Effective Sorption of Europium Ions by Interpolymer System Based on Industrial Ion-Exchanger Resins Amberlite IR120 and AB-17-8
Abstract
:1. Introduction
- Each ion exchanger is selective relatively to one certain ion (absence of selectivity). In other words, it cannot be used successfully for selective sorption of another metal ion;
- After each cycle of sorption ion-exchange resins require complex process of regeneration.
- Possibility of fast development of several sorbents which are selective to one certain ion;
- Possibility of creation of selective sorbents based on non-expensive industrial ion exchangers;
- One interpolymer system can be used for selective sorption of several ions;
- Effective separation groups of cations from anions.
2. Materials and Methods
2.1. Materials
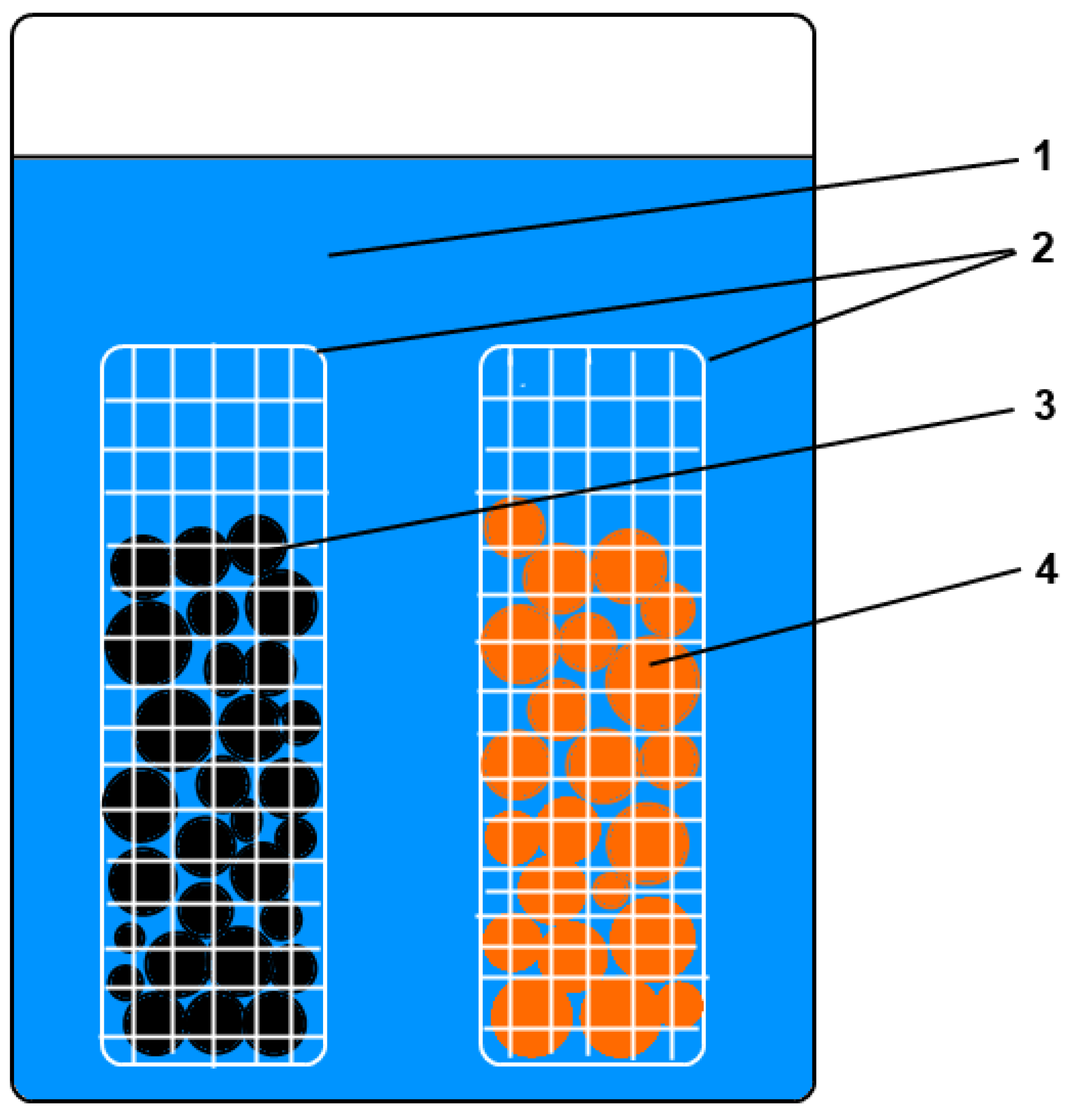
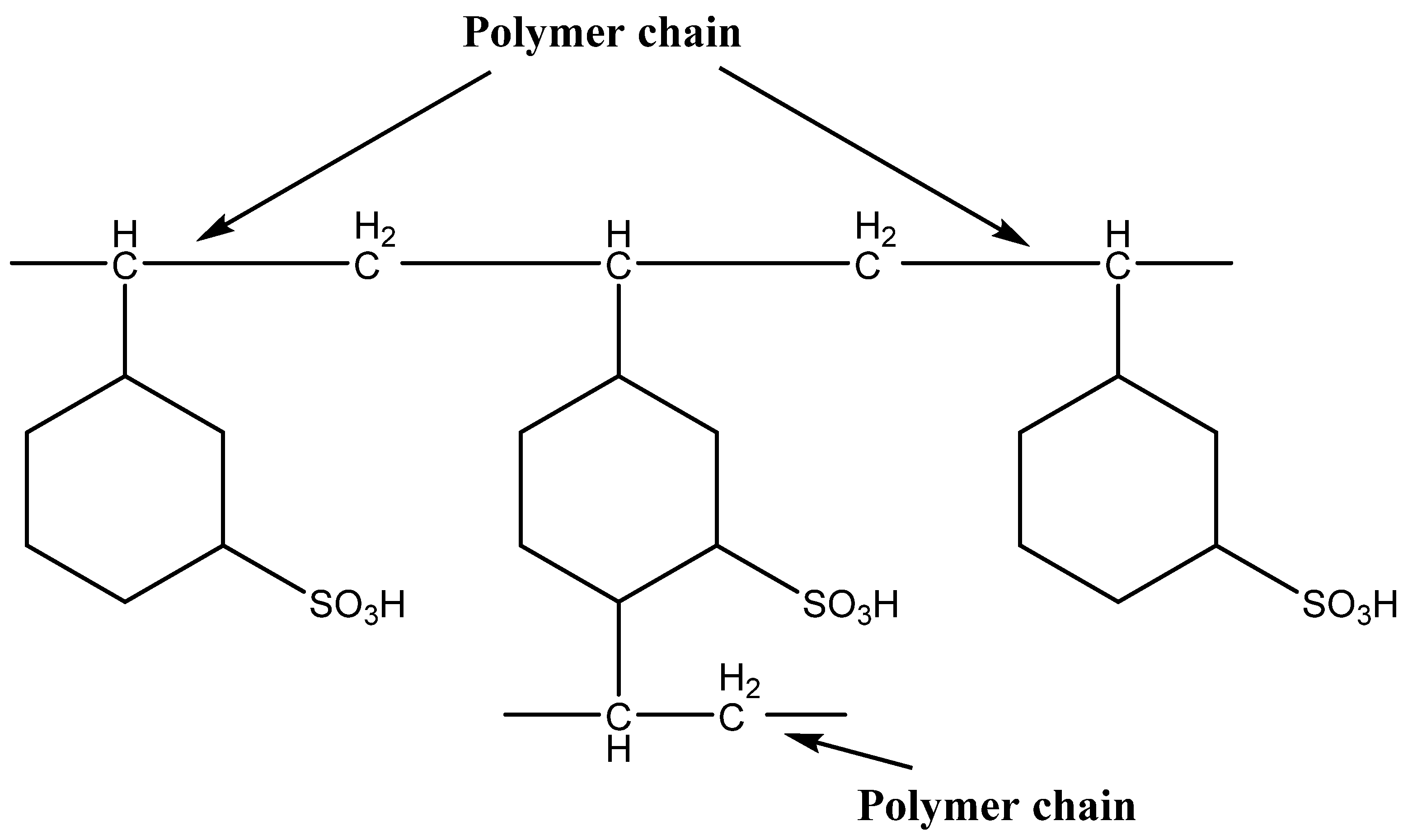
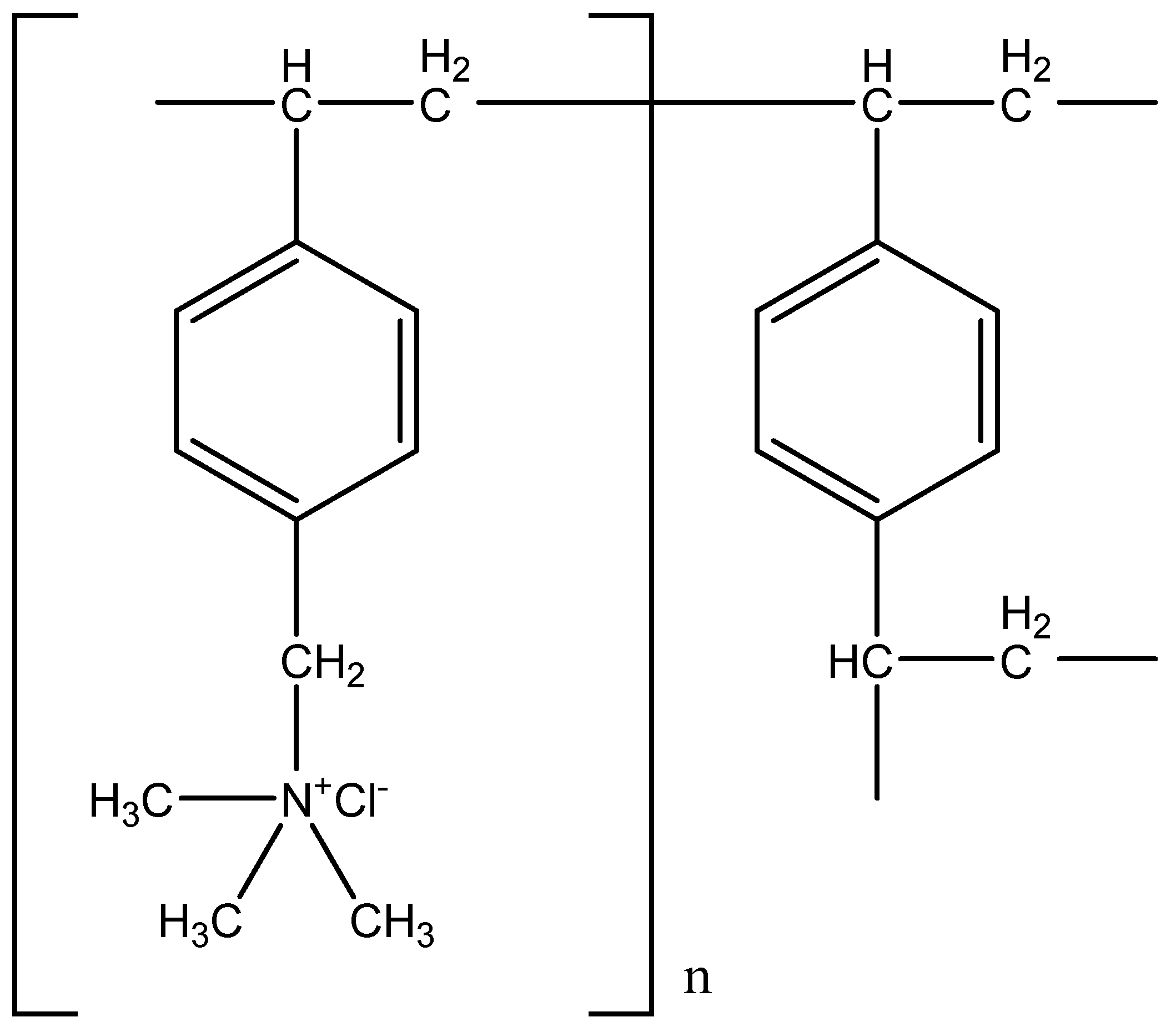
2.1.1. Ion-Exchange Resins
2.1.2. Preparation of Interpolymer Systems
2.1.3. Calculation of Sorption Parameters
