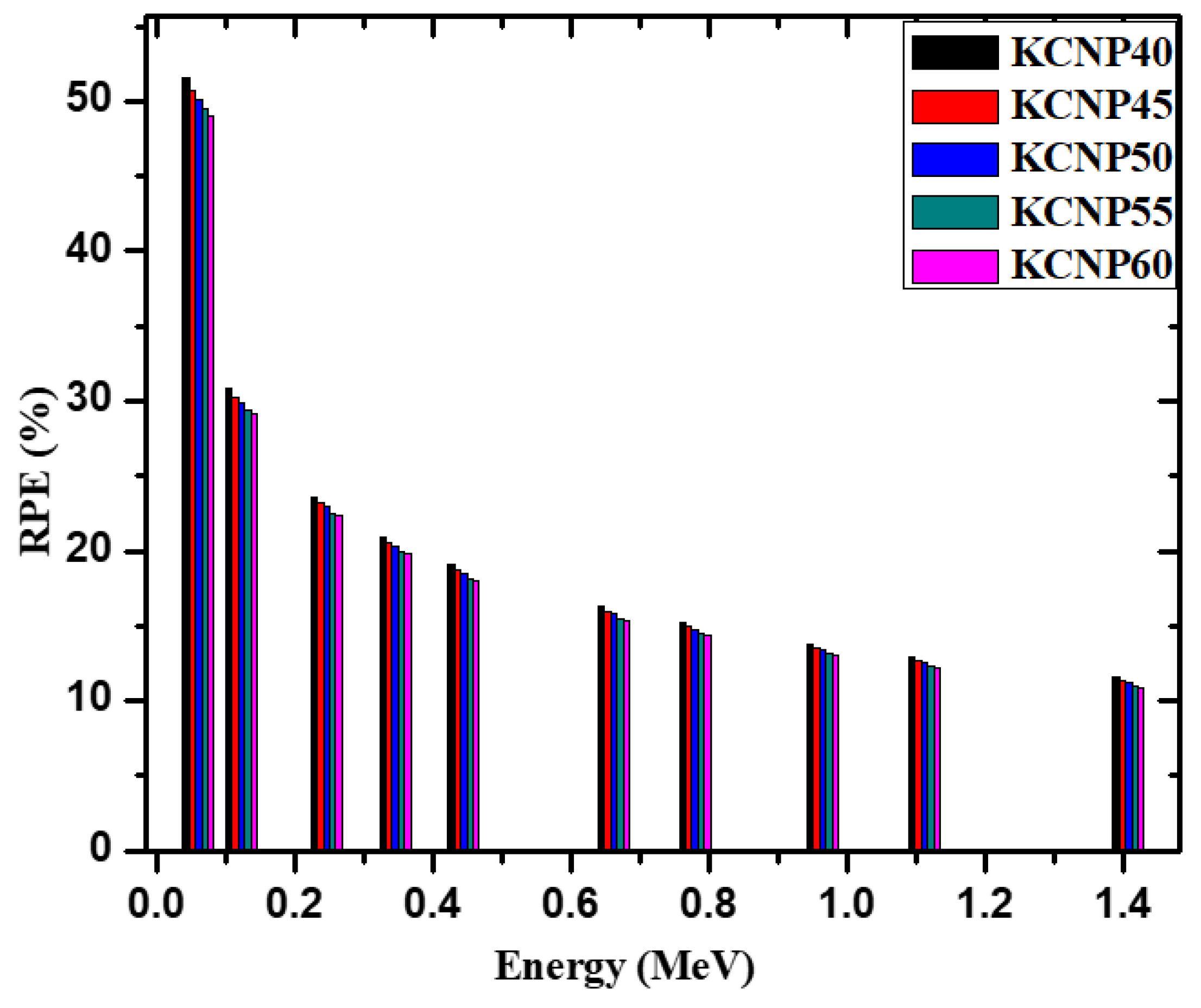
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rachniyom, W.; Chaiphaksa, W.; Limkitjaroeanporn, P.; Tuschaoen, S.; Sangwaranatee, N.; Kaewkhao, J. Effect of Bi2O3 on radiation shielding properties of glasses from coal fly ash. Mater. Today Proc. 2018, 5, 14046–14051. [Google Scholar] [CrossRef]
- Kavaz, E. An experimental study on gamma ray shielding features of lithium borate glasses doped with dolomite, hematite and goethite minerals. Radiat. Phys. Chem. 2019, 160, 112–123. [Google Scholar] [CrossRef]
- Ashok, K. Gamma ray shielding properties of PbO-Li2O-B2O3 glasses. Radiat. Phys. Chem. 2017, 136, 50–53. [Google Scholar]
- Chanthima, N.; Kaewkhao, J.; Limkitjaroenporn, P.; Tuscharoen, S.; Kothan, S.; Tungjai, M.; Kaewjaeng, S.; Sarachai, S.; Limsuwan, P. Development of BaO–ZnO–B2O3 glasses as a radiation shielding material. Radiat. Phys. Chem. 2017, 137, 72–77. [Google Scholar] [CrossRef]
- Sayyed, M.I.; Al-Hadeethi, Y.; AlShammari, M.M.; Ahmed, M.; Al-Heniti, S.H.; Rammah, Y.S. Physical, optical and gamma radiation shielding competence of newly borotellurite based glasses: TeO2–B2O3–ZnO–Li2O3–Bi2O3. Ceram. Int. 2021, 47, 611–618. [Google Scholar] [CrossRef]
- Sayyed, M.I.; Mhareb, M.H.A.; Alajerami, Y.S.M.; Mahmoud, K.A.; Imheidat, M.A.; Alshahri, F.; Alqahtani, M.; Al-Abdullah, T. Optical and radiation shielding features for a new series of borate glass samples. Optik 2021, 239, 166790. [Google Scholar] [CrossRef]
