Clinical, Radiographic, and Histomorphometric Evaluation of a Vertical Ridge Augmentation Procedure Using a Titanium-Reinforced Microporous Expanded Polytetrafluoroethylene Membrane: A Prospective Case Series with 1-Year Follow-Up
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
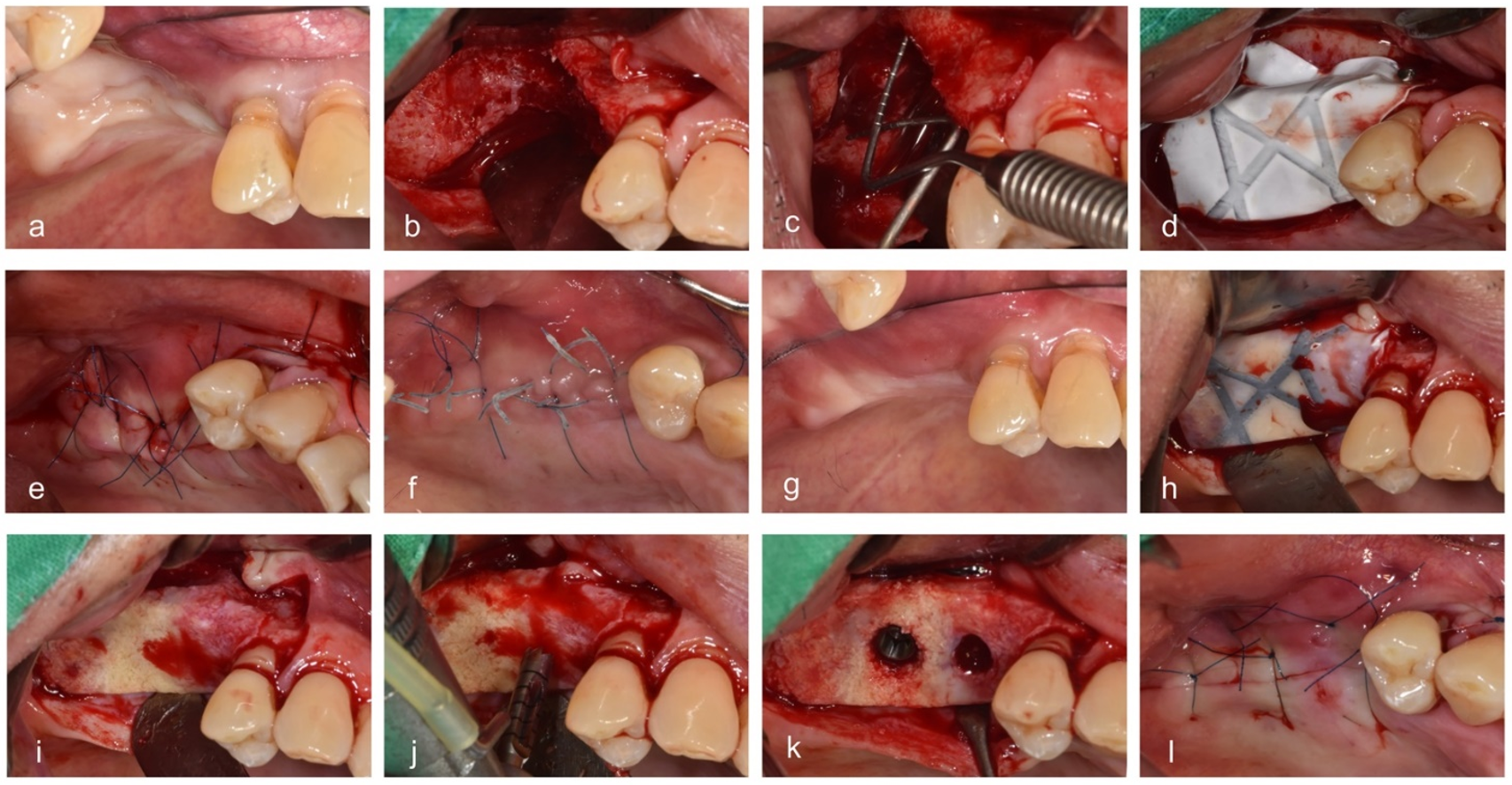
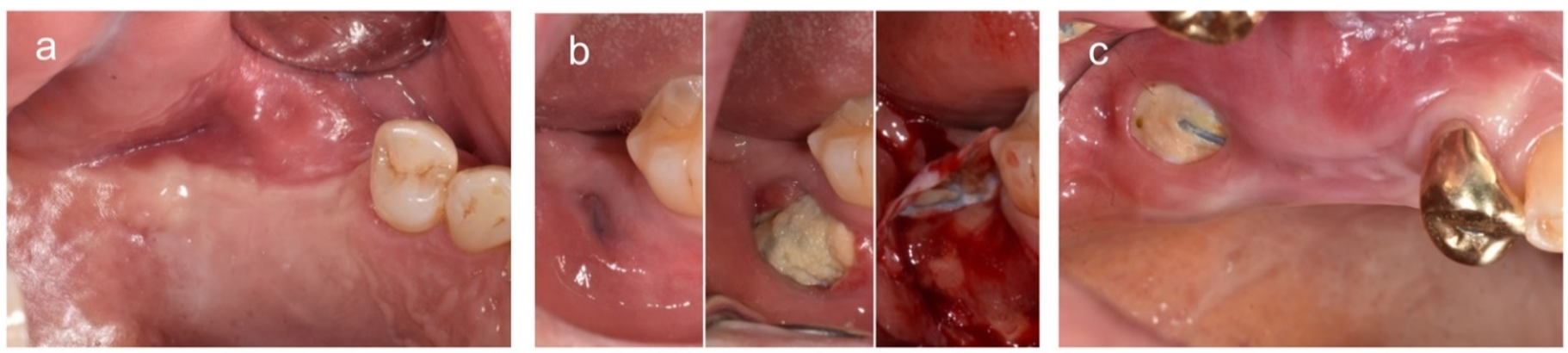
2.2. Surgical Procedures
2.3. Clinical Analysis
2.3.1. Exposure
2.3.2. Primary Stability at the Time of Implant Placement
2.3.3. Additional Bone Grafts
2.4. Radiographic Analysis
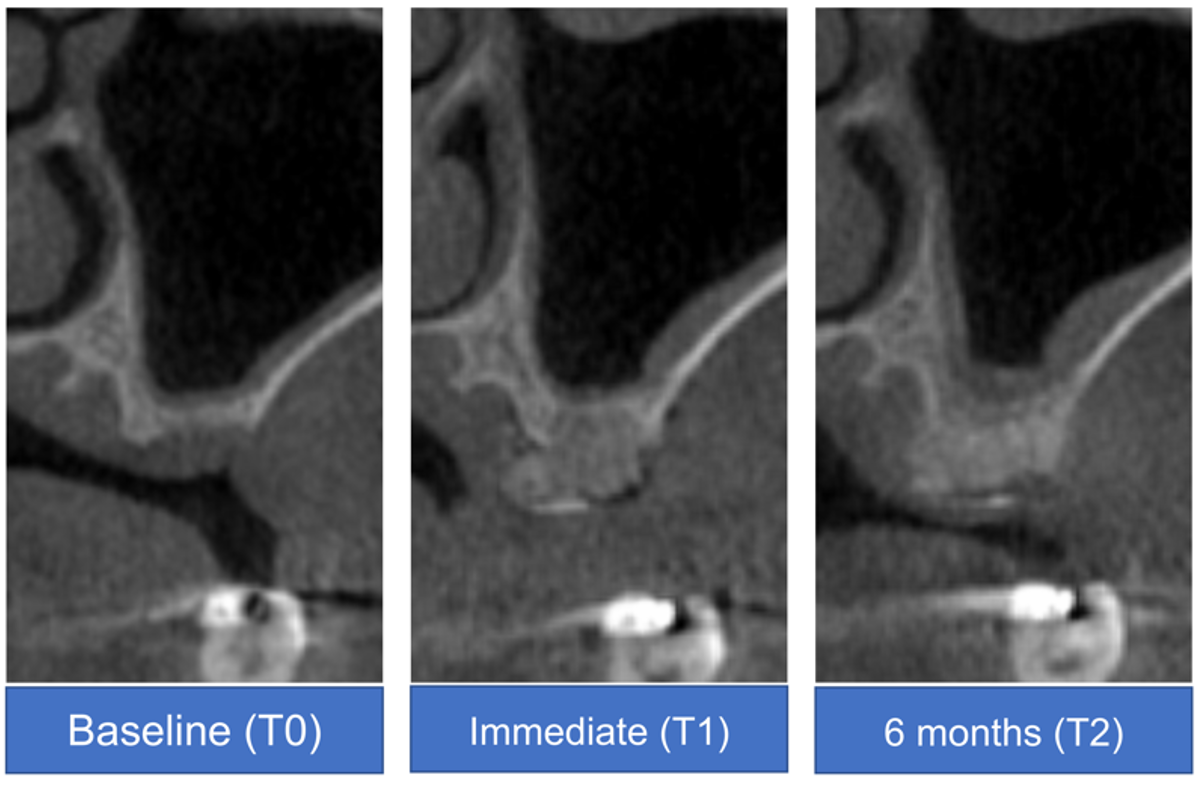
2.4.1. CBCT
2.4.2. Periapical Radiography
2.5. Histological Processing and Histomorphometry Analysis
2.6. Statistical Analysis
3. Results
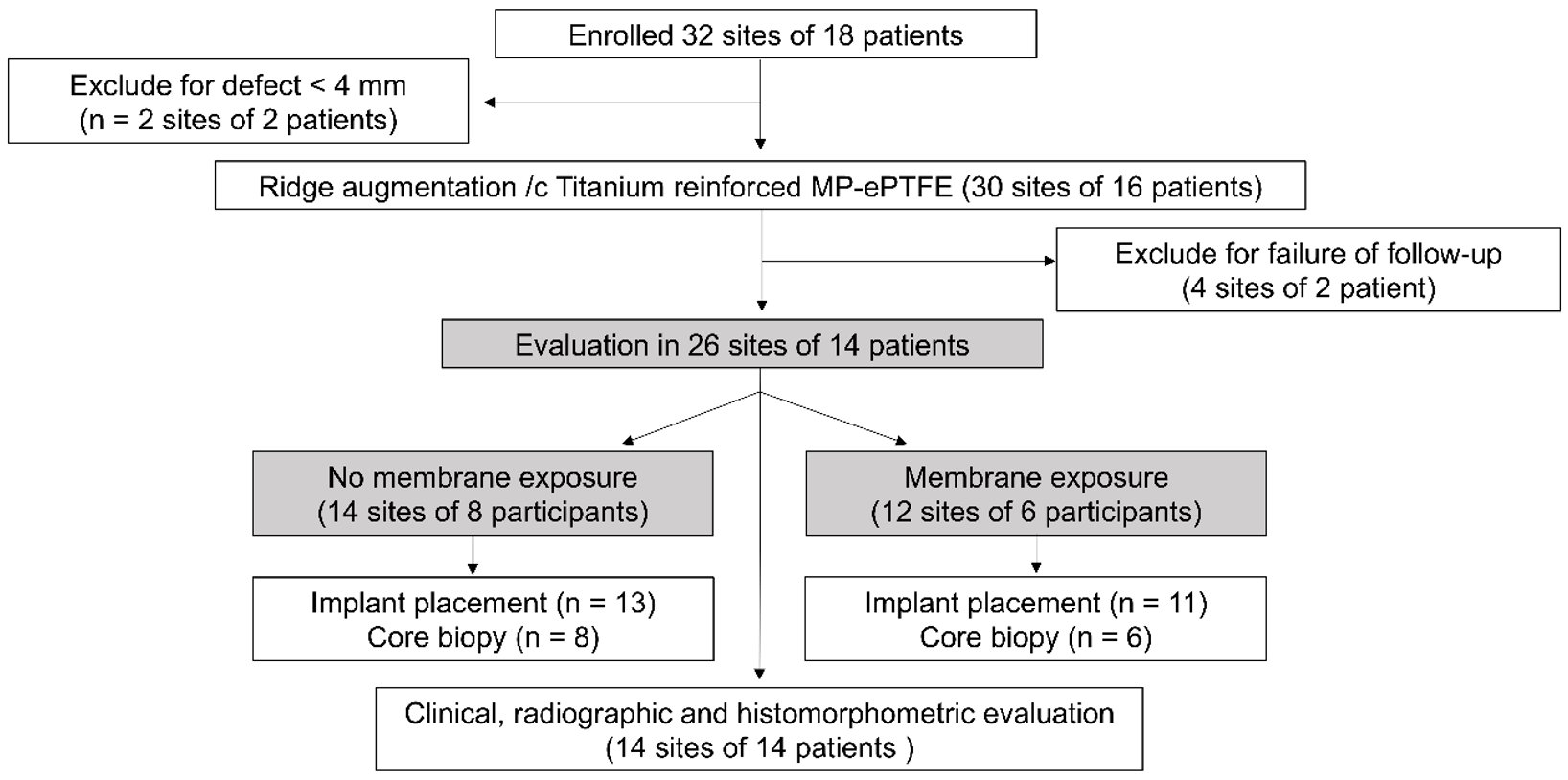
3.1. Demographic Information
3.2. Clinical Analysis
3.3. Radiographic Analysis
3.3.1. CBCT
3.3.2. Periapical Radiography
3.4. Histomorphometric Analysis
3.5. Statistical Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Esposito, M.; Grusovin, M.G.; Worthington, H. Interventions for replacing missing teeth: Treatment of peri-implantitis. Cochrane Database Syst. Rev. 2012, 1, CD004970. [Google Scholar] [CrossRef] [PubMed]
- Khojasteh, A.; Kheiri, L.; Motamedian, S.R.; Khoshkam, V. Guided bone regeneration for the reconstruction of alveolar bone defects. Ann. Maxillofac. Surg. 2017, 7, 263–277. [Google Scholar] [CrossRef] [PubMed]
- Chiapasco, M.; Zaniboni, M.; Boisco, M. Augmentation procedures for the rehabilitation of deficient edentulous ridges with oral implants. Clin. Oral Implant. Res. 2006, 17, 136–159. [Google Scholar] [CrossRef]