2.2. Methods
2.2.1. Measurement of Electrochemical Properties
2.2.2. Measurement of Concentration
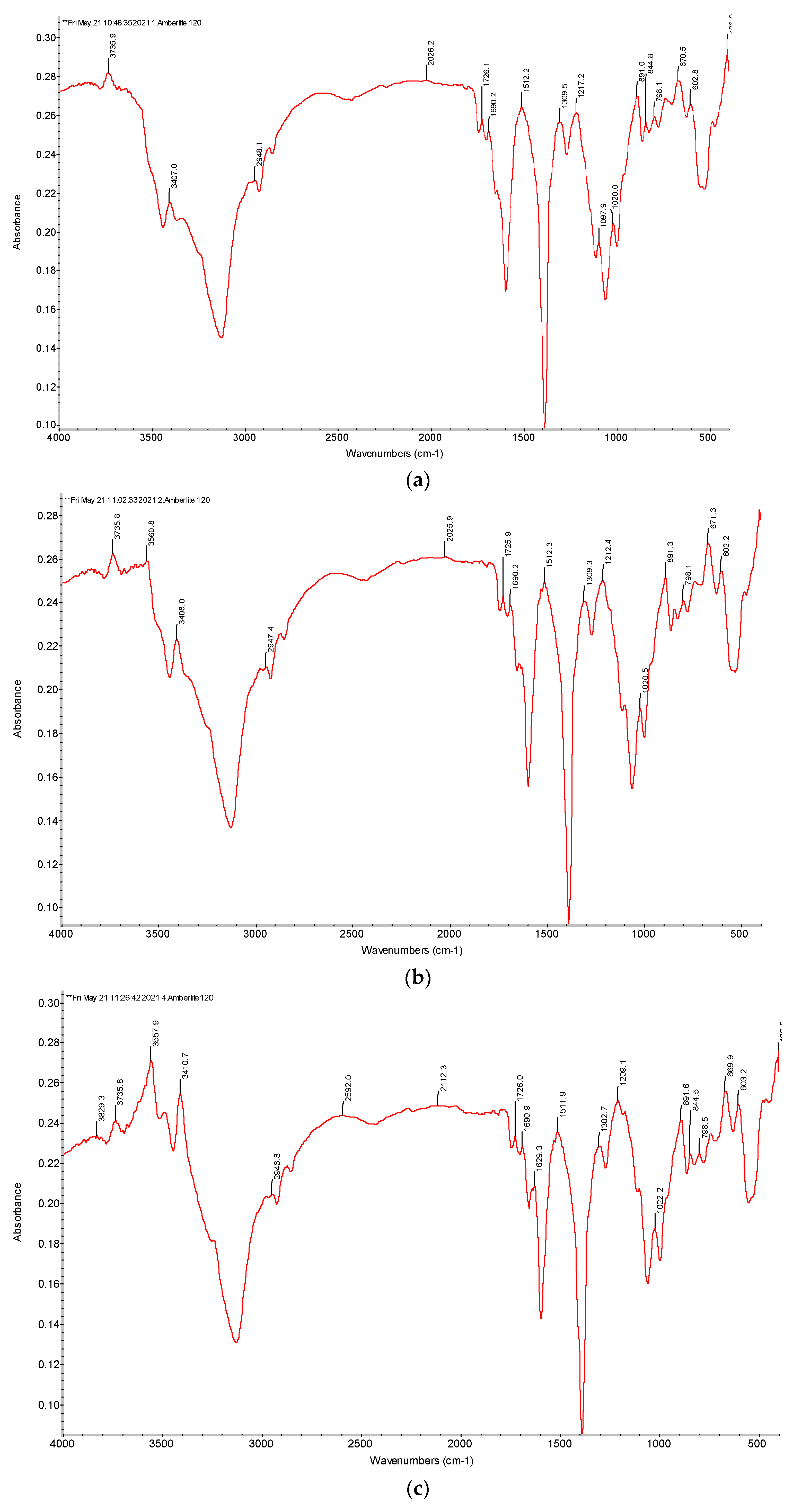
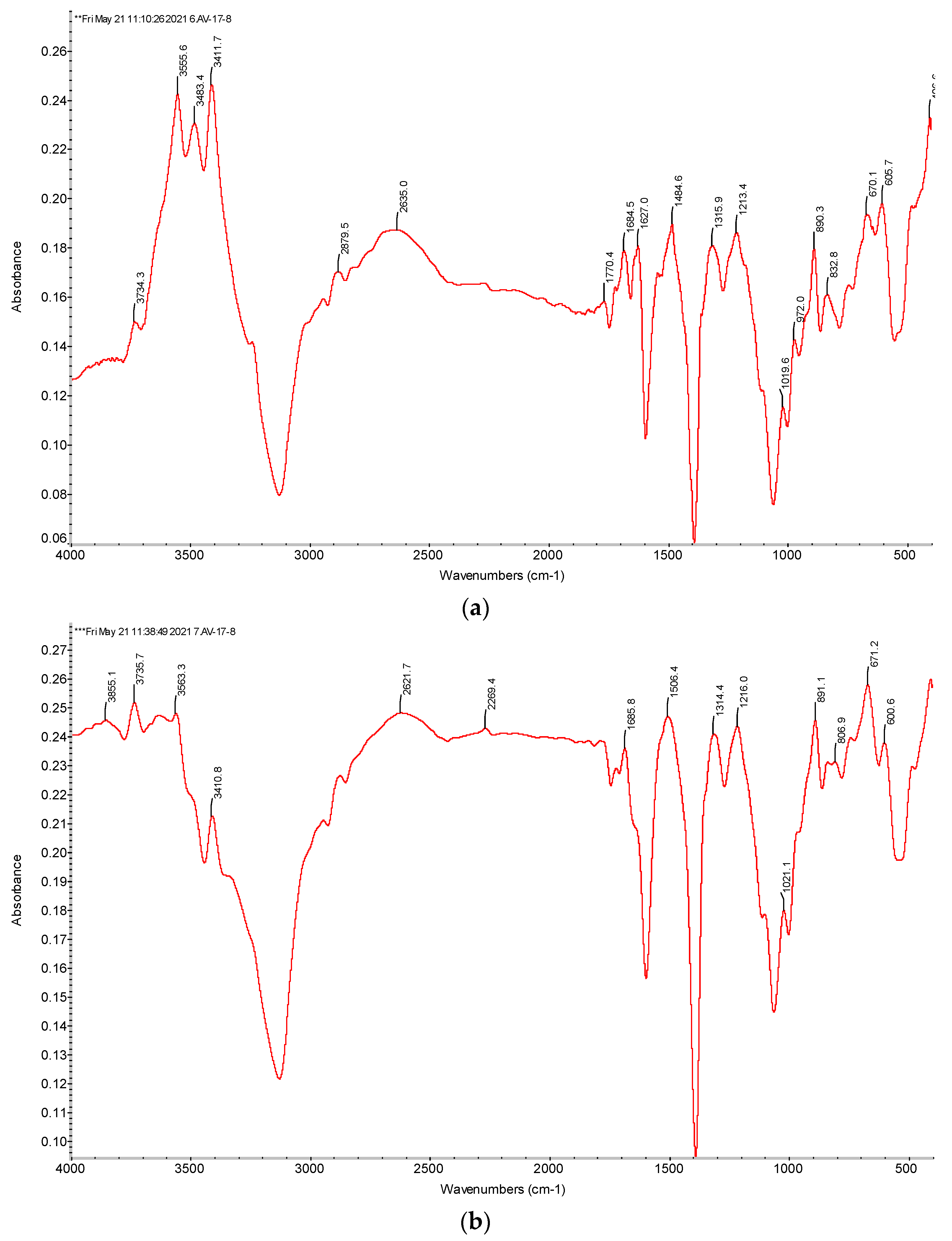
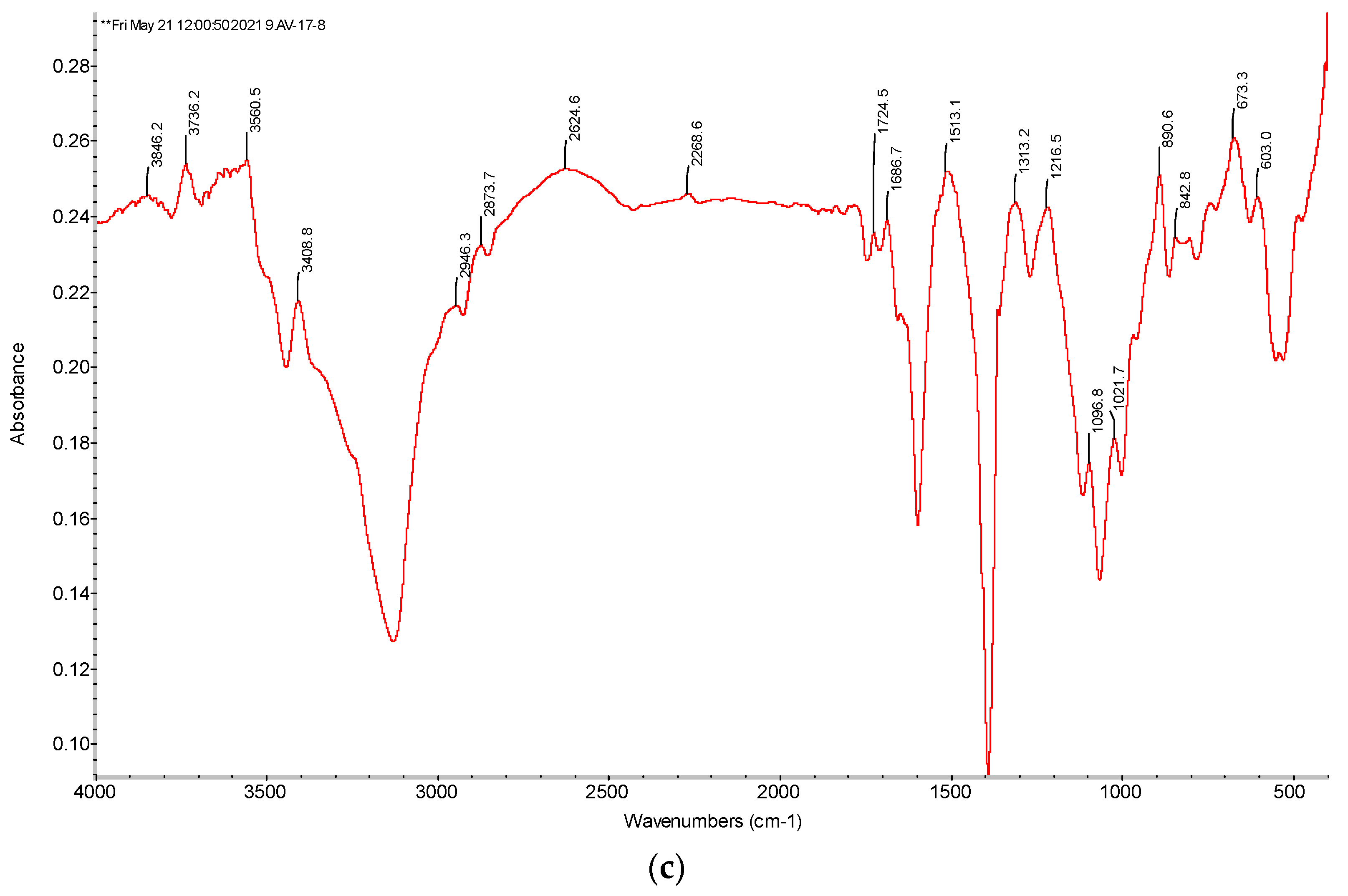
2.2.3. Fourier Transform Infrared (FTIR) Spectra of Ion Exchangers Amberlite IR120 and AB-17-8
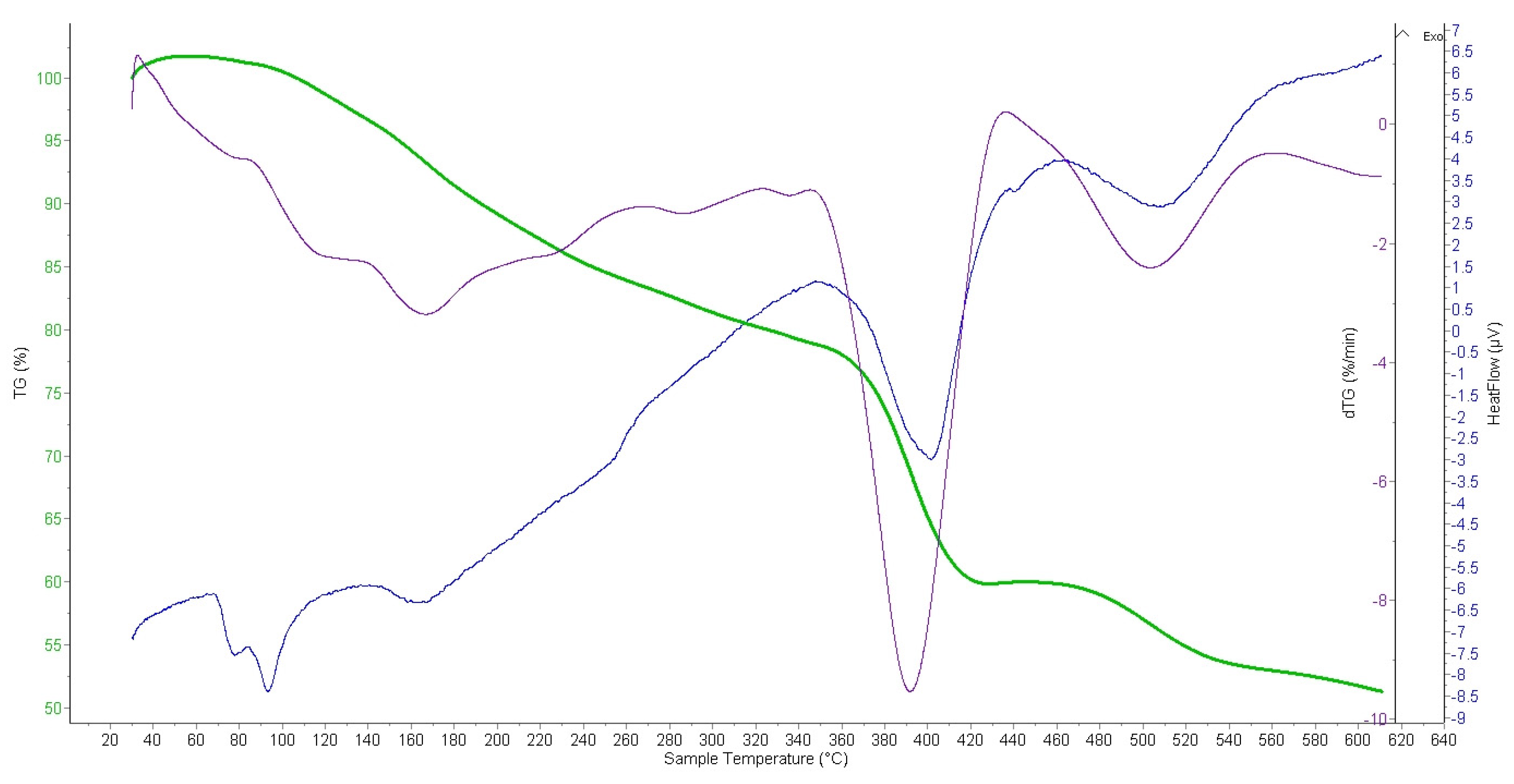
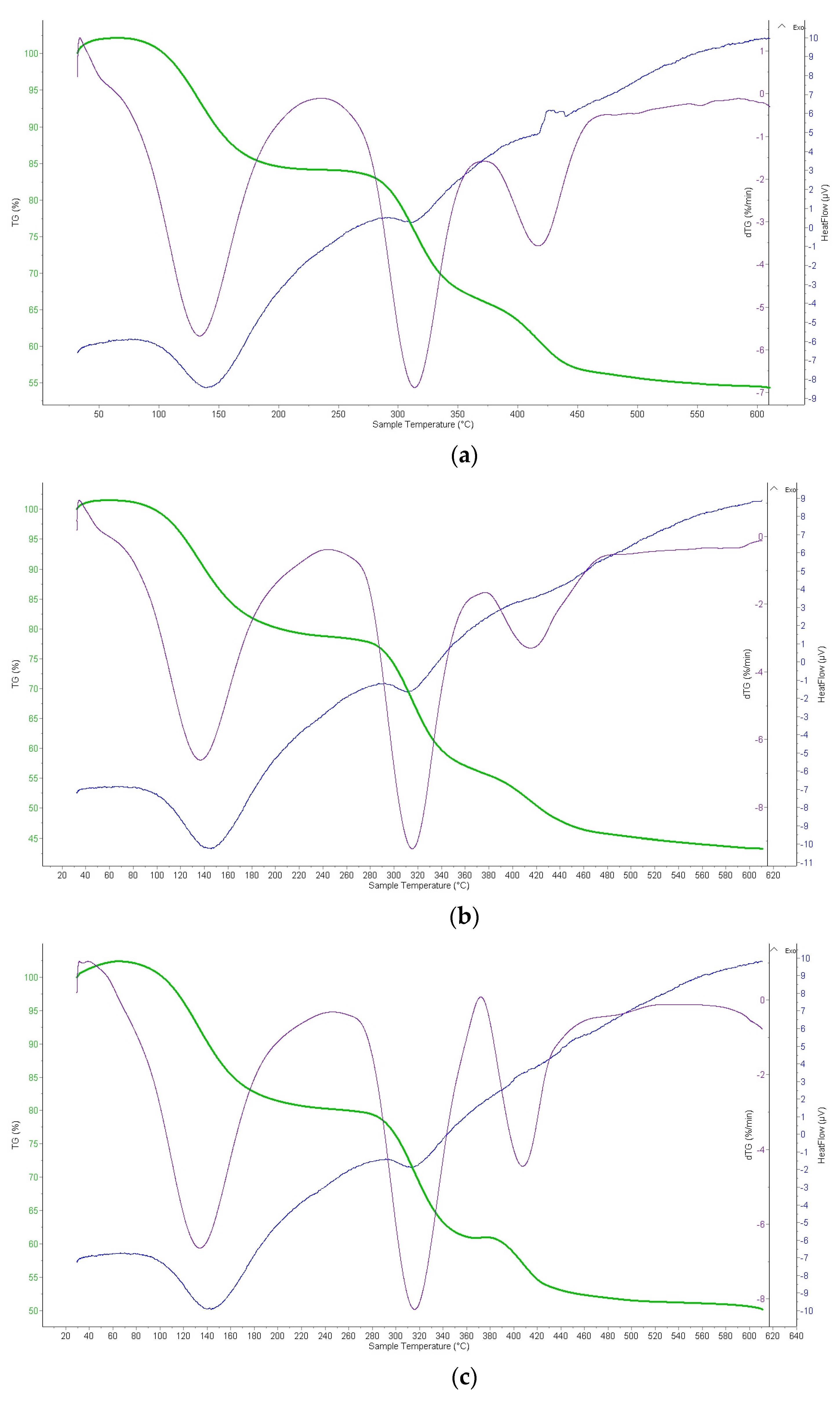
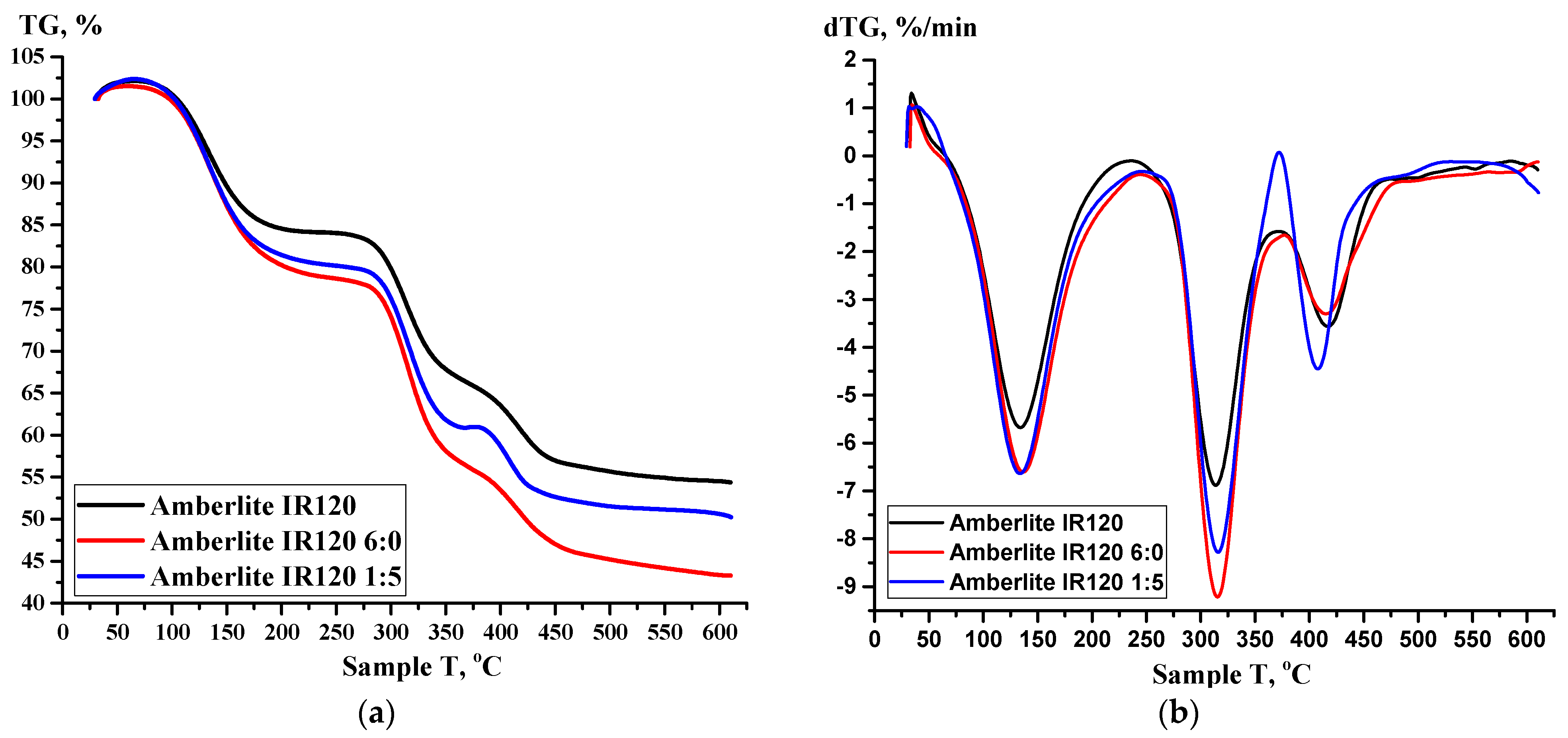
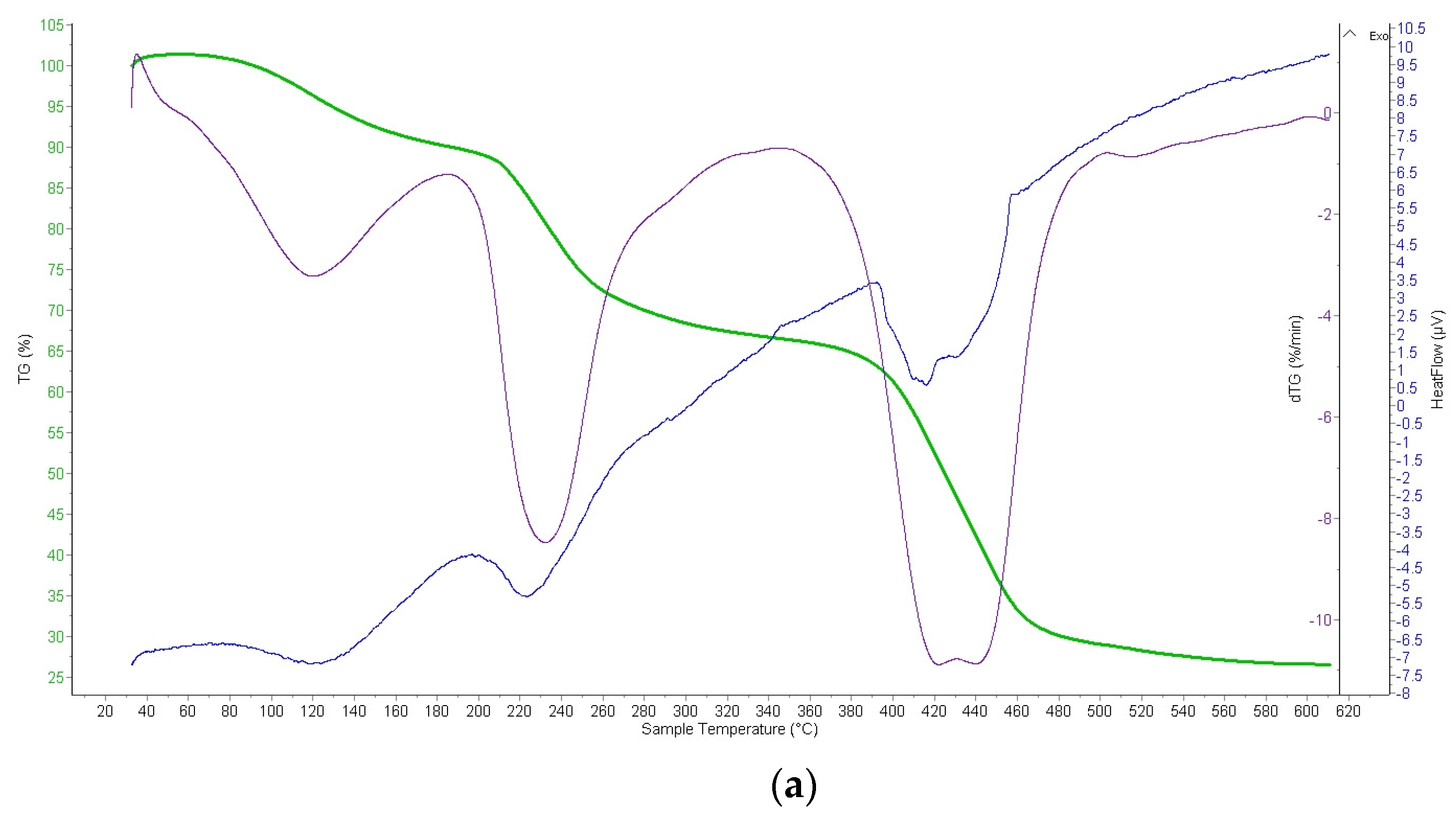