- Kurudirek, M.; Chutithanapanon, N.; Laopaiboon, R.; Yenchai, C.; Bootjomchai, C. Effect of Bi2O3 on gamma ray shielding and structural properties of borosilicate glasses recycled from high pressure sodium lamp glass. J. Alloys Compd. 2018, 745, 355–364. [Google Scholar] [CrossRef]
- Aljawhara, H.; Almuqrin, M.I. Sayyed, Radiation shielding characterizations and investigation of TeO2–WO3–Bi2O3 and TeO2–WO3–PbO glasses. Appl. Phys. A 2021, 127, 190. [Google Scholar]
- Tijani, S.A.; Kamal, S.M.; Al-Hadeethi, Y.; Arib, M.; Hussein, M.A.; Wageh, S.; Dim, L.A. Radiation shielding properties of transparent erbium zinc tellurite glass system determined at medical diagnostic energies. J. Alloys Compd. 2018, 741, 293–299. [Google Scholar] [CrossRef]
- El-Samrah, M.G.; Abdel-Rahman, M.A.; El Shazly, R.M. Effect of heating on physical, mechanical, and nuclear radiation shielding properties of modified concrete mixes. Radiat. Phys. Chem. 2018, 153, 104–110. [Google Scholar] [CrossRef]
- Roman, J.; Michał, A.G.; Wojciech, K.; Mariusz, D. Application of a non-stationary method in determination of the thermal properties of radiation shielding concrete with heavy and hydrous aggregate. Int. J. Heat Mass Transf. 2019, 130, 882–892. [Google Scholar]
- Reza, B.; Alireza, K.M.; Seyed, P.S.; Bakhtiar, A.; Mojtaba, S. Determination of gamma-ray shielding properties for silicate glasses containing Bi2O3, PbO, and BaO. J. Non-Cryst. Solids 2018, 479, 62–71. [Google Scholar]
- Halimah, M.K.; Azuraida, A.; Ishak, M.; Hasnimulyati, L. Influence of bismuth oxide on gamma radiation shielding properties of borotellurite glass. J. Non-Cryst. Solids 2019, 512, 140–147. [Google Scholar] [CrossRef]