- Buser, D.; Dula, K.; Belser, U.C.; Hirt, H.P.; Berthold, H. Localized ridge augmentation using guided bone regeneration. II. Surgical procedure in the mandible. Int. J. Periodontics Restor. Dent. 1995, 15, 10–29. [Google Scholar]
- Urban, I.A.; Montero, E.; Monje, A.; Sanz-Sanchez, I. Effectiveness of vertical ridge augmentation interventions: A systematic review and meta-analysis. J. Clin. Periodontol. 2019, 46, 319–339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ballini, A.; Boccaccio, A.; Saini, R.; Van Pham, P.; Tatullo, M. Dental-Derived Stem Cells and Their Secretome and Interactions with Bioscaffolds/Biomaterials in Regenerative Medicine: From the In Vitro Research to Translational Applications; Hindawi: London, UK, 2017. [Google Scholar]
- Inchingolo, F.; Tatullo, M.; Marrelli, M.; Inchingolo, A.; Inchingolo, A.; Dipalma, G.; Flace, P.; Girolamo, F.; Tarullo, A.; Laino, L. Regenerative surgery performed with platelet-rich plasma used in sinus lift elevation before dental implant surgery: An useful aid in healing and regeneration of bone tissue. Eur. Rev. Med. Pharmacol. Sci. 2012, 16, 1222–1226. [Google Scholar]
- Tatullo, M.; Spagnuolo, G.; Codispoti, B.; Zamparini, F.; Zhang, A.; Esposti, M.D.; Aparicio, C.; Rengo, C.; Nuzzolese, M.; Manzoli, L. PLA-based mineral-doped scaffolds seeded with human periapical cyst-derived MSCs: A promising tool for regenerative healing in dentistry. Materials 2019, 12, 597. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marrelli, M.; Codispoti, B.; Shelton, R.M.; Scheven, B.A.; Cooper, P.R.; Tatullo, M.; Paduano, F. Dental pulp stem cell mechanoresponsiveness: Effects of mechanical stimuli on dental pulp stem cell behavior. Front. Physiol. 2018, 9, 1685. [Google Scholar] [CrossRef] [PubMed]
