Qualitative Analysis of Remineralization Capabilities of Bioactive Glass (NovaMin) and Fluoride on Hydroxyapatite (HA) Discs: An In Vitro Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Specimen Preparation and Treatment
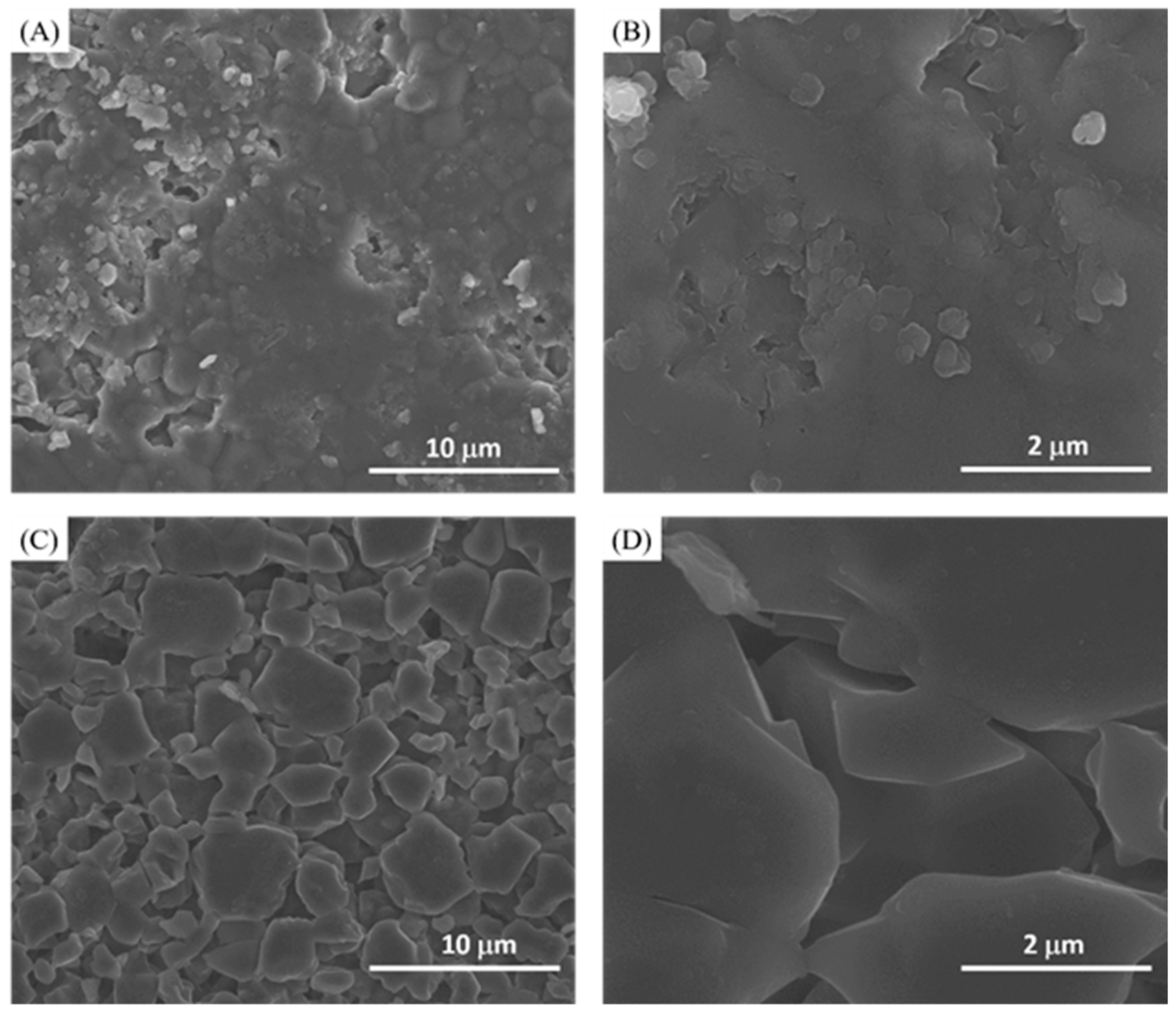
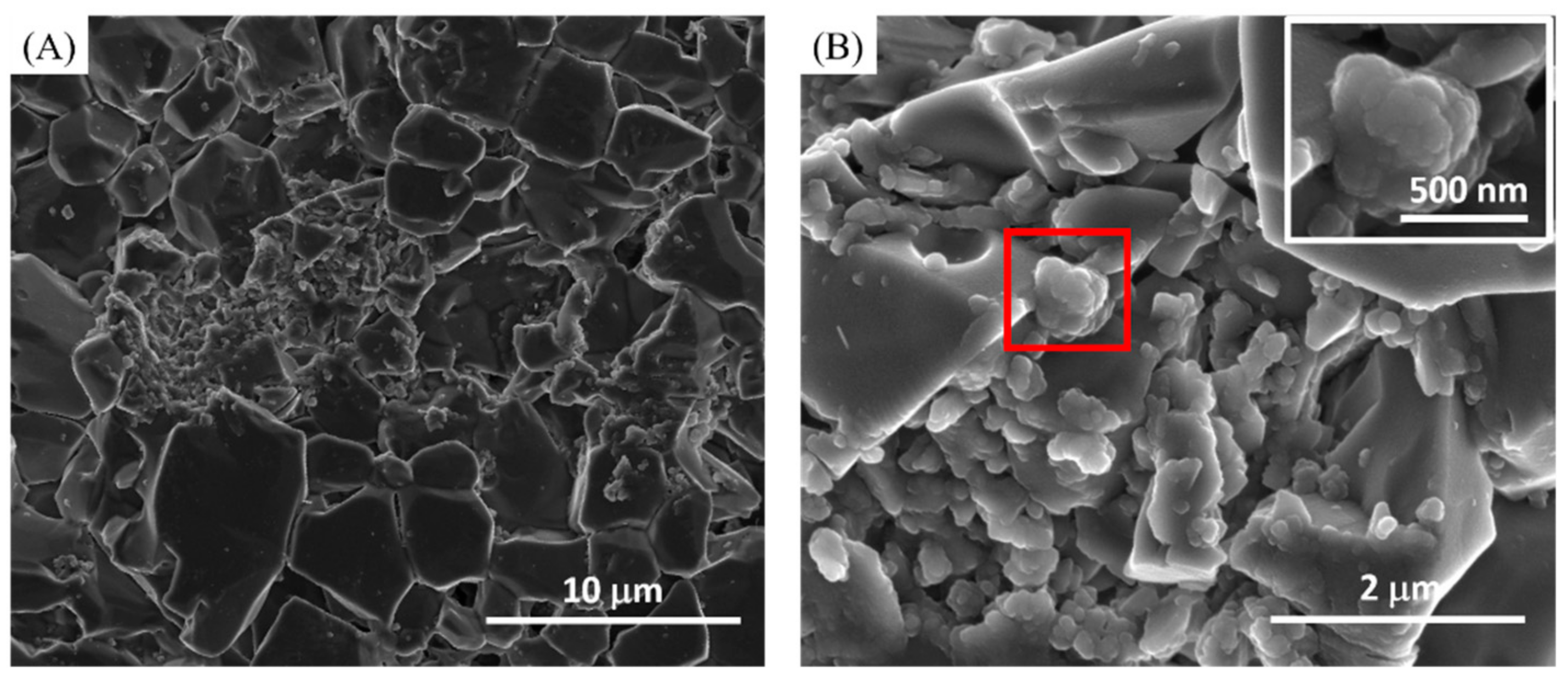
2.2. Scanning Electron Microscopy (SEM) Analysis
2.3. X-ray Photoelectron Spectroscopy Analysis (XPS)
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Higham, S.; Hope, C.; Valappil, S.; Smith, P. Caries Process and Prevention Strategies: The Agent—A Multi Factorial Disease. Available online: https://www.dentalcare.com/en-us/professional-education/ce-courses/ce369 (accessed on 7 July 2021).
- Featherstone, J. The Continuum of Dental Caries—Evidence for a Dynamic Disease Process. J. Dent. Res. 2004, 83, 39–42. [Google Scholar] [CrossRef]
- Edelstein, B.L. The Dental Caries Pandemic and Disparities Problem. BMC Oral Health 2006, 6, S2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- U.S. Department of Health and Human Services. Oral Health in America: A Report of the Surgeon General; U.S. Department of Health and Human Services, National Institute of Dental and Craniofacial Research, National Institutes of Health: Rockville, MD, USA, 2000.