- Sayyed, M.I.; Mahmoud, K.A.; Lacomme, E.; AlShammari, M.M.; Dwaikat, N.; Alajerami, Y.S.M.; Alqahtani, M.; El-bashir, B.O.; Mhareb, M.H.A. Development of a novel MoO3-doped borate glass network for gamma-ray shielding applications. Eur. Phys. J. Plus 2021, 136, 108. [Google Scholar] [CrossRef]
- Sayyed, M.I.; Mahmoud, K.A.; Tashlykov, O.L.; Khandaker, M.U.; Faruque, M.R.I. Enhancement of the Shielding Capability of Soda–Lime Glasses with Sb2O3 Dopant: A Potential Material for Radiation Safety in Nuclear Installations. Appl. Sci. 2021, 11, 326. [Google Scholar] [CrossRef]
- Sayyed, M.I.; Olarinoye, O.I.; Elsafi, M. Assessment of gamma-radiation attenuation characteristics of Bi2O3–B2O3–SiO2–Na2O glasses using Geant4 simulation code. Eur. Phys. J. Plus 2021, 136, 535. [Google Scholar] [CrossRef]
- Hasnaoui, S.; Sdiri, N.; Horchani-Naifer, K.; Férid, M. Study of the physical properties of 90 P2O5 + xV2O5 (10-x) BaO (0≤x≤3%) glasses. Sens. Actuators A 2021, 319, 112540. [Google Scholar] [CrossRef]
- Sastry, S.S.; Rupa, B.; Rao, B.R.V. Structural and optical properties of vanadium doped alkaline earth lead zinc phosphate glasses. Indian J. Pure Appl. Phys. 2014, 52, 491–498. [Google Scholar]
- Sayyed, M.I.; Almuqrin, A.H.; Kurtulus, R.; Javier-Hila, A.M.V.; Kaky, K.; Kavas, T. X-ray shielding characteristics of P2O5–Nb2O5 glass doped with Bi2O3 by using EPICS2017 and Phy-X/PSD. Appl. Phys. A 2021, 127, 243. [Google Scholar] [CrossRef]
- Alotaibi, B.M.; Abouhaswa, A.S.; Sayyed, M.I.; Mahmoud, K.A.; Al-Yousef, H.A.; Hila, F.C.; Al-Hadeethi, Y. Structural, optical, and gamma-ray shielding properties of a newly fabricated P2O5–B2O3–Bi2O3–Li2O–ZrO2 glass system. Eur. Phys. J. Plus 2021, 136, 224. [Google Scholar] [CrossRef]