- Naung, N.Y.; Shehata, E.; Van Sickels, J.E. Resorbable versus nonresorbable membranes: When and why? Dent. Clin. 2019, 63, 419–431. [Google Scholar] [CrossRef]
- Pellegrino, G.; Lizio, G.; Corinaldesi, G.; Marchetti, C. Titanium mesh technique in rehabilitation of totally edentulous atrophic maxillae: A retrospective case series. J. Periodontol. 2016, 87, 519–528. [Google Scholar] [CrossRef]
- Rakhmatia, Y.D.; Ayukawa, Y.; Furuhashi, A.; Koyano, K. Current barrier membranes: Titanium mesh and other membranes for guided bone regeneration in dental applications. J. Prosthodont. Res. 2013, 57, 3–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ronda, M.; Rebaudi, A.; Torelli, L.; Stacchi, C. Expanded vs. dense polytetrafluoroethylene membranes in vertical ridge augmentation around dental implants: A prospective randomized controlled clinical trial. Clin. Oral Implant. Res. 2014, 25, 859–866. [Google Scholar] [CrossRef] [PubMed]
- Barber, H.D.; Lignelli, J.; Smith, B.M.; Bartee, B.K. Using a dense PTFE membrane without primary closure to achieve bone and tissue regeneration. J. Oral Maxillofac. Surg. 2007, 65, 748–752. [Google Scholar] [CrossRef] [PubMed]
- Fontana, F.; Maschera, E.; Rocchietta, I.; Simion, M. Clinical classification of complications in guided bone regeneration procedures by means of a nonresorbable membrane. Int. J. Periodontics Restor. Dent. 2011, 31, 265–273. [Google Scholar]
- Gallo, P.; Díaz-Báez, D. Management of 80 complications in vertical and horizontal ridge augmentation with nonresorbable membrane (d-PTFE): A cross-sectional study. Int. J. Oral Maxillofac. Implant. 2019, 34, 927–935. [Google Scholar] [CrossRef] [PubMed]