- Margolis, H.C.; Zhang, Y.P.; Van Houte, J.; Moreno, E.C. Effect of Sucrose Concentration on the Cariogenic Potential of Pooled Plaque Fluid from Caries-Free and Caries-Positive Individuals. Caries Res. 1993, 27, 467–473. [Google Scholar] [CrossRef]
- Ran, T.; Chattopadhyay, S.K.; Force, C.P.S.T. Economic Evaluation of Community Water Fluoridation: A Community Guide Systematic Review. Am. J. Prev. Med. 2016, 50, 790–796. [Google Scholar] [CrossRef]
- Cate, J.M.T. Contemporary perspective on the use of fluoride products in caries prevention. Br. Dent. J. 2013, 214, 161–167. [Google Scholar] [CrossRef]
- ADA. Oral Health Topics. Topical and Systemic Supplements. Available online: https://www.ada.org/en/member-center/oral-health-topics/fluoride-topical-and-systemic-supplements (accessed on 12 July 2020).
- Council, N.R. Chapter: 7 Neurotoxicity and Neurobe-Havioral Effects. In Fluoride in Drinking Water: A Scientific Review of EPA’s Standards; The National Academies Press: Washington, DC, USA, 2006; pp. 205–223. [Google Scholar]
- Choi Anna, L.; Sun, G.; Zhang, Y.; Grandjean, P. Developmental Fluoride Neurotoxicity: A Systematic Review and Meta-Analysis. Environ. Health Perspect. 2012, 120, 1362–1368. [Google Scholar] [CrossRef] [Green Version]
- Rahaman, M.N.; Day, D.E.; Sonny Bal, B.; Fu, Q.; Jung, S.B.; Bonewald, L.F.; Tomsia, A.P. Bioactive glass in tissue engineering. Acta Biomater. 2011, 7, 2355–2373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baino, F.; Hamzehlou, S.; Kargozar, S. Bioactive Glasses: Where Are We and Where Are We Going? J. Funct. Biomater. 2018, 9, 25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jones, J.R. Review of bioactive glass: From Hench to hybrids. Acta Biomater. 2013, 9, 4457–4486. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.Z.; Thompson, I.D.; Boccaccini, A.R. 45S5 Bioglass®-derived glass–ceramic scaffolds for bone tissue engineering. Biomaterials 2006, 27, 2414–2425. [Google Scholar] [CrossRef] [PubMed]
- GSK. Novamin Technology Science. Available online: https://www.gskhealthpartner.com/en-gb/oral-health/brands/sensodyne/science/novamin/ (accessed on 7 July 2021).
- Palaniswamy, U.K.; Prashar, N.; Kaushik, M.; Lakkam, S.R.; Arya, S.; Pebbeti, S. A comparative evaluation of remineralizing ability of bioactive glass and amorphous calcium phosphate casein phosphopeptide on early enamel lesion. Dent. Res. J. 2016, 13, 297. [Google Scholar] [CrossRef]
- Dai, L.L.; Mei, M.L.; Chu, C.H.; Lo, E.C.M. Mechanisms of Bioactive Glass on Caries Management: A Review. Materials 2019, 12, 4183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bakry, A.; Takahashi, H.; Otsuki, M.; Tagami, J. Evaluation of new treatment for incipient enamel demineralization using 45S5 bioglass. Dent. Mater. 2014, 30, 314–320. [Google Scholar] [CrossRef] [PubMed]
- Gocha, A. BioMin Bioglass Toothpaste May Better Protect Sensitive Teeth and Find Its Way into US Market. Available online: https://ceramics.org/ceramic-tech-today/biomaterials/biomin-bioglass-toothpaste-may-better-protect-sensitive-teeth-and-find-its-way-into-us-market (accessed on 7 July 2021).