- Aygün, B. High alloyed new stainless steel shielding material for gamma and fast neutron radiation. Nucl. Eng. Technol. 2020, 52, 647–653. [Google Scholar] [CrossRef]
- Dong, M.; Zhou, S.; Xue, X.; Feng, X.; Sayyed, M.I.; Khandaker, M.U.; Bradley, D.A. The potential use of boron containing resources for protection against nuclear radiation. Radiat. Phys. Chem. 2021, 188, 109601. [Google Scholar] [CrossRef]
- Sayyed, M.I.; Jecong, J.F.M.; Hila, F.C.; Balderas, C.V.; Alhuthali, A.M.S.; Guillermo, N.R.D.; Al-Hadeethi, Y. Radiation shielding characteristics of selected ceramics using the EPICS2017 library. Ceram. Int. 2021, 47, 13181–13186. [Google Scholar] [CrossRef]
- Sayyed, M.I.; Kumar, A.; Alhuthali, A.M.S.; Mahmoud, K.A.; Al-Hadeethi, Y. Tailoring Dy3+/Tb3+-doped lead telluride borate glasses for gamma-ray shielding applications. Eur. Phys. J. Plus 2021, 136, 1–16. [Google Scholar]
- Almuqn, A.H.; Sayyed, M.I.; Kumar, A.; El-bashir, B.O.; Akkurt, I. Optical, mechanical properties and gamma ray shielding behavior of TeO2-Bi2O3-PbO-MgO-B2O3 glasses using FLUKA simulation code. Opt. Mater. 2021, 113, 110900. [Google Scholar] [CrossRef]
- Al-Harbi, N.; Sayyed, M.I.; Kurtulus, R.; Kamışlıoğlu, M.; Kumar, A.; Alhuthali, A.M.; Kavas, T.; Al-Hadeethi, Y. Understanding the role of Bi2O3 in the P2O5–CaO–Na2O–K2O glass system in terms of physical, structural and radiation shielding properties. J. Mater. Sci. Mater. Electron. 2021, 32, 11649–11665. [Google Scholar] [CrossRef]