- Simion, M.; Trisi, P.; Maglione, M.; Piattelli, A. Bacterial penetration in vitro through GTAM membrane with and without topical chlorhexidine application: A light and scanning electron microscopic study. J. Clin. Periodontol. 1995, 22, 321–331. [Google Scholar] [CrossRef] [PubMed]
- Jung, R.E.; Philipp, A.; Annen, B.M.; Signorelli, L.; Thoma, D.S.; Hammerle, C.H.; Attin, T.; Schmidlin, P. Radiographic evaluation of different techniques for ridge preservation after tooth extraction: A randomized controlled clinical trial. J. Clin. Periodontol. 2013, 40, 90–98. [Google Scholar] [CrossRef]
- Cho, I.W.; Park, J.C.; Shin, H.S. A comparison of different compressive forces on graft materials during alveolar ridge preservation. J. Periodontal Implant. Sci. 2017, 47, 51–63. [Google Scholar] [CrossRef] [Green Version]
- Cheon, G.B.; Kang, K.L.; Yoo, M.K.; Yu, J.A.; Lee, D.W. Alveolar ridge preservation using allografts and dense polytetrafluoroethylene membranes with open membrane technique in unhealthy extraction socket. J. Oral Implantol. 2017, 43, 267–273. [Google Scholar] [CrossRef]
- Urban, I.A.; Nagursky, H.; Lozada, J.L. Horizontal ridge augmentation with a resorbable membrane and particulated autogenous bone with or without anorganic bovine bone-derived mineral: A prospective case series in 22 patients. Int. J. Oral Maxillofac. Implant. 2011, 26, 404–414. [Google Scholar]
- Madhuri, S.V. Membranes for periodontal regeneration. Int. J. Pharm. Sci. Invent. 2016, 5, 19–24. [Google Scholar]
- Chao, Y.-C.; Chang, P.; Fu, J.; Wang, H.; Chan, H. Surgical site assessment for soft tissue management in ridge augmentation procedures. Int. J. Periodontics Restor. Dent. 2015, 35, e75–e83. [Google Scholar] [CrossRef] [Green Version]