2.2.4. Thermogravimetric Analysis (TGA) of Ion Exchangers and Interpolymer System
3. Results and Discussion
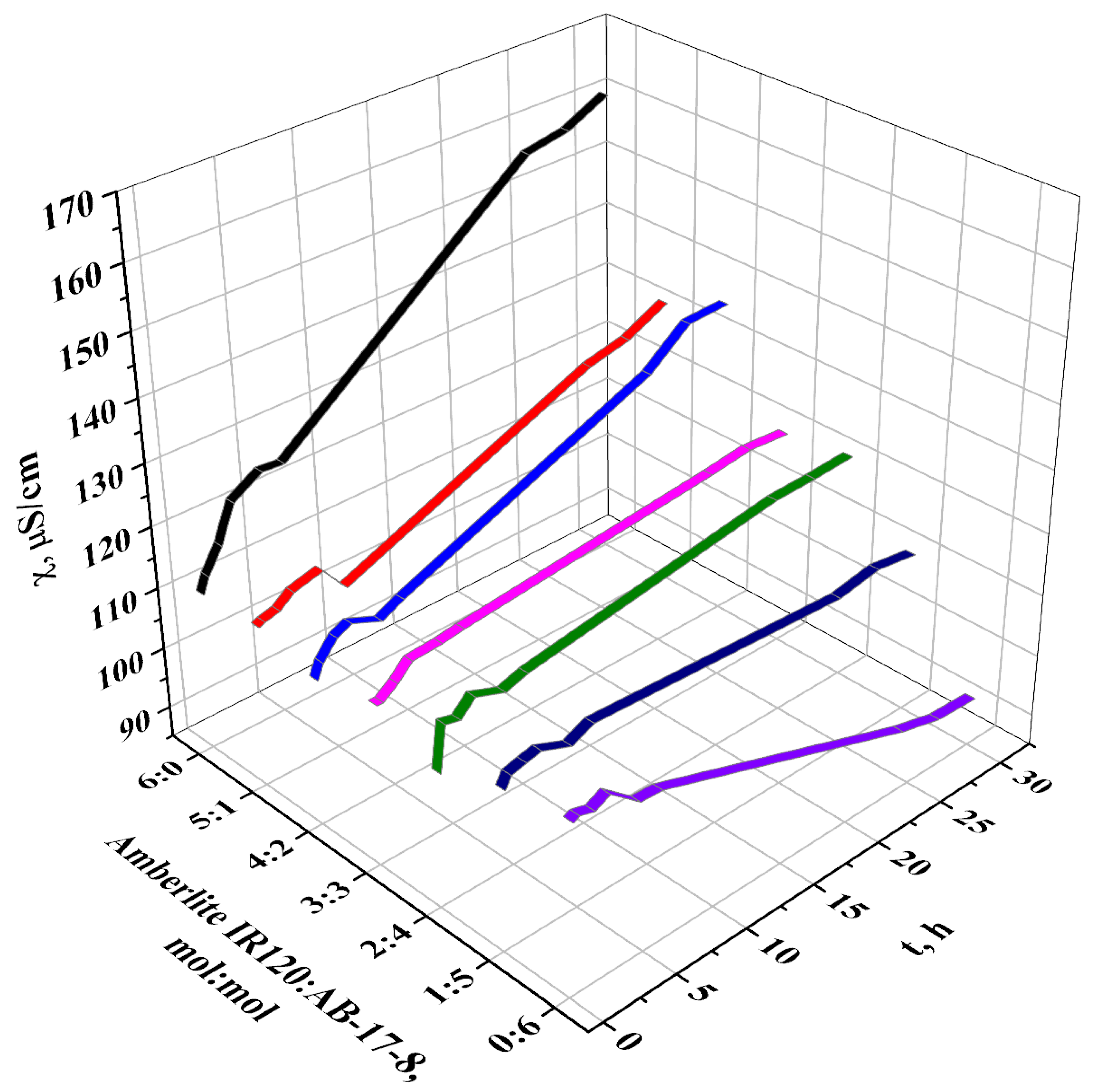
3.1. Electrochemical Properties of the Interpolymer System Amberlite IR120-AB-17-8
- Dissociation of functional groups along with formation of water molecules while binding OH-groups;
- Conformational changes of links of the ion exchangers stimulated by remote interaction provides destruction of interchain associates releasing functional groups with subsequent additional dissociation.