- Abbas, M.I.; Elsafi, M. NaI cubic detector full-energy peak efficiency, including coincidence and self-absorption corrections for rectangular sources using analytical method. J. Radioanal. Nucl. Chem. 2021, 327, 251–258. [Google Scholar] [CrossRef]
- Badawi, M.; Noureddine, S.; Kopatch, Y.; Abbas, M.; Ruskov, I.; Grozdanov, D.; Thabet, A.; Fedorov, N.; Gouda, M.; Hramco, C.; et al. Characterization of the efficiency of a cubic NaI detector with rectangular cavity for axially positioned sources. J. Instrum. 2020, 15, P0201. [Google Scholar] [CrossRef]
- Abbas, M.I.; Badawi, M.S.; Thabet, A.A.; Kopatch, Y.N.; Ruskov, I.N.; Grozdanov, D.N.; Noureddine, S.; Fedorov, N.A.; Gouda, M.M.; Hramco, C. Efficiency of a cubic NaI(Tl) detector with rectangular cavity using standard radioactive point sources placed at non-axial position. Appl. Radiat. Isot. 2020, 163, 109139. [Google Scholar] [CrossRef]
- Alharshan, G.A.; Aloraini, D.A.; Elzaher, M.A.; Badawi, M.S.; Alabsy, M.T.; Abbas, M.I.; El-Khatib, A.M. A comparative study between nano-cadmium oxide and lead oxide reinforced in high density polyethylene as gamma rays shielding composites. Nucl. Technol. Radiat. Prot. 2020, 35, 42–49. [Google Scholar] [CrossRef]
- Mahmoud, K.A.; Sayyed, M.I.; Tashlykov, O.L. Gamma ray shielding characteristics and exposure buildup factor for some natural rocks using MCNP-5 code. Nucl. Eng. Technol. 2019, 51, 1835–1841. [Google Scholar] [CrossRef]
- Kiani, M.A.; Ahmadi, S.J.; Outokesh, M.; Adeli, R.; Kiani, H. Study on physico-mechanical and gamma-ray shielding characteristics of new ternary nanocomposites. Appl. Radiat. Isot. 2019, 143, 141–148. [Google Scholar] [CrossRef]