- BMR Plus. First ever fluoride-containing bioglass toothpaste wins FDA approval. Br. Dent. J. 2021, 230, 179. [Google Scholar] [CrossRef]
- Featherstone, J.D.B. Dental caries: A dynamic disease process. Aust. Dent. J. 2008, 53, 286–291. [Google Scholar] [CrossRef] [PubMed]
- Thomas, M.V.; Puleo, D.A.; Al-Sabbagh, M. Bioactive Glass Three Decades On. J. Autom. Inf. Sci. 2005, 15, 585–597. [Google Scholar] [CrossRef]
- Min, Q.D.; Bian, Z.; Jiang, H.; Greenspan, D.C.; Burwell, A.K.; Zhong, J.; Tai, B.J. Clinical evaluation of a dentifrice containing calcium sodium phosphosilicate (novamin) for the treatment of dentin hypersensitivity. Am. J. Dent. 2008, 21, 210–214. [Google Scholar]
- Burwell, A.; Jennings, D.; Muscle, D.; Greenspan, D.C. NovaMin and dentin hypersensitivity—In vitro evidence of efficacy. J. Clin. Dent. 2010, 21, 66–71. [Google Scholar]
- Zero, D.T. Dentifrices, mouthwashes, and remineralization/caries arrestment strategies. BMC Oral Health 2006, 6, S9. [Google Scholar] [CrossRef] [Green Version]
- Fincham, A.; Moradian-Oldak, J.; Simmer, J. The Structural Biology of the Developing Dental Enamel Matrix. J. Struct. Biol. 1999, 126, 270–299. [Google Scholar] [CrossRef]
- Robinson, C.; Brookes, S.j.; Shore, R.C.; Kirkham, J. The developing enamel matrix: Nature and function. J. Oral Sci. 1998, 106, 282–291. [Google Scholar] [CrossRef] [PubMed]
- Vranic, E.; Lacevic, A.; Mehmedagic, A.; Uzunovic, A. Formulation ingredients for tootpates and mouthwashes. Bosn. J. Basic Med. Sci. 2004, 4, 51–58. [Google Scholar] [CrossRef]
- Hamza, B.; Tanner, M.; Attin, T.; Wegehaupt, F.J. Dentin Abrasivity and Cleaning Efficacy of Novel/Alternative Tooth-pastes. Oral Health Prev. Dent. 2020, 18, 713–718. [Google Scholar] [PubMed]
- Golpayegani, M.V.; Sohrabi, A.; Biria, M.; Ansari, G. Remineralization Effect of Topical NovaMin Versus Sodium Fluoride (1.1%) on Caries-Like Lesions in Permanent Teeth. J. Dent.) 2012, 9, 68–75. [Google Scholar]
- Burwell, A.K.; Litkowski, L.J.; Greenspan, D.C. Calcium Sodium Phosphosilicate (NovaMin®): Remineralization Potential. Adv. Dent. Res. 2009, 21, 35–39. [Google Scholar] [CrossRef] [PubMed]
- Aasenden, R.; Brudevold, F.; Richardson, B. Clearance of fluoride from the mouth after topical treatment or the use of a fluoride mouthrinse. Arch. Oral Biol. 1968, 13, 625–636. [Google Scholar] [CrossRef]
- Farooq, I.; Majeed, A.; AlShwaimi, E.; Dammam, K.A. Efficacy of A Novel Fluoride Containing Bioactive Glass Based Dentifrice In Remineralizing Artificially Induced Demineralization In Human Enamel. Fluoride 2019, 52, 447–455. [Google Scholar]
- Andersson, O.H.; Kangasniemi, I. Calcium phosphate formation at the surface of bioactive glass in vitro. J. Biomed. Mater. Res. 1991, 25, 1019–1030. [Google Scholar] [CrossRef]
- Shah, F.A.; Brauer, D.S.; Desai, N.; Hill, R.G.; Hing, K.A. Fluoride-containing bioactive glasses and Bioglass® 45S5 form apatite in low pH cell culture medium. Mater. Lett. 2014, 119, 96–99. [Google Scholar] [CrossRef]
- Ali, S.; Farooq, I.; Al-Thobity, A.M.; Al-Khalifa, K.; Alhooshani, K.; Sauro, S. An in-vitro evaluation of fluoride content and enamel remineralization potential of two toothpastes containing different bioactive glasses. Biomed. Mater. Eng. 2020, 30, 487–496. [Google Scholar] [CrossRef]
- Mony, S.; Rao, A.; Shenoy, R.; Suprabha, B.S. Comparative evaluation of the remineralizing efficacy of calcium sodium phosphosilicate agent and fluoride based on quantitative and qualitative analysis. J. Indian Soc. Pedod. Prev. Dent. 2015, 33, 291–295. [Google Scholar] [CrossRef] [PubMed]