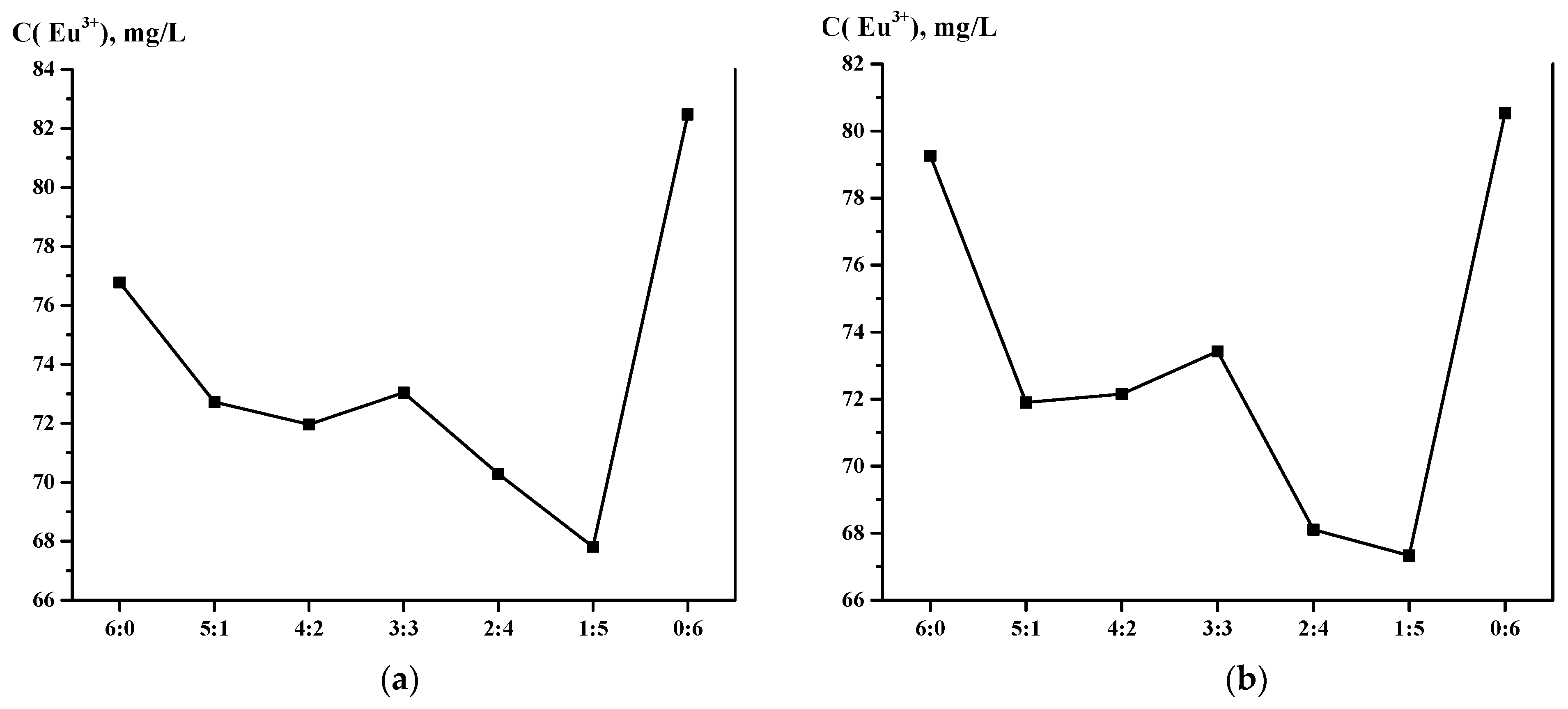
3.2. Sorption Properties of the Interpolymer System Amberlite IR120-AB-17-8
3.3. Characterisation of the Ion Exchangers Amberlite IR120 and AB-17-8 before and after Europium Ions Sorption
3.3.1. FTIR Spectra of the Initial Ion Exchangers and the Interpolymer Pair Amberlite IR120:AB-17-8 = 1:5
3.3.2. Thermogravimetric Analysis (TGA) of Amberlite IR120; AB-17-8 and the Interpolymer Pair Amberlite IR120:AB-17-8 = 1:5
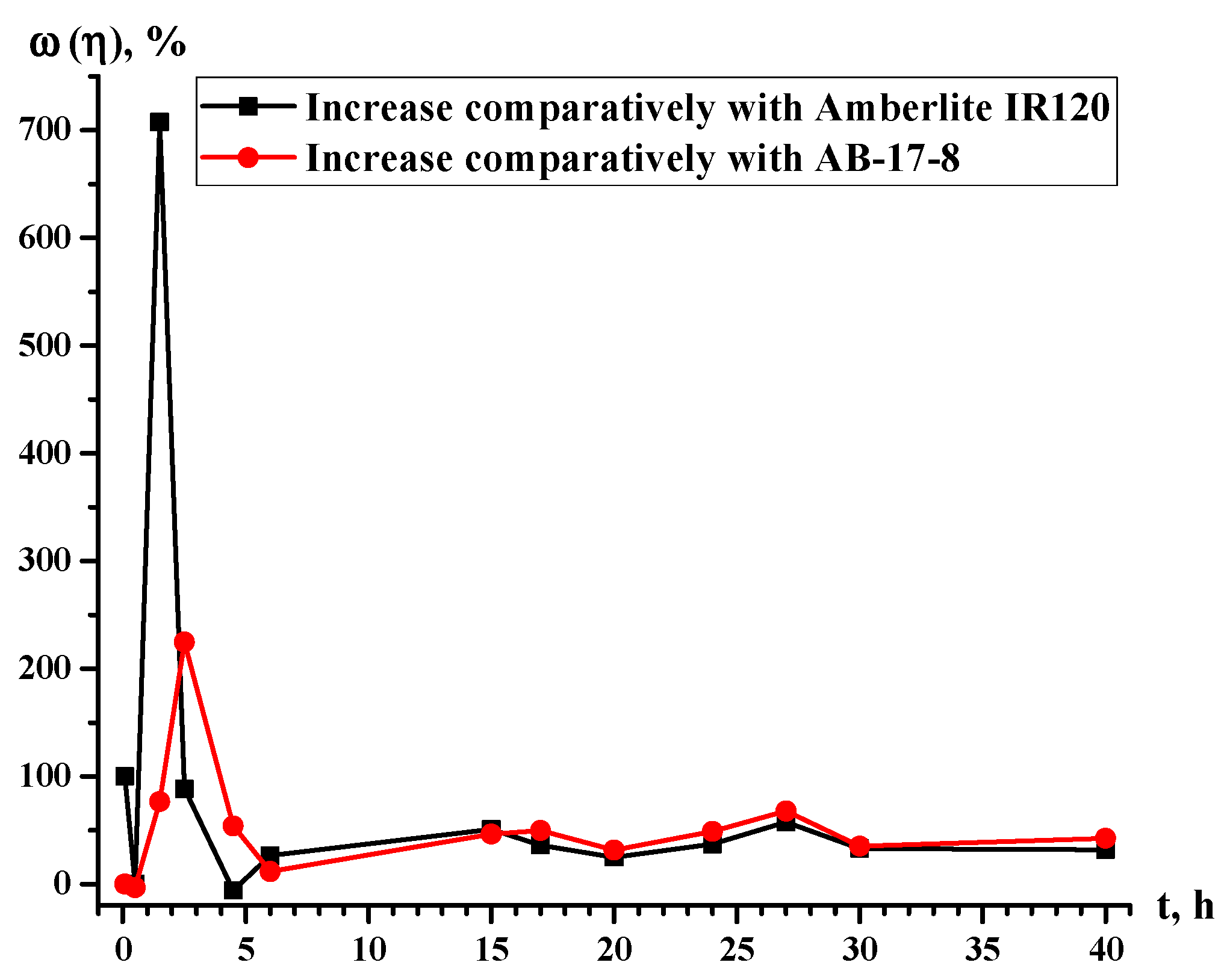
3.4. Growth of the Sorption Properties in Interpolymer Pair Amberlite IR120:AB-17-8 = 1:5
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kryukov, V.A.; Yatsenko, V.A.; Kryukov, Y.V. Rare-earth industry—To realize available opportunities. Min. Ind. 2020, 5, 68–84. [Google Scholar]
- Norman, A.; Zou, X.; Barnett, J. Critical Minerals: Rara Earths and the U.S. Economy. Natl. Cent. Policy Anal. 2014, 175, 16. [Google Scholar]
- Cotton, S. Lanthanides and Actinides; Oxford University Press: Oxford, UK, 1991. [Google Scholar]
- Hedrick, J.B. Rare earths. In Minerals Year-Book—Metals and Minerals; United States Geological Survey: Reston, VA, USA, 1999. [Google Scholar]
- Jones, A.P.; Wall, F.; Williams, C.T. Rare Earth Minerals: Chemistry, Origin, and Ore Deposits; Chapman and Hall: London, UK, 1996. [Google Scholar]
- Kilbourn, B.T. A Lanthanide Lanthology; Molycorp, Inc.: New York, NY, USA, 1993; pp. 1–61. [Google Scholar]
- Lipin, B.R.; McKay, G.A. Geochemistry and mineralogy of the rare earth elements. Rev. Mineral. 1989, 21, 348. [Google Scholar]
- Rossotti, H. Diverse Atoms: Profiles of the Chemical Elements; Oxford University Press: Oxford, UK, 1998. [Google Scholar]
- Alimbekova, B.T.; Korganbayeva, Z.K.; Himersen, H.; Kondaurov, R.G.; Jumadilov, T.K. Features of polymethacrylic acid and poly-2-methyl-5-vinylpyridine hydrogels remote interaction in an aqueous medium. Chem. Chem. Eng. 2014, 8, 265–269. [Google Scholar] [CrossRef] [Green Version]
- Jumadilov, T.K.; Himersen, H.; Kaldayeva, S.S.; Kondaurov, R.G. Features of Electrochemical and Conformational Behavior of Intergel System Based on Polyacrylic Acid and Poly-4-Vinylpyridine Hydrogels in an Aqueous Medium. J. Mater. Sci. Eng. B. 2014, 4, 147–151. [Google Scholar]
- Jumadilov, T.; Abilov, Z.; Kondaurov, R.; Himersen, H.; Yeskalieva, G.; Akylbekova, M.; Akimov, A. Influence of Hydrogels Initial State on their Electrochemical and Volume-Gravimetric Properties in Intergel System Polyacrylic Acid Hydrogel and Poly-4-vinylpyridine Hydrogel. Chem. Chem. Technol. 2015, 9, 459–462. [Google Scholar] [CrossRef]
- Laatikainen, M.; Branger, C.; Laatikainen, K.; Sainio, T. Ion exchange of lanthanides with conventional and ion-imprinted resins containing sulfonic or iminodiacetic acid groups. Sep. Sci. Technol. 2021, 56, 203–216. [Google Scholar] [CrossRef]
- Botelho Junior, A.B.; Pinheiro, É.F.; Espinosa, D.C.R.; Tenório, J.A.S.; Baltazar, M.d.P.G. Adsorption of lanthanum and cerium on chelating ion exchange resins: Kinetic and thermodynamic studies. Sep. Sci. Technol. 2021, in press. [Google Scholar] [CrossRef]
- Kon’Kova, T.V.; Quynh, T.N. Sorption Recovery of Lanthanum, Iron, Aluminum, and Calcium Ions from Phosphoric Acid. Russ. J. Appl. Chem. 2020, 93, 1868–1872. [Google Scholar] [CrossRef]
- Sert, Ş.; Altaş, Y.; Tel, H.; Inan, S.; Çetinkaya, B.; Sengül, S.; Özkan, B. Investigation of sorption behaviors of La, Pr, Nd, Sm, Eu and Gd on D2EHPA-impregnated XAD7 resin in nitric acid medium. Sep. Sci. Technol. 2021, 56, 26–35. [Google Scholar] [CrossRef]