- Rammah, Y.S.; Ali, A.A.; El-Mallawany, R.; El-Agawany, F.I. Fabrication, physical, optical characteristics and gamma-ray competence of novel bismo-borate glasses doped with Yb2O3 rare earth. Phys. B Condens. Matter. 2020, 583, 412055. [Google Scholar] [CrossRef]
- Akman, F.; Khattari, Z.Y.; Kaçal, M.R.; Sayyed, M.I.; Afaneh, F. The radiation shielding features for some silicide, boride and oxide types ceramics. Radiat. Phys. Chem. 2019, 160, 9–14. [Google Scholar] [CrossRef]
- Mhareb, M.H.A. Physical, optical and shielding features of Li2O–B2O3–MgO–Er2O3 glasses co-doped of Sm2O3. Appl. Phys. A 2020, 126, 71. [Google Scholar] [CrossRef]
- Mengge, D.; Xiangxin, X.; He, Y.; Zhefu, L. Highly cost-effective shielding composite made from vanadium slag and boron-rich slag and its properties. Radiat. Phys. Chem. 2017, 141, 239–244. [Google Scholar]
- Dong, M.; Xue, X.; Yang, H.; Liu, D.; Wang, C.; Li, Z. A novel comprehensive utilization of vanadium slag: As gamma ray shielding material. J. Hazard. Mater. 2016, 318, 751–757. [Google Scholar] [CrossRef]
- Yasmin, S.; Barua, B.S.; Khandaker, M.U.; Rashid, M.A.; Bradley, D.A.; Olatunji, M.A.; Kamal, M. Studies of ionizing radiation shielding effectiveness of silica-based commercial glasses used in Bangladeshi dwellings. Results Phys. 2018, 9, 541–549. [Google Scholar] [CrossRef]
- Speit, B. Radiation-shielding Glasses Providing Safety Against Electrical Discharge and Being Resistant to Discoloration, 1991 Google Patents. Available online: https://patents.google.com/patent/US5073524A/en (accessed on 2 July 2021).