- Machtei, E.E. The effect of membrane exposure on the outcome of regenerative procedures in humans: A meta-analysis. J. Periodontol. 2001, 72, 512–516. [Google Scholar] [CrossRef] [PubMed]
- Mendoza-Azpur, G.; Gallo, P.; Mayta-Tovalino, F.; Alva, R.; Valdivia, E. A case series of vertical ridge augmentation using a nonresorbable membrane: A multicenter study. Int. J. Periodontics Restor. Dent. 2018, 38, 811–816. [Google Scholar] [CrossRef]
- Lim, G.; Lin, G.H.; Monje, A.; Chan, H.L.; Wang, H.L. Wound healing complications following guided bone regeneration for ridge augmentation: A systematic review and meta-analysis. Int. J. Oral Maxillofac. Implant. 2018, 33, 41–50. [Google Scholar] [CrossRef]
- Buser, D.; Brägger, U.; Lang, N.; Nyman, S. Regeneration and enlargement of jaw bone using guided tissue regeneration. Clin. Oral Implant. Res. 1990, 1, 22–32. [Google Scholar] [CrossRef]
- Duyck, J.; Vandamme, K. The effect of loading on peri-implant bone: A critical review of the literature. J. Oral Rehabil. 2014, 41, 783–794. [Google Scholar] [CrossRef]
- Peñarrocha, M.; Palomar, M.; Sanchis, J.M.; Guarinos, J.; Balaguer, J. Radiologic study of marginal bone loss around 108 dental implants and its relationship to smoking, implant location, and morphology. Int. J. Oral Maxillofac. Implant. 2004, 19, 861–867. [Google Scholar]
- Becker, W.; Becker, B.E.; Israelson, H.; Lucchini, J.P.; Handelsman, M.; Ammons, W.; Rosenberg, E.; Rose, L.; Tucker, L.M.; Lekholm, U. One-step surgical placement of brånemark implants: A prospective multicenter clinical study. Int. J. Oral Maxillofac. Implant. 1997, 12, 454–462. [Google Scholar]