- Martin, D.M.; Jalaff, L.D.; Garcia, M.A.; Faccini, M. Selective Recovery of Europium and Yttrium Ions with Cyanex 272-Polyacrylonitrile Nanofibers. Nanomaterials 2021, 9, 1648. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abu Elgoud, E.M.; Ismail, Z.H.; Ahmad, M.I.; El-Nadi, Y.A.; Abdelwahab, S.M.; Aly, H.F.; Abu Elgoud, E.M.; Ismail, Z.; Ahmad, M.I.; El-Nadi, Y.A.; et al. Sorption of Lanthanum(III) and Neodymium(III) from Concentrated Phosphoric Acid by Strongly Acidic Cation Exchange Resin (SQS-6). Russ. J. Appl. Chem. 2019, 92, 1581–1592. [Google Scholar] [CrossRef]
- Kolodynska, D.; Hubicki, Z.; Fila, D. Recovery of rare earth elements from acidic solutions using macroporous ion exchangers. Sep. Sci. Technol. 2019, 54, 2059–2076. [Google Scholar] [CrossRef]
- Allahkarami, E.; Rezai, B. Removal of cerium from different aqueous solutions using different adsorbents: A review. Process. Saf. Environ. Prot. 2019, 124, 345–362. [Google Scholar] [CrossRef]
- Russo, V.; Taddeo, F.; Cogliano, T.; Vitiello, R.; Esposito, R.; Tesser, R.; Salmi, T.; Di Serio, M. Investigation of the intrinsic reaction kinetics and the mass transfer phenomena of nonanoic acid esterification with 2-ethylhexanol promoted by sulfuric acid or Amberlite IR120. Chem. Eng. J. 2020, 408, 127236. [Google Scholar] [CrossRef]
- Gossuin, Y.; Hantson, A.L.; Vuong, Q.L. Low resolution benchtop nuclear magnetic resonance for the follow-up of the removal of Cu2+ and Cr3+ from water by Amberlite IR120 ion exchange resin. J. Wat. Proc. Eng. 2020, 33, 101024. [Google Scholar] [CrossRef]
- Kumar, S.; Batra, S.; Datta, D. Use of polymeric adsorbent Amberlite IR120 H resin for isonicotinic adsorption. J. Mol. Liq. 2017, 247, 289–293. [Google Scholar] [CrossRef]
- Elabd, A.A.; Zidan, W.I.; Abo-Aly, M.M.; Bakier, E.; Attia, M.S. Uranyl ions adsorption by novel metal hydroxides loaded Amberlite IR120. J. Environ. Radioact. 2014, 134, 99–108. [Google Scholar] [CrossRef]
- Negrea, A.; Ciopec, M.; Davidescu, C.M.; Muntean, C.; Negrea, P.; Lupa, L. Kinetic, equilibrium and thermodynamic studies of cesium removal from aqueous solutions using Amberjet UP1400 and Amberlite IR120 resins. Environ. Eng. Manag. J. 2013, 12, 991–998. [Google Scholar] [CrossRef]
- Petrov, G.; Zotova, I.; Nikitina, T.; Fokina, S. Sorption recovery of platinum metals from production solutions of sulfate-chloride leaching of chromite wastes. Metals 2021, 11, 569. [Google Scholar] [CrossRef]
- Jumadilov, T.; Yskak, L.; Imangazy, A.; Suberlyak, O. Ion Exchange Dynamics in Cerium Nitrate Solution Regulated by Re-motely Activated Industrial Ion Exchangers. Materials 2021, 14, 3491. [Google Scholar] [CrossRef]
- Altshuler, H.N.; Ostapova, E.V.; Malyshenko, N.V.; Altshuler, O.H. Sorption of nicotinic and isonicotinic acids by the strongly basic anion exchanger AB-17-8. Russ. Chem. Bull. 2017, 66, 1854–1859. [Google Scholar] [CrossRef]
- Ivanovich, T.G. Technology to modernizations filtration equipment for cleaning water from the engine room of icebreakers. Mar. Int. Technol. 2016, 1, 186–191. [Google Scholar]
- Jumadilov, T.K.; Kondaurov, R.G.; Abilov, Z.A.; Grazulevicius, J.V.; Akimov, A.A. Influence of polyacrylic acid and poly-4-vinylpyridine hydrogels mutual activation in intergel system on their sorption properties in relation to lanthanum (III) ions. Polym. Bull. 2017, 74, 4701–4713. [Google Scholar] [CrossRef]
- Jumadilov, T.; Abilov, Z.; Grazulevicius, J.; Zhunusbekova, N.; Kondaurov, R.; Agibayeva, L.; Akimov, A. Mutual activation and sorption ability of rare cross-linked networks in intergel system based on polymethacrylic acid and poly-4-vinylpyridine hydrogels in relation to lanthanum ions. Chem. Chem. Technol. 2017, 11, 188–194. [Google Scholar] [CrossRef] [Green Version]