- Körner, P.; Schleich, A.J.; Wiedemeier, D.B.; Attin, T.; Wegehaupt, F.J. Effects of Additional Use of Bioactive Glasses or a Hydroxyapatite Toothpaste on Remineralization of Artificial Lesions in vitro. Caries Res. 2020, 54, 1–7. [Google Scholar] [CrossRef]
- Raikar, G.N.; Ong, J.L.; Lucas, L.C. Hydroxyapatite Characterized by XPS. Surf. Sci. Spectra 1996, 4, 9–13. [Google Scholar] [CrossRef]
- Food and Drug Administration. Anticaries Drug Products for Over-the-Counter Human Use; Final Monograph. Fed. Regist. 1995, 60, 52474–52510. [Google Scholar]




| Groups | Toothpaste | Ingredients |
|---|---|---|
| Group 1: 1–5 HA etched discs | Sensodyne with fluoride | Active ingredient: Stannous fluoride, 0.454% (0.15% w/v fluoride ion). Antihypersensitivity, anticavity. Inactive ingredients: Hydrated silica, glycerin, polyacrylic acid, pentasodium triphosphate, sodium saccharin, sodium lauryl sulfate, titanium dioxide, PEG-8, cocamidopropyl betaine, flavor. |
| Group 2: 6–10 HA etched discs | Sensodyne with fluoride and NovaMin | Medical ingredients: Calcium sodium phosphosilicate (Novamin) 5.0% w/w, sodium monofluorophosphate 0.788% w/w (fluoride 0.104% w/w). Nonmedical ingredients: (Alpha) carbomer, DL-Limonene, glycerin, linalool, mint flavor, PEG-8, silica, sodium lauryl sulfate, sodium saccharin, titanium dioxide. |
| Group 3: 11–15 HA etched discs | Tom’s with no fluoride or NovaMin | Arginine bicarbonate, calcium carbonate, hydrated silica, xanthan, peppermint oil, benzyl alcohol, sodium bicarbonate, sodium lauryl sulfate, titanium dioxide, water, gum, xylitol, sorbitol. |
| Group 4: 16–20 HA etched discs | Tom’s with NovaMin | Tom’s: Arginine bicarbonate, calcium carbonate, hydrated silica, sodium bicarbonate, sodium lauryl sulfate, peppermint oil, benzyl alcohol, sorbitol, titanium dioxide, water, xanthan gum, xylitol. Bioactive glass (NovaMin): The powder ingredient is calcium sodium phosphosilicate. The glass is composed of 45 wt% SiO2, 24.5 wt% Na2O, 24.5 wt% CaO, and 6.0 wt% P2O5. |
| Group | Samples | O 1s | Ca 2p | P 2p | Na 1s | Si 2s | N 1s | S 2p | Ca/P |
|---|---|---|---|---|---|---|---|---|---|
| Control | Control | 59.48 | 20.05 | 12.41 | 5.06 | 3.00 | 1.62 | ||
| Group 1 | Sensodyne with fluoride | 62.66 | 18.30 | 12.71 | 1.18 | 4.56 | 0.59 | 1.44 | |
| Group 2 | Sensodyne with fluoride and NovaMin | 60.13 | 21.39 | 13.00 | 2.97 | 1.27 | 0.51 | 0.73 | 1.65 |
| Group 3 | Tom’s with no fluoride or NovaMin | 55.7 | 18.68 | 9.82 | 9.27 | 2.29 | 4.24 | 1.90 | |
| Group 4 | Tom’s with NovaMin | 57.69 | 24.32 | 14.11 | 0.66 | 2.68 | 0.54 | 1.72 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsu, S.-M.; Alsafadi, M.; Vasconez, C.; Fares, C.; Craciun, V.; O’Neill, E.; Ren, F.; Clark, A.; Esquivel-Upshaw, J. Qualitative Analysis of Remineralization Capabilities of Bioactive Glass (NovaMin) and Fluoride on Hydroxyapatite (HA) Discs: An In Vitro Study. Materials 2021, 14, 3813. https://doi.org/10.3390/ma14143813
Hsu S-M, Alsafadi M, Vasconez C, Fares C, Craciun V, O’Neill E, Ren F, Clark A, Esquivel-Upshaw J. Qualitative Analysis of Remineralization Capabilities of Bioactive Glass (NovaMin) and Fluoride on Hydroxyapatite (HA) Discs: An In Vitro Study. Materials. 2021; 14(14):3813. https://doi.org/10.3390/ma14143813
Chicago/Turabian StyleHsu, Shu-Min, Muhammad Alsafadi, Christina Vasconez, Chaker Fares, Valentin Craciun, Edgar O’Neill, Fan Ren, Arthur Clark, and Josephine Esquivel-Upshaw. 2021. "Qualitative Analysis of Remineralization Capabilities of Bioactive Glass (NovaMin) and Fluoride on Hydroxyapatite (HA) Discs: An In Vitro Study" Materials 14, no. 14: 3813. https://doi.org/10.3390/ma14143813