- Yasmin, S.; Rozaila, Z.S.; Khandaker, M.U.; Barua, B.S.; Chowdhury, F.U.Z.; Rashid, M.A.; Bradley, D.A. The radiation shielding offered by the commercial glass installed in Bangladeshi dwellings. Radiat. Effects Defects Solids 2018, 173, 657–672. [Google Scholar] [CrossRef]


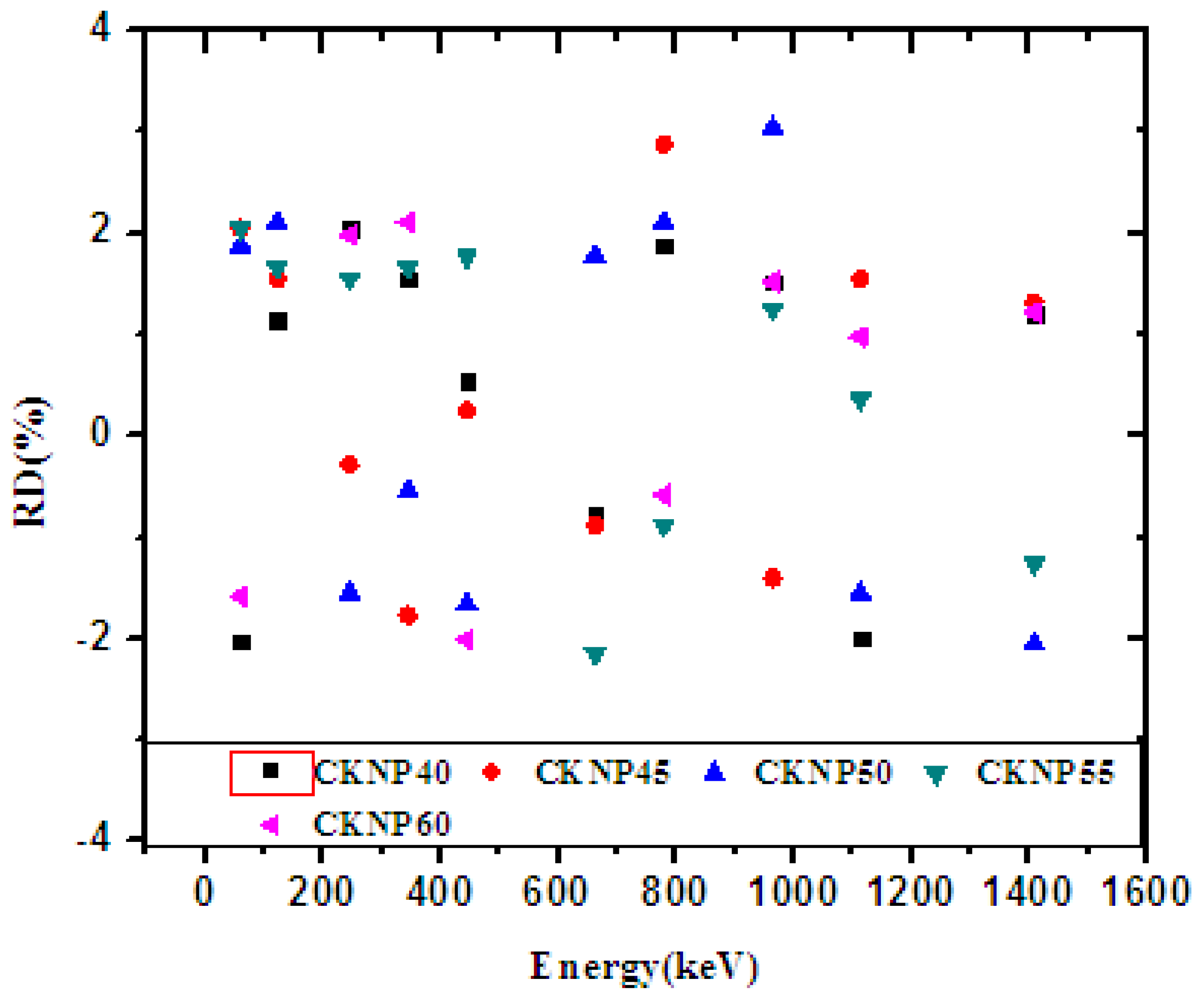
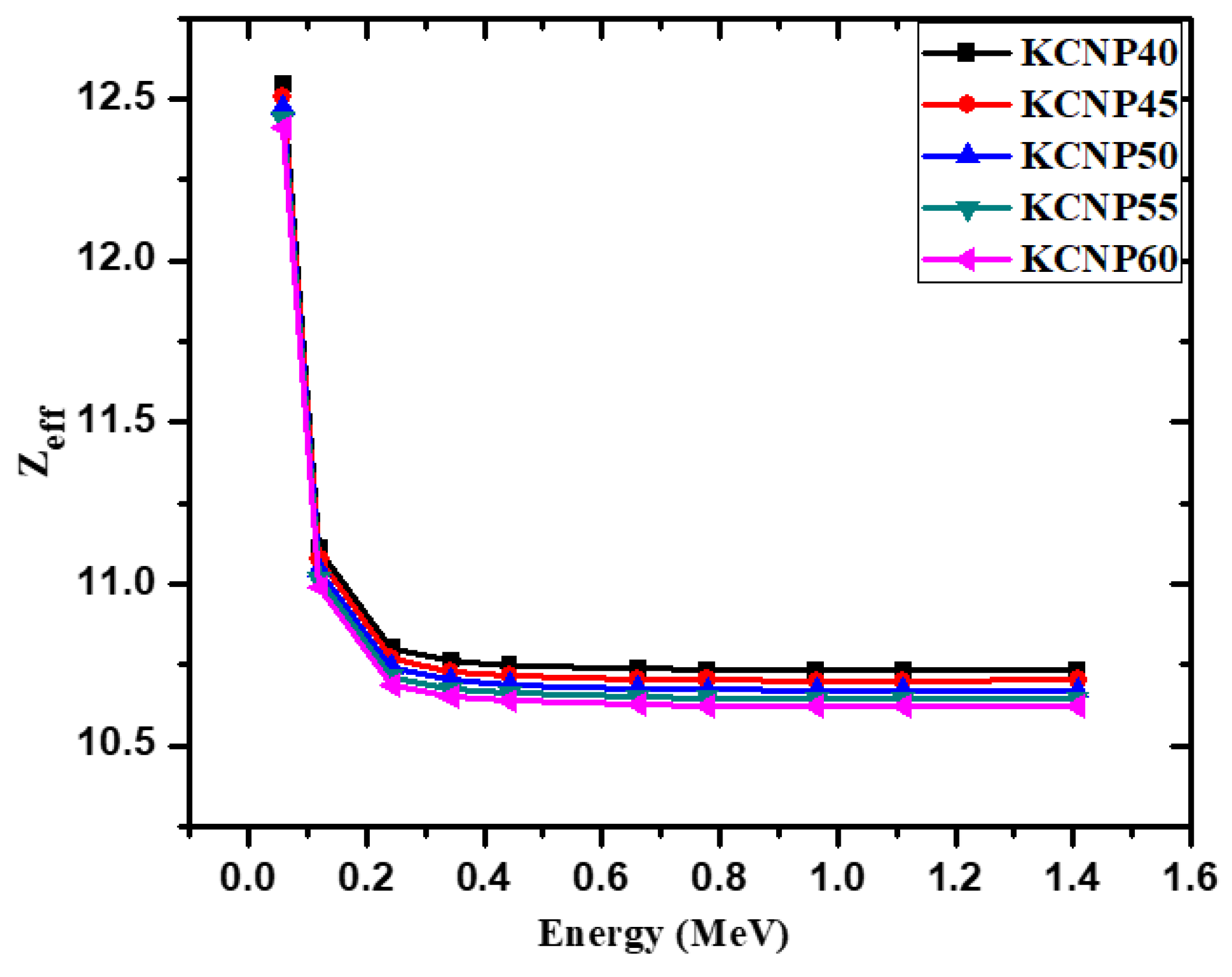
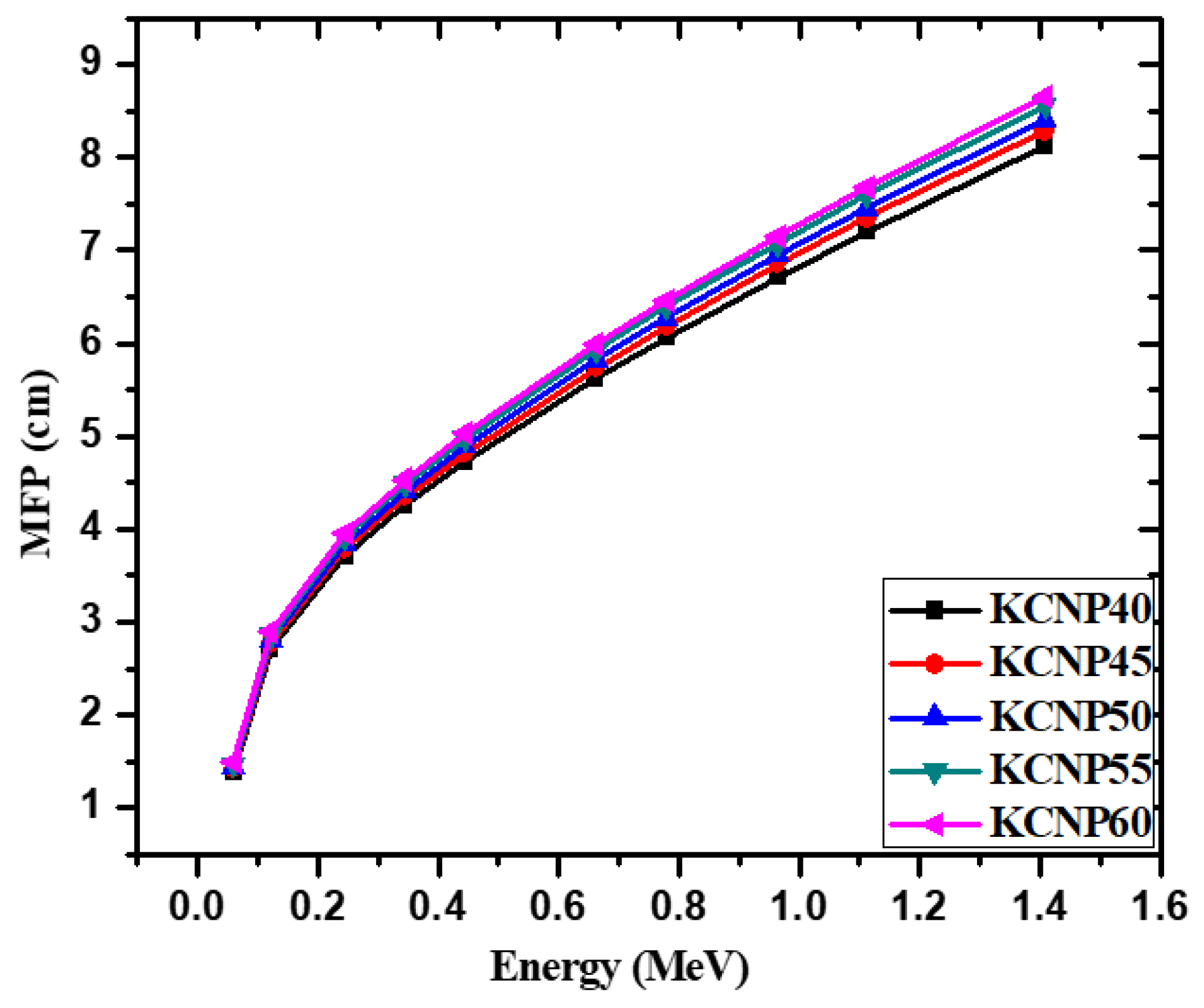
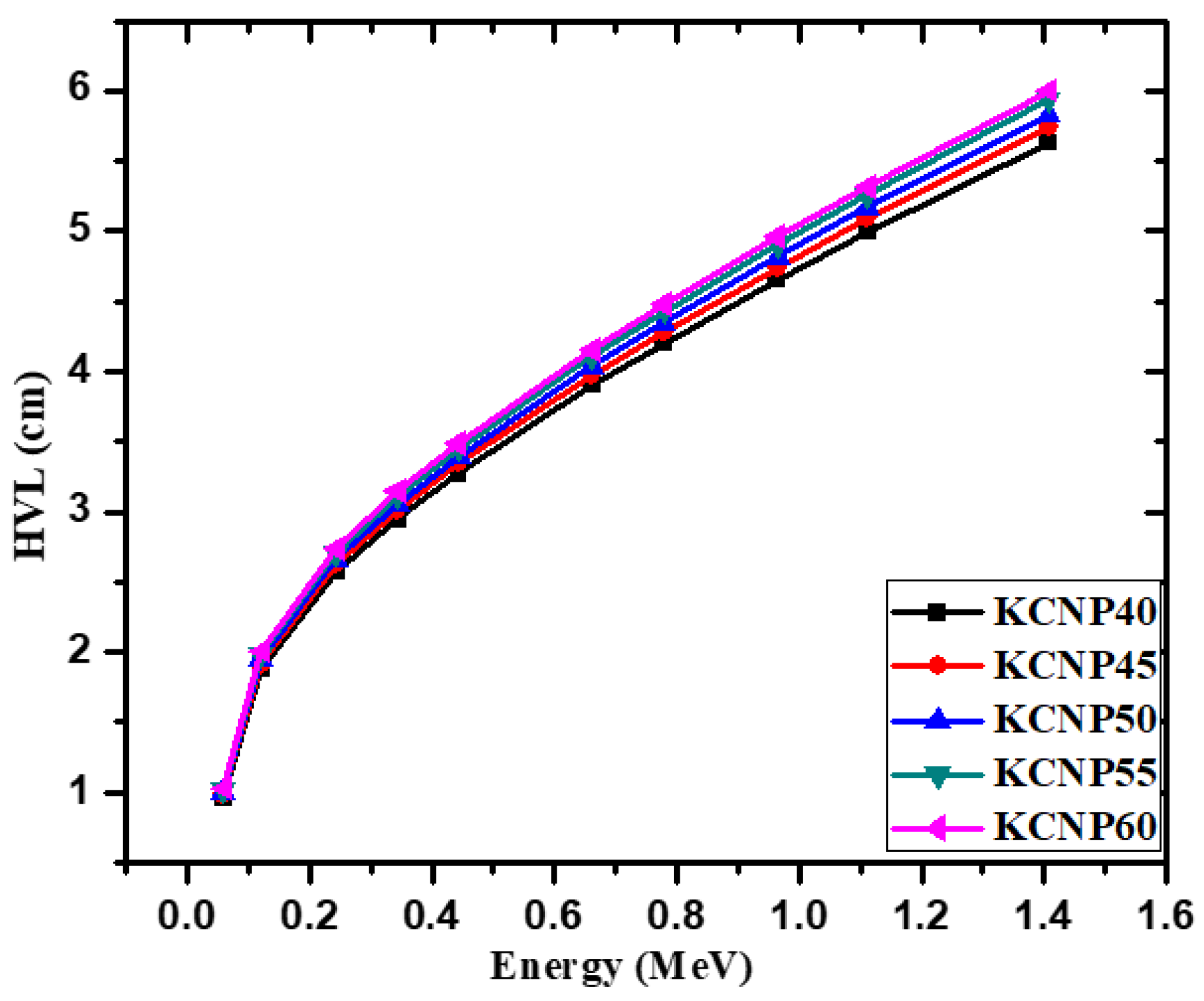

| Energy (keV) | CKNP40 | CKNP45 | CKNP50 | CKNP55 | CKNP60 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Experimental | XCOM | Experimental | XCOM | Experimental | XCOM | Experimental | XCOM | Experimental | XCOM | |
| 59.54 | 0.317 ± 0.030 | 0.310 | 0.303 ± 0.022 | 0.310 | 0.303 ± 0.038 | 0.309 | 0.302 ± 0.041 | 0.309 | 0.313 ± 0.022 | 0.308 |
| 121.80 | 0.156 ± 0.011 | 0.158 | 0.155 ± 0.011 | 0.158 | 0.155 ± 0.026 | 0.158 | 0.155 ± 0.033 | 0.158 | 0.052 ± 0.030 | 0.158 |
| 244.70 | 0.113 ± 0.009 | 0.116 | 0.116 ± 0.025 | 0.116 | 0.117 ± 0.014 | 0.116 | 0.114 ± 0.038 | 0.116 | 0.113 ± 0.028 | 0.116 |
| 344.30 | 0.099 ± 0.021 | 0.101 | 0.103 ± 0.040 | 0.101 | 0.101 ± 0.025 | 0.101 | 0.099 ± 0.022 | 0.101 | 0.099 ± 0.021 | 0.101 |
| 444.00 | 0.090 ± 0.035 | 0.091 | 0.091 ± 0.009 | 0.091 | 0.092 ± 0.014 | 0.091 | 0.089 ± 0.030 | 0.091 | 0.093 ± 0.019 | 0.091 |
| 661.70 | 0.077 ± 0.007 | 0.076 | 0.077 ± 0.026 | 0.076 | 0.075 ± 0.022 | 0.076 | 0.078 ± 0.025 | 0.076 | 3.819 ± 0.017 | 0.076 |
| 778.90 | 0.069 ± 0.008 | 0.071 | 0.069 ± 0.031 | 0.071 | 0.069 ± 0.027 | 0.071 | 0.071 ± 0.018 | 0.071 | 0.071 ± 0.028 | 0.071 |