- Jumadilov, T.K.; Kondaurov, R.G.; Kozhabekov, S.S.; Tolegen, G.A.; Eskalieva, G.K.; Khakimzhanov, S.A. Influence of poly-acrylic acid hydrogel’s swelling degree on sorption ability of intergel system polyacrylic acid hydrogel–poly-4-vinylpyridine hydrogel in relation to neodymium ions. Chem. Technol. Met. 2018, 53, 88–93. [Google Scholar]
- Jumadilov, T.; Kondaurov, R.; Imangazy, A.; Myrzakhmetova, N.; Saparbekova, I. Phenomenon of Remote Interaction and Sorption Ability of Rare Cross-linked Hydrogels of Polymethacrylic Acid and Poly-4-vinylpyridine in Relation to Erbium Ions. Chem. Chem. Technol. 2019, 13, 451–458. [Google Scholar] [CrossRef]
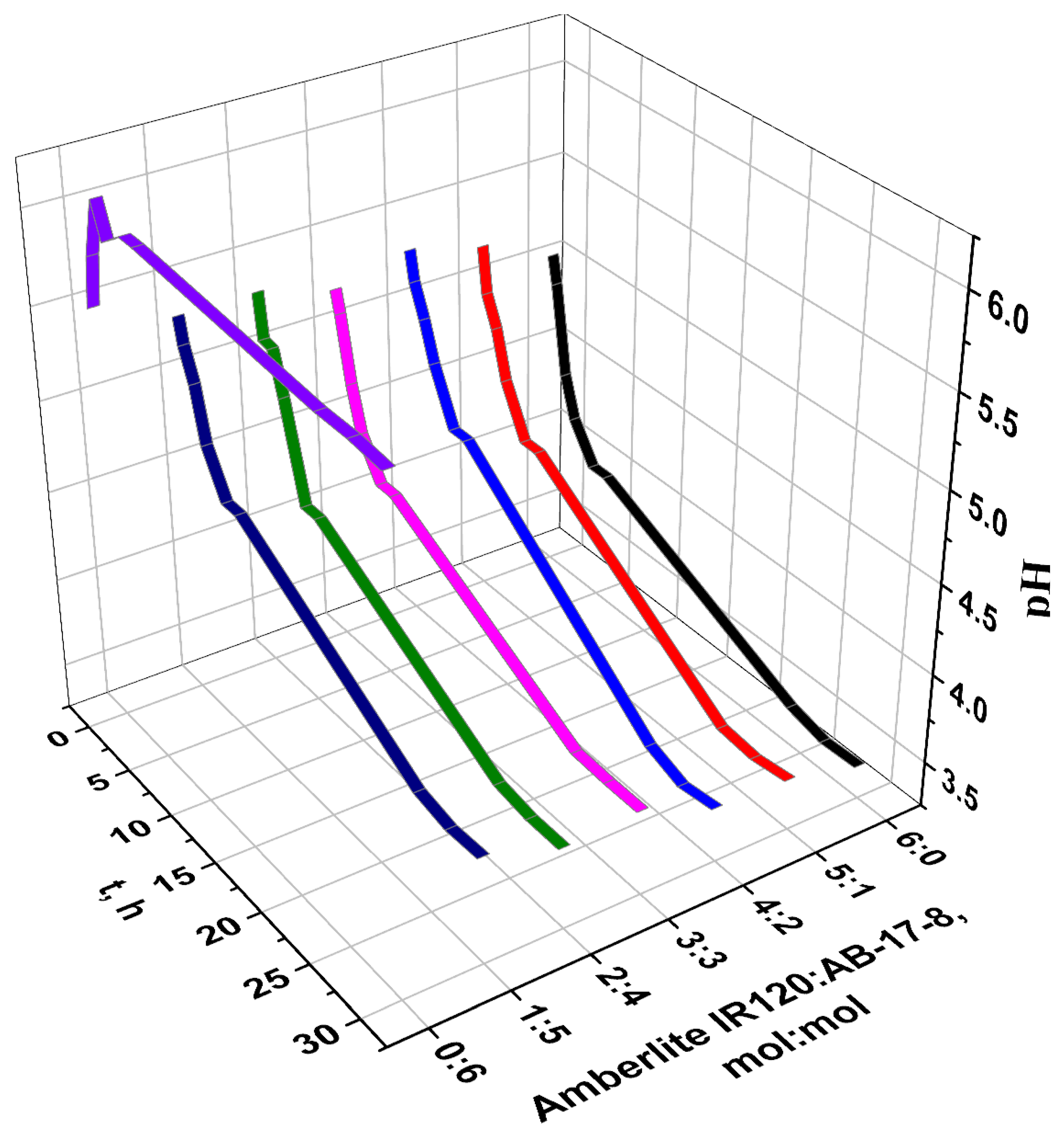

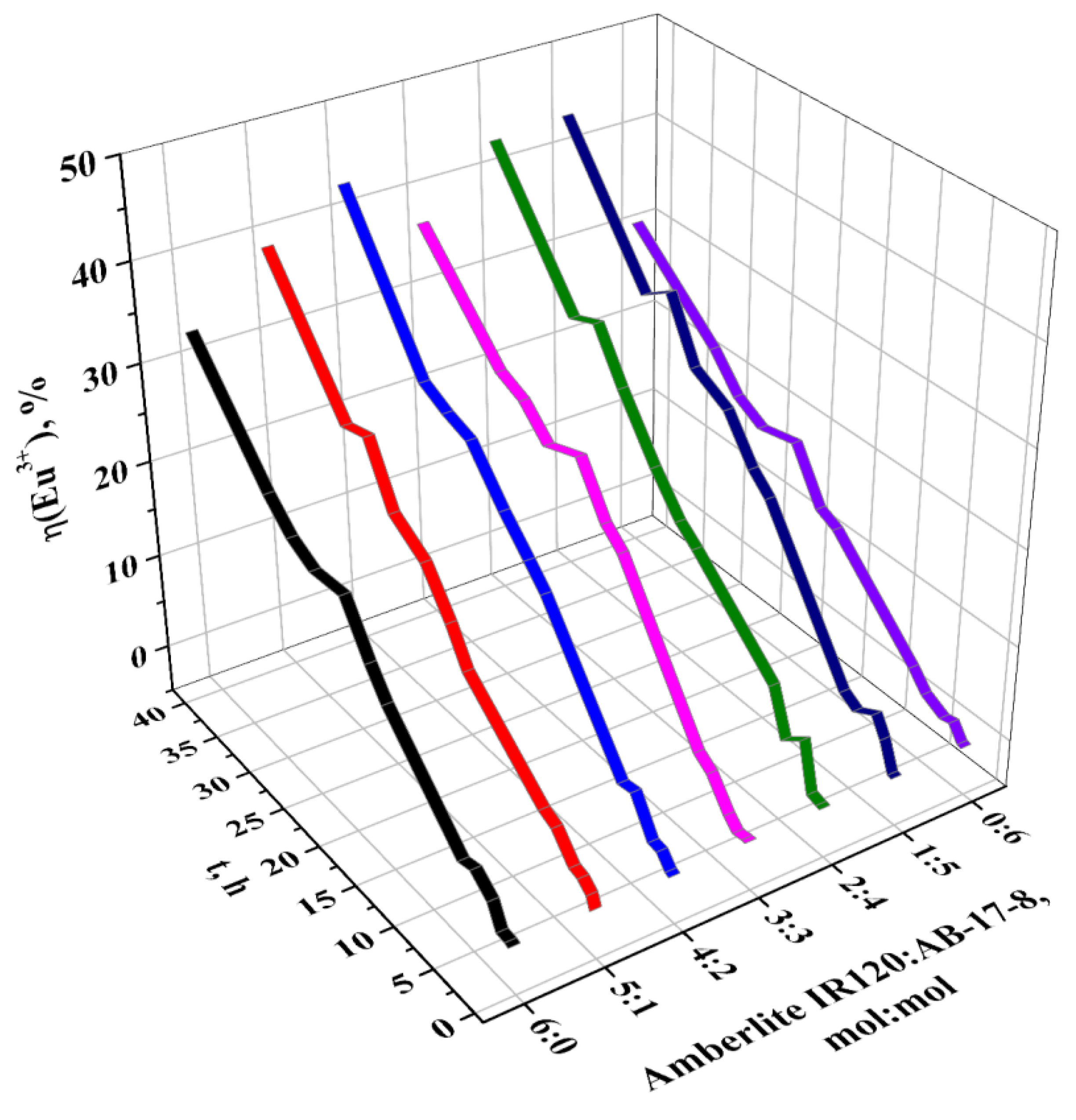
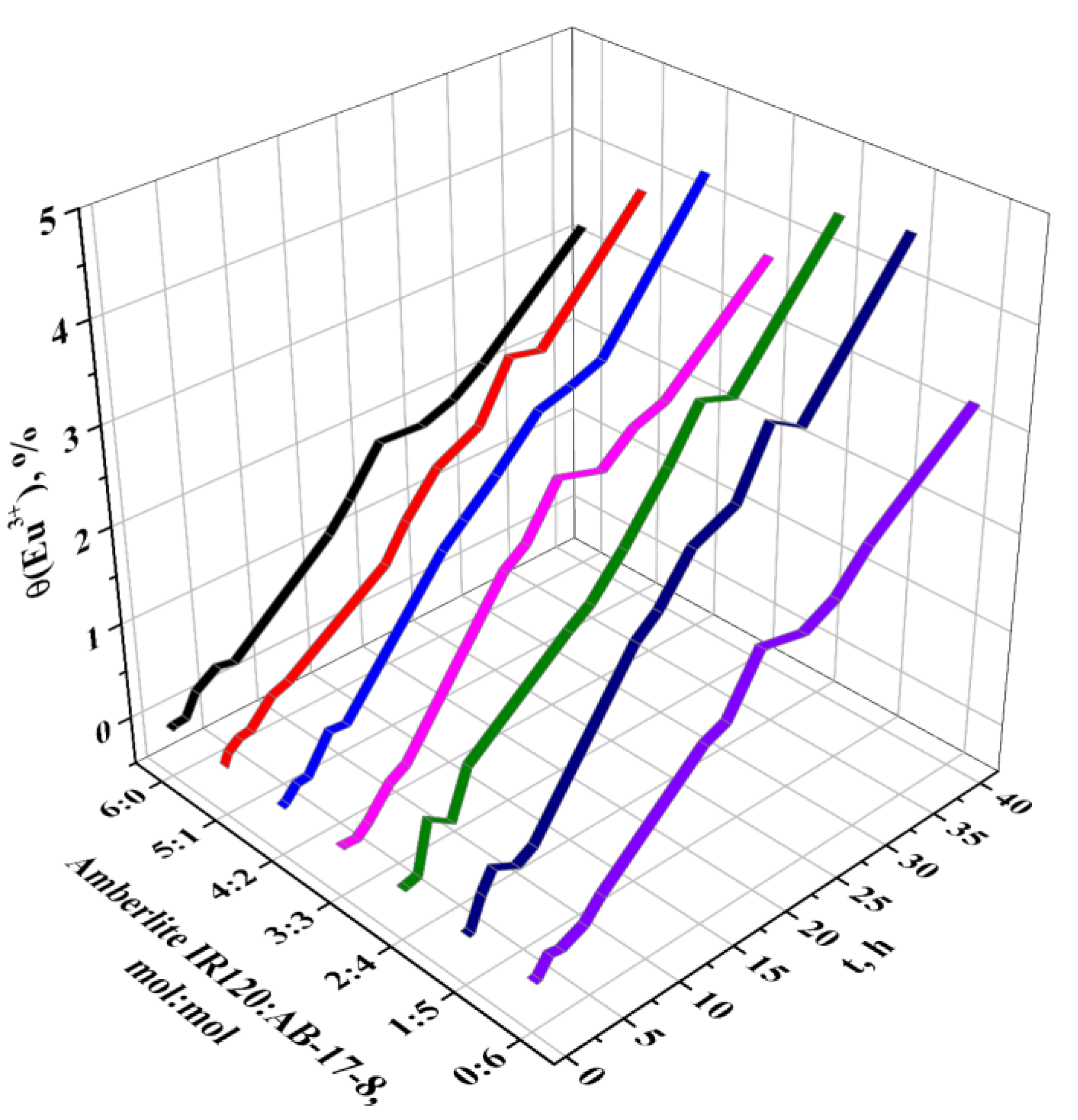



| t. h | η (Eu3+), % | ||||||
|---|---|---|---|---|---|---|---|
| Amberlite IR120:AB-17-8 mol:mol | |||||||
| 6:0 | 5:1 | 4:2 | 3:3 | 2:4 | 1:5 | 0:6 | |
| 0.08 | 0.1 | 0.2 | 0.1 | 0.2 | 0.3 | 0.2 | 0.2 |
| 0.5 | 0.3 | 1.45 | 0.3 | 0.2 | 0.4 | 0.3 | 0.31 |
| 1.5 | 0.4 | 2.34 | 1.58 | 0.05 | 0.7 | 3.23 | 1.83 |
| 2.5 | 2.72 | 2.59 | 1.7 | 1.07 | 5.64 | 5.13 | 1.58 |
| 4.5 | 4.24 | 5 | 5.26 | 3.86 | 4.11 | 3.99 | 2.59 |
| 6 | 3.86 | 5.64 | 5 | 4.88 | 8.68 | 4.88 | 4.37 |
| 15 | 12.5 | 12.74 | 17.57 | 18.83 | 16.42 | 18.84 | 12.87 |
| 17 | 15.15 | 16.17 | 19.6 | 20.48 | 17.92 | 20.61 | 13.76 |
| 20 | 19.85 | 20.2 | 22.5 | 25.3 | 21.5 | 24.8 | 18.84 |
| 24 | 19.47 | 22.39 | 27 | 23.91 | 27.08 | 26.7 | 17.95 |
| 27 | 20.74 | 28.1 | 27.85 | 26.58 | 31.9 | 32.67 | 19.47 |
| 30 | 23.15 | 27.34 | 29.11 | 27.72 | 30.76 | 30.76 | 22.77 |
| 40 | 33.17 | 39.14 | 43.07 | 37.1 | 43.2 | 43.7 | 30.64 |
| t. h | θ (Eu3+), % | ||||||
|---|---|---|---|---|---|---|---|
| Amberlite IR120:AB-17-8 mol:mol | |||||||
| 6:0 | 5:1 | 4:2 | 3:3 | 2:4 | 1:5 | 0:6 | |
| 0.08 | 0.01 | 0.02 | 0.01 | 0.02 | 0.03 | 0.02 | 0.02 |
| 0.5 | 0.03 | 0.14 | 0.03 | 0.02 | 0.04 | 0.03 | 0.03 |
| 1.5 | 0.04 | 0.23 | 0.16 | 0.01 | 0.07 | 0.33 | 0.19 |
| 2.5 | 0.26 | 0.25 | 0.17 | 0.11 | 0.58 | 0.53 | 0.17 |
| 4.5 | 0.41 | 0.49 | 0.52 | 0.39 | 0.42 | 0.41 | 0.27 |
| 6 | 0.37 | 0.55 | 0.50 | 0.49 | 0.89 | 0.51 | 0.46 |
| 15 | 1.21 | 1.25 | 1.75 | 1.90 | 1.68 | 1.95 | 1.35 |
| 17 | 1.47 | 1.59 | 1.95 | 2.06 | 1.83 | 2.13 | 1.44 |
| 20 | 1.92 | 1.98 | 2.24 | 2.55 | 2.20 | 2.57 | 1.98 |
| 24 | 1.89 | 2.20 | 2.68 | 2.41 | 2.77 | 2.76 | 1.88 |
| 27 | 2.01 | 2.76 | 2.77 | 2.68 | 3.26 | 3.38 | 2.04 |
| 30 | 2.24 | 2.68 | 2.89 | 2.79 | 3.14 | 3.18 | 2.39 |
| 40 | 3.21 | 3.84 | 4.28 | 3.74 | 4.41 | 4.52 | 3.22 |
| t. h | Q (Eu3+), mg/g | ||||||
|---|---|---|---|---|---|---|---|
| Amberlite IR120:AB-17-8 mol:mol | |||||||
| 6:0 | 5:1 | 4:2 | 3:3 | 2:4 | 1:5 | 0:6 | |
| 0.08 | 4.17 | 8.33 | 4.17 | 8.33 | 12.50 | 8.33 | 8.33 |
| 0.5 | 12.50 | 60.42 | 12.50 | 8.33 | 16.67 | 12.50 | 12.92 |
| 1.5 | 16.67 | 97.50 | 65.83 | 2.08 | 29.17 | 134.58 | 76.25 |
| 2.5 | 113.33 | 107.92 | 70.83 | 44.58 | 235.00 | 213.75 | 65.83 |
| 4.5 | 176.67 | 208.33 | 219.17 | 160.83 | 171.25 | 166.25 | 107.92 |
| 6 | 160.83 | 235.00 | 208.33 | 203.33 | 361.67 | 203.33 | 182.08 |
| 15 | 520.83 | 530.83 | 732.08 | 784.58 | 684.17 | 785.00 | 536.25 |
| 17 | 631.25 | 673.75 | 816.67 | 853.33 | 746.67 | 858.75 | 573.33 |
| 20 | 827.08 | 841.67 | 937.50 | 1054.17 | 895.83 | 1033.33 | 785.00 |
| 24 | 811.25 | 932.92 | 1125.00 | 996.25 | 1128.33 | 1112.50 | 747.92 |
| 27 | 864.17 | 1170.83 | 1160.42 | 1107.50 | 1329.17 | 1361.25 | 811.25 |
| 30 | 964.58 | 1139.17 | 1212.92 | 1155.00 | 1281.67 | 1281.67 | 948.75 |
| 40 | 1382.08 | 1630.83 | 1794.58 | 1545.83 | 1800.00 | 1820.83 | 1276.67 |