- Roos, J.; Sennerby, L.; Lekholm, U.; Jemt, T.; Gröndahl, K.; Albrektsson, T. A qualitative and quantitative method for evaluating implant success: A 5-year retrospective analysis of the Brånemark implant. Int. J. Oral Maxillofac. Implant. 1997, 12, 504–514. [Google Scholar]
- Chan, H.L.; Benavides, E.; Tsai, C.Y.; Wang, H.L. A titanium mesh and particulate allograft for vertical ridge augmentation in the posterior mandible: A pilot study. Int. J. Periodontics Restor. Dent. 2015, 35, 515–522. [Google Scholar] [CrossRef] [PubMed] [Green Version]
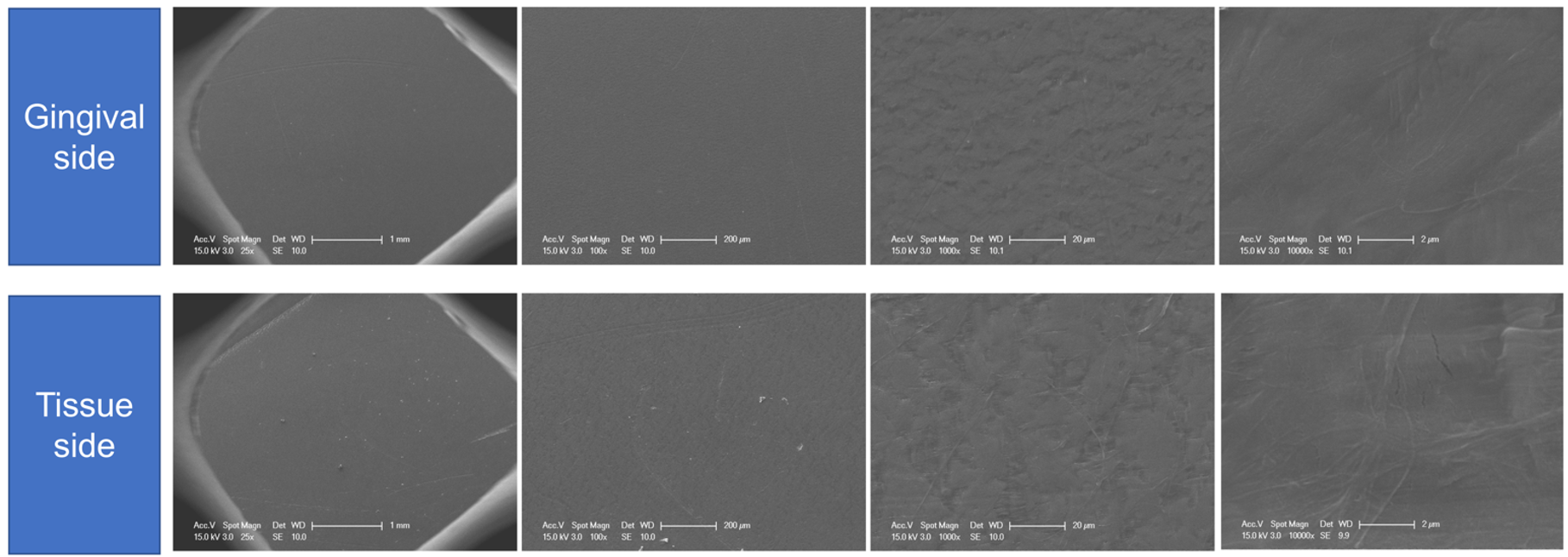
- Cucchi, A.; Sartori, M.; Parrilli, A.; Aldini, N.N.; Vignudelli, E.; Corinaldesi, G. Histological and histomorphometric analysis of bone tissue after guided bone regeneration with non-resorbable membranes vs resorbable membranes and titanium mesh. Clin. Implant. Dent. Relat. Res. 2019, 21, 693–701. [Google Scholar] [CrossRef] [PubMed]
- Proussaefs, P.; Lozada, J. Use of titanium mesh for staged localized alveolar ridge augmentation: Clinical and histologic-histomorphometric evaluation. J. Oral Implantol. 2006, 32, 237–247. [Google Scholar] [CrossRef] [Green Version]
- Simion, M.; Fontana, F.; Rasperini, G.; Maiorana, C. Vertical ridge augmentation by expanded-polytetrafluoroethylene membrane and a combination of intraoral autogenous bone graft and deproteinized anorganic bovine bone (Bio Oss). Clin. Oral Implant. Res. 2007, 18, 620–629. [Google Scholar] [CrossRef] [PubMed]
- Lang, N.; Hämmerle, C.; Brägger, U.; Lehmann, B.; Nyman, S. Guided tissue regeneration in jawbone defects prior to implant placement. Clin. Oral Implant. Res. 1994, 5, 92–97. [Google Scholar] [CrossRef]