| 964.10 | 0.063 ± 0.025 | 0.064 | 0.065 ± 0.030 | 0.064 | 0.062 ± 0.013 | 0.064 | 0.063 ± 0.012 | 0.064 | 0.063 ± 0.012 | 0.064 |
| 1112.00 | 0.061 ± 0.009 | 0.059 | 0.059 ± 0.024 | 0.060 | 0.061 ± 0.009 | 0.060 | 0.059 ± 0.014 | 0.060 | 0.059 ± 0.010 | 0.060 |
| 1408.00 | 0.052 ± 0.011 | 0.053 | 0.052 ± 0.009 | 0.053 | 0.054 ± 0.010 | 0.053 | 0.302 ± 0.041 | 0.309 | 0.313 ± 0.022 | 0.308 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sayyed, M.I.; Albarzan, B.; Almuqrin, A.H.; El-Khatib, A.M.; Kumar, A.; Tishkevich, D.I.; Trukhanov, A.V.; Elsafi, M. Experimental and Theoretical Study of Radiation Shielding Features of CaO-K2O-Na2O-P2O5 Glass Systems. Materials 2021, 14, 3772. https://doi.org/10.3390/ma14143772
Sayyed MI, Albarzan B, Almuqrin AH, El-Khatib AM, Kumar A, Tishkevich DI, Trukhanov AV, Elsafi M. Experimental and Theoretical Study of Radiation Shielding Features of CaO-K2O-Na2O-P2O5 Glass Systems. Materials. 2021; 14(14):3772. https://doi.org/10.3390/ma14143772
Chicago/Turabian StyleSayyed, M. I., Badriah Albarzan, Aljawhara H. Almuqrin, Ahmed M. El-Khatib, Ashok Kumar, Daria I. Tishkevich, Alex V. Trukhanov, and Mohamed Elsafi. 2021. "Experimental and Theoretical Study of Radiation Shielding Features of CaO-K2O-Na2O-P2O5 Glass Systems" Materials 14, no. 14: 3772. https://doi.org/10.3390/ma14143772
APA StyleSayyed, M. I., Albarzan, B., Almuqrin, A. H., El-Khatib, A. M., Kumar, A., Tishkevich, D. I., Trukhanov, A. V., & Elsafi, M. (2021). Experimental and Theoretical Study of Radiation Shielding Features of CaO-K2O-Na2O-P2O5 Glass Systems. Materials, 14(14), 3772. https://doi.org/10.3390/ma14143772