| t. h | η (Eu3+), % | ω (η), % | |||
|---|---|---|---|---|---|
| Amberlite IR120 | Amberlite IR120-AB-17-8 = 1:5 | AB-17-8 | Growth Relative to Amberlite IR120 | Growth Relative to AB-17-8 | |
| 0.08 | 0.1 | 0.20 | 0.20 | 100.00 | 0.00 |
| 0.5 | 0.3 | 0.30 | 0.31 | 0.00 | −3.23 |
| 1.5 | 0.4 | 3.23 | 1.83 | 707.50 | 76.50 |
| 2.5 | 2.72 | 5.13 | 1.58 | 88.60 | 224.68 |
| 4.5 | 4.24 | 3.99 | 2.59 | −5.90 | 54.05 |
| 6 | 3.86 | 4.88 | 4.37 | 26.42 | 11.67 |
| 15 | 12.5 | 18.84 | 12.87 | 50.72 | 46.39 |
| 17 | 15.15 | 20.61 | 13.76 | 36.04 | 49.78 |
| 20 | 19.85 | 24.80 | 18.84 | 24.94 | 31.63 |
| 24 | 19.47 | 26.70 | 17.95 | 37.13 | 48.75 |
| 27 | 20.74 | 32.67 | 19.47 | 57.52 | 67.80 |
| 30 | 23.15 | 30.76 | 22.77 | 32.87 | 35.09 |
| 40 | 33.17 | 43.70 | 30.64 | 31.75 | 42.62 |
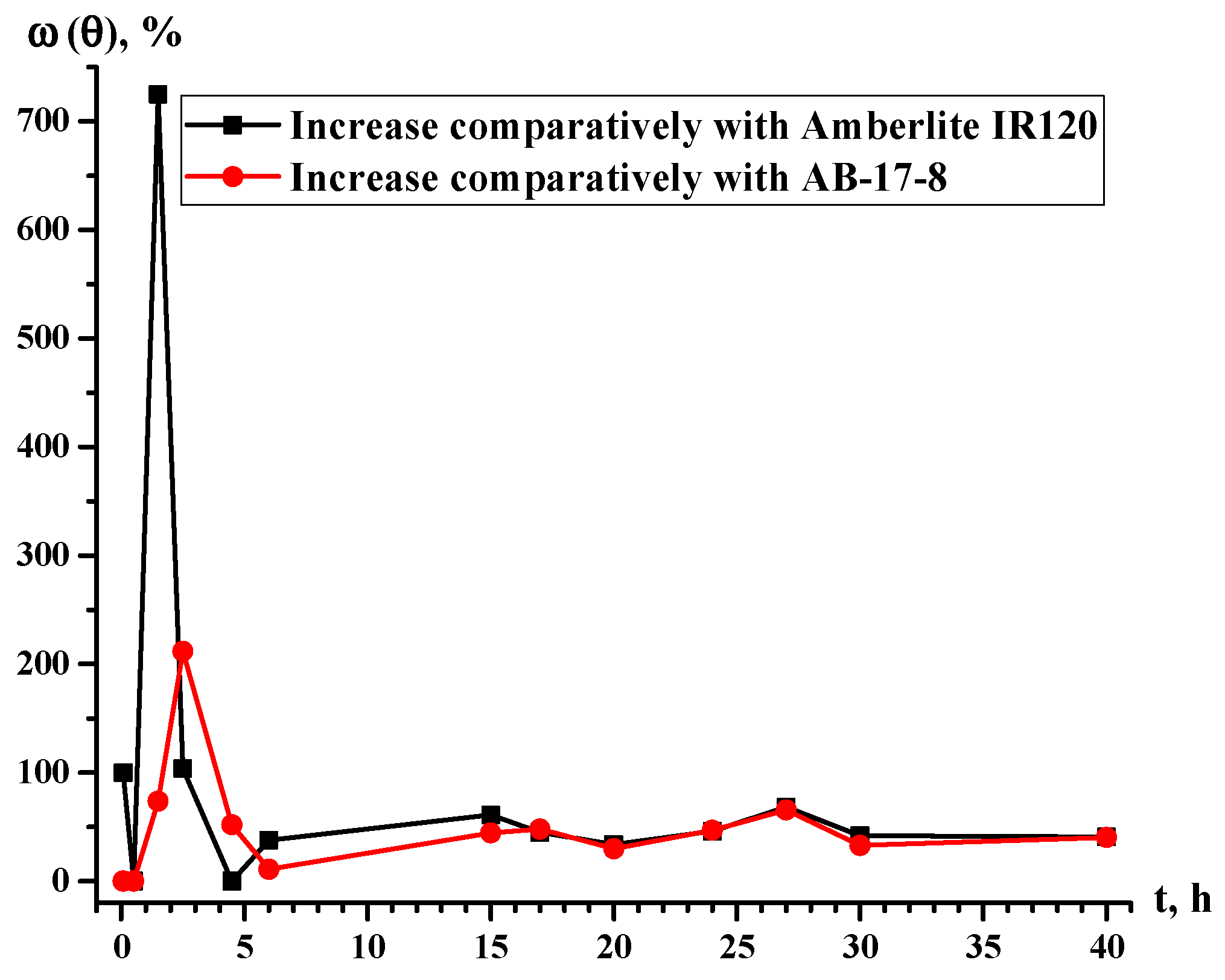
| t. h | θ (Eu3+), % | ω (θ), % | |||
|---|---|---|---|---|---|
| Amberlite IR120 | Amberlite IR120-AB-17-8 = 1:5 | AB-17-8 | Growth Relative to Amberlite IR120 | Growth Relative to AB-17-8 | |
| 0.08 | 0.01 | 0.02 | 0.02 | 100.00 | 0.00 |
| 0.5 | 0.03 | 0.03 | 0.03 | 0.00 | 0.00 |
| 1.5 | 0.04 | 0.33 | 0.19 | 725.00 | 73.68 |
| 2.5 | 0.26 | 0.53 | 0.17 | 103.85 | 211.76 |
| 4.5 | 0.41 | 0.41 | 0.27 | 0.00 | 51.85 |
| 6 | 0.37 | 0.51 | 0.46 | 37.84 | 10.87 |
| 15 | 1.21 | 1.95 | 1.35 | 61.16 | 44.44 |
| 17 | 1.47 | 2.13 | 1.44 | 44.90 | 47.92 |
| 20 | 1.92 | 2.57 | 1.98 | 33.85 | 29.80 |
| 24 | 1.89 | 2.76 | 1.88 | 46.03 | 46.81 |
| 27 | 2.01 | 3.38 | 2.04 | 68.16 | 65.69 |
| 30 | 2.24 | 3.18 | 2.39 | 41.96 | 33.05 |
| 40 | 3.21 | 4.52 | 3.22 | 40.81 | 40.37 |
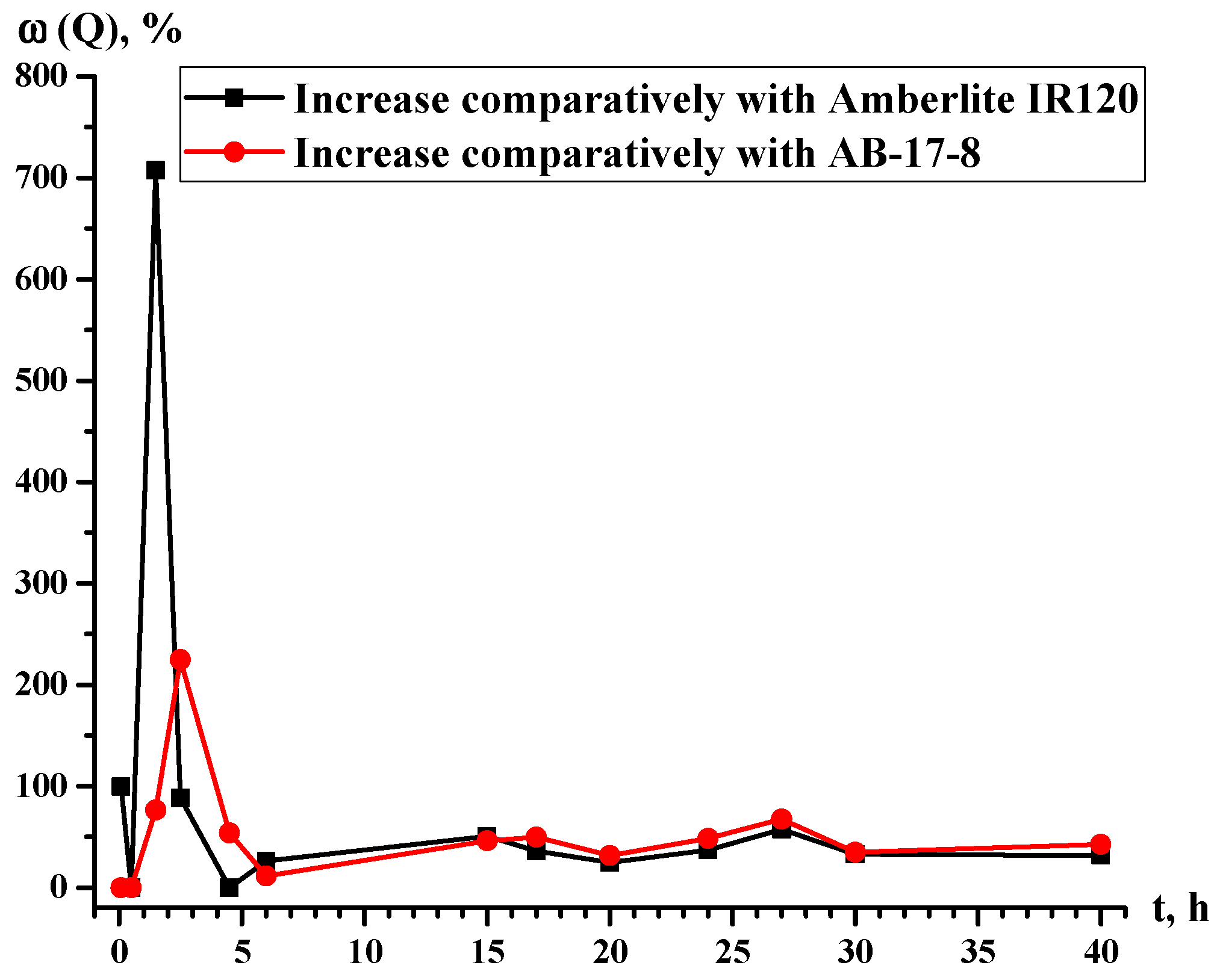
| t. h | Q (Eu3+), mg/g | ω (Q), % | |||
|---|---|---|---|---|---|
| Amberlite IR120 | Amberlite IR120-AB-17-8 = 1:5 | AB-17-8 | Growth Relatively to Amberlite IR120 | Growth Relatively to AB-17-8 | |
| 0.08 | 4.17 | 8.33 | 8.33 | 99.76 | 0.00 |
| 0.5 | 12.50 | 12.50 | 12.50 | 0.00 | 0 |
| 1.5 | 16.67 | 134.58 | 76.25 | 707.32 | 76.50 |
| 2.5 | 113.33 | 213.75 | 65.83 | 88.61 | 224.70 |
| 4.5 | 166.25 | 166.25 | 107.92 | 0 | 54.05 |
| 6 | 160.83 | 203.33 | 182.08 | 26.43 | 11.67 |
| 15 | 520.83 | 785.00 | 536.25 | 50.72 | 46.39 |
| 17 | 631.25 | 858.75 | 573.33 | 36.04 | 49.78 |
| 20 | 827.08 | 1033.33 | 785.00 | 24.94 | 31.63 |
| 24 | 811.25 | 1112.50 | 747.92 | 37.13 | 48.75 |
| 27 | 864.17 | 1361.25 | 811.25 | 57.52 | 67.80 |
| 30 | 964.58 | 1281.67 | 948.75 | 32.87 | 35.09 |
| 40 | 1382.08 | 1820.83 | 1276.67 | 31.75 | 42.62 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jumadilov, T.; Khimersen, K.; Malimbayeva, Z.; Kondaurov, R. Effective Sorption of Europium Ions by Interpolymer System Based on Industrial Ion-Exchanger Resins Amberlite IR120 and AB-17-8. Materials 2021, 14, 3837. https://doi.org/10.3390/ma14143837
Jumadilov T, Khimersen K, Malimbayeva Z, Kondaurov R. Effective Sorption of Europium Ions by Interpolymer System Based on Industrial Ion-Exchanger Resins Amberlite IR120 and AB-17-8. Materials. 2021; 14(14):3837. https://doi.org/10.3390/ma14143837
Chicago/Turabian StyleJumadilov, Talkybek, Khuangul Khimersen, Zamira Malimbayeva, and Ruslan Kondaurov. 2021. "Effective Sorption of Europium Ions by Interpolymer System Based on Industrial Ion-Exchanger Resins Amberlite IR120 and AB-17-8" Materials 14, no. 14: 3837. https://doi.org/10.3390/ma14143837
APA StyleJumadilov, T., Khimersen, K., Malimbayeva, Z., & Kondaurov, R. (2021). Effective Sorption of Europium Ions by Interpolymer System Based on Industrial Ion-Exchanger Resins Amberlite IR120 and AB-17-8. Materials, 14(14), 3837. https://doi.org/10.3390/ma14143837