| Study Variable | Descriptive Statistics |
|---|---|
| Sample size (patients/tooth sites) | 14/26 |
| Sex (male/female) | 9/5 |
| Age (years) | 67 ± 9.3 (range, 50–79) |
| Arch (maxilla/mandible) | 10/4 |
| Site (nonmolar/molar) | 11/15 |
| Group | No Exposure | Exposure | p-Value |
|---|---|---|---|
| (N = 8) | (N = 6) | ||
| Sex | 0.301 | ||
| Female | 4 (50.0%) | 1 (16.7%) | |
| Male | 4 (50.0%) | 5 (83.3%) | |
| Age | 63.5 ± 11.2 | 71.8 ± 4.1 | 0.084 |
| Sites | 1 | ||
| Maxillary premolar | 2 (25.0%) | 1 (16.7%) | |
| Maxillary posterior | 4 (50.0%) | 3 (50.0%) | |
| Mandibular posterior | 2 (25.0%) | 2 (33.3%) | |
| Single vs. multiple | 0.091 | ||
| Single | 1 (12.5%) | 4 (66.7%) | |
| Multiple | 7 (87.5%) | 2 (33.3%) | |
| Smoking | 1 | ||
| Yes | 1 (12.5%) | 1 (16.7%) | |
| No | 7 (87.5%) | 5 (83.3%) | |
| Entry period (weeks) | 180.2 ± 16.0 | 190.2 ± 43.4 | 0.613 |
| Primary stability (N/cm) | 0.473 | ||
| <30 | 6 (75.0%) | 6 (100.0%) | |
| ≥30 | 2 (25.0%) | 0 (0.0%) | |
| Change in marginal level (mm) | |||
| Mesial | 0.3 ± 0.3 | 0.4 ± 0.1 | 0.771 |
| Distal | 0.2 ± 0.2 | 0.3 ± 0.2 | 0.482 |
| Histomorphometric (area %) | |||
| New bone | 28.6 ± 7.8 | 28.0 ± 4.0 | 0.857 |
| Residual bone graft | 8.7 ± 1.7 | 4.8 ± 2.3 | 0.003 * |
| Soft tissue | 62.7 ± 8.4 | 67.2 ± 3.2 | 0.23 |
| Vertical Height | Variable | Beta Coefficient | Standard Error of Beta Coefficient | p-Value |
|---|---|---|---|---|
| VHB | Intercept | 9.312 | 3.363 | 0.009 |
| Exposure | 1.288 | 5.138 | 0.804 | |
| T1 | 4.438 | 0.652 | <0.001 | |
| T2 | 3.750 | 0.713 | <0.001 | |
| Exposure × T1 | 2.229 | 0.996 | 0.032 | |
| Exposure × T2 | 0.983 | 1.089 | 0.373 | |
| VHM * | Intercept | 9.312 | 3.363 | 0.009 |
| T1 | 4.438 | 0.652 | <0.001 | |
| T2 | 3.750 | 0.713 | <0.001 | |
| VHL * | Intercept | 9.312 | 3.363 | 0.009 |
| T1 | 4.438 | 0.652 | <0.001 | |
| T2 | 3.750 | 0.713 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ji, J.-G.; Yu, J.-A.; Choi, S.-H.; Lee, D.-W. Clinical, Radiographic, and Histomorphometric Evaluation of a Vertical Ridge Augmentation Procedure Using a Titanium-Reinforced Microporous Expanded Polytetrafluoroethylene Membrane: A Prospective Case Series with 1-Year Follow-Up. Materials 2021, 14, 3828. https://doi.org/10.3390/ma14143828
Ji J-G, Yu J-A, Choi S-H, Lee D-W. Clinical, Radiographic, and Histomorphometric Evaluation of a Vertical Ridge Augmentation Procedure Using a Titanium-Reinforced Microporous Expanded Polytetrafluoroethylene Membrane: A Prospective Case Series with 1-Year Follow-Up. Materials. 2021; 14(14):3828. https://doi.org/10.3390/ma14143828
Chicago/Turabian StyleJi, Jung-Gu, Jung-A Yu, Seong-Ho Choi, and Dong-Woon Lee. 2021. "Clinical, Radiographic, and Histomorphometric Evaluation of a Vertical Ridge Augmentation Procedure Using a Titanium-Reinforced Microporous Expanded Polytetrafluoroethylene Membrane: A Prospective Case Series with 1-Year Follow-Up" Materials 14, no. 14: 3828. https://doi.org/10.3390/ma14143828
APA StyleJi, J.-G., Yu, J.-A., Choi, S.-H., & Lee, D.-W. (2021). Clinical, Radiographic, and Histomorphometric Evaluation of a Vertical Ridge Augmentation Procedure Using a Titanium-Reinforced Microporous Expanded Polytetrafluoroethylene Membrane: A Prospective Case Series with 1-Year Follow-Up. Materials, 14(14), 3828. https://doi.org/10.3390/ma14143828






