Change of Oxidation Mechanisms by Laser Chemical Machined Rim Zone Modifications of 42CrMo4 Steel
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
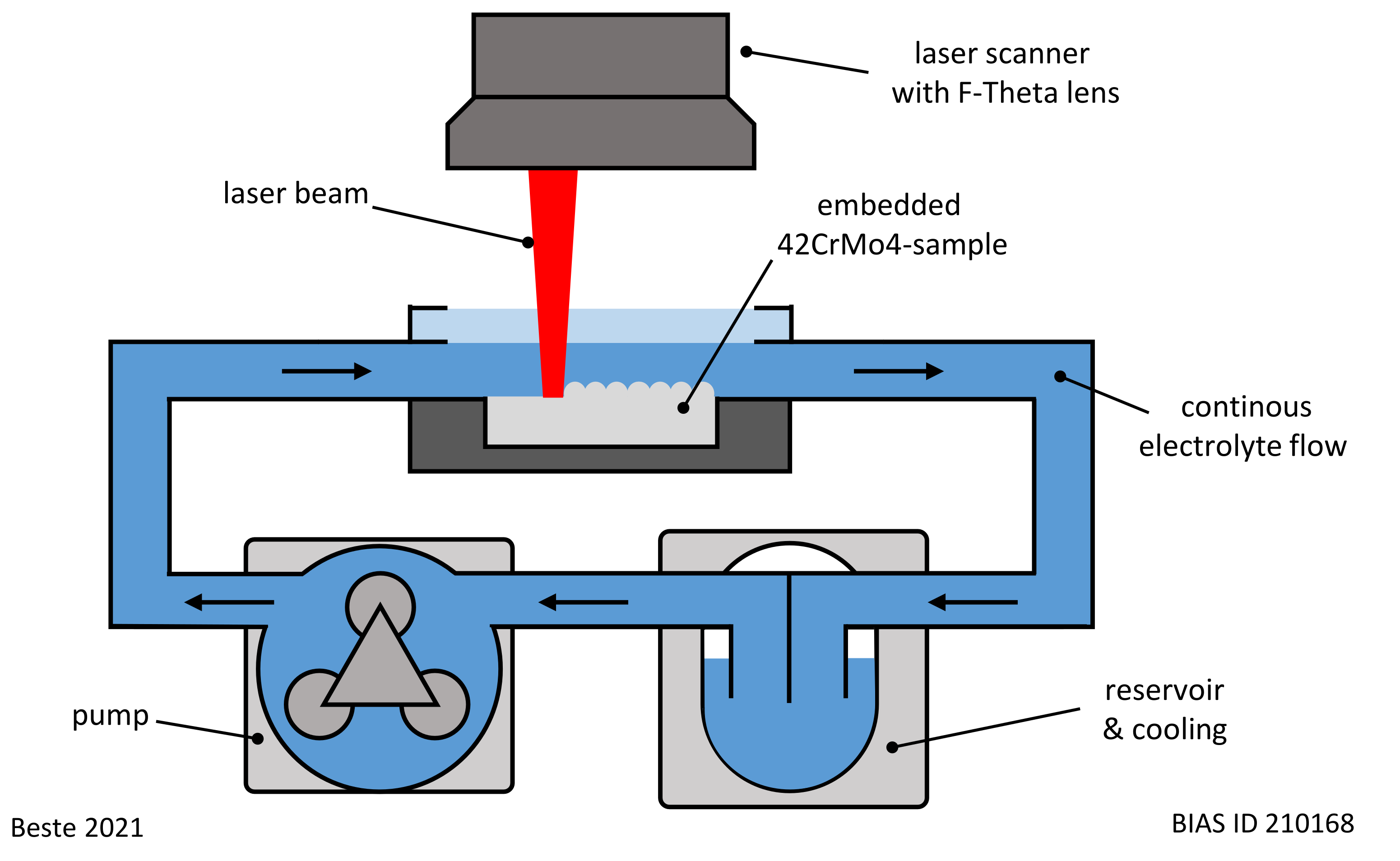
2.2. LCM
2.3. Methods
3. Results
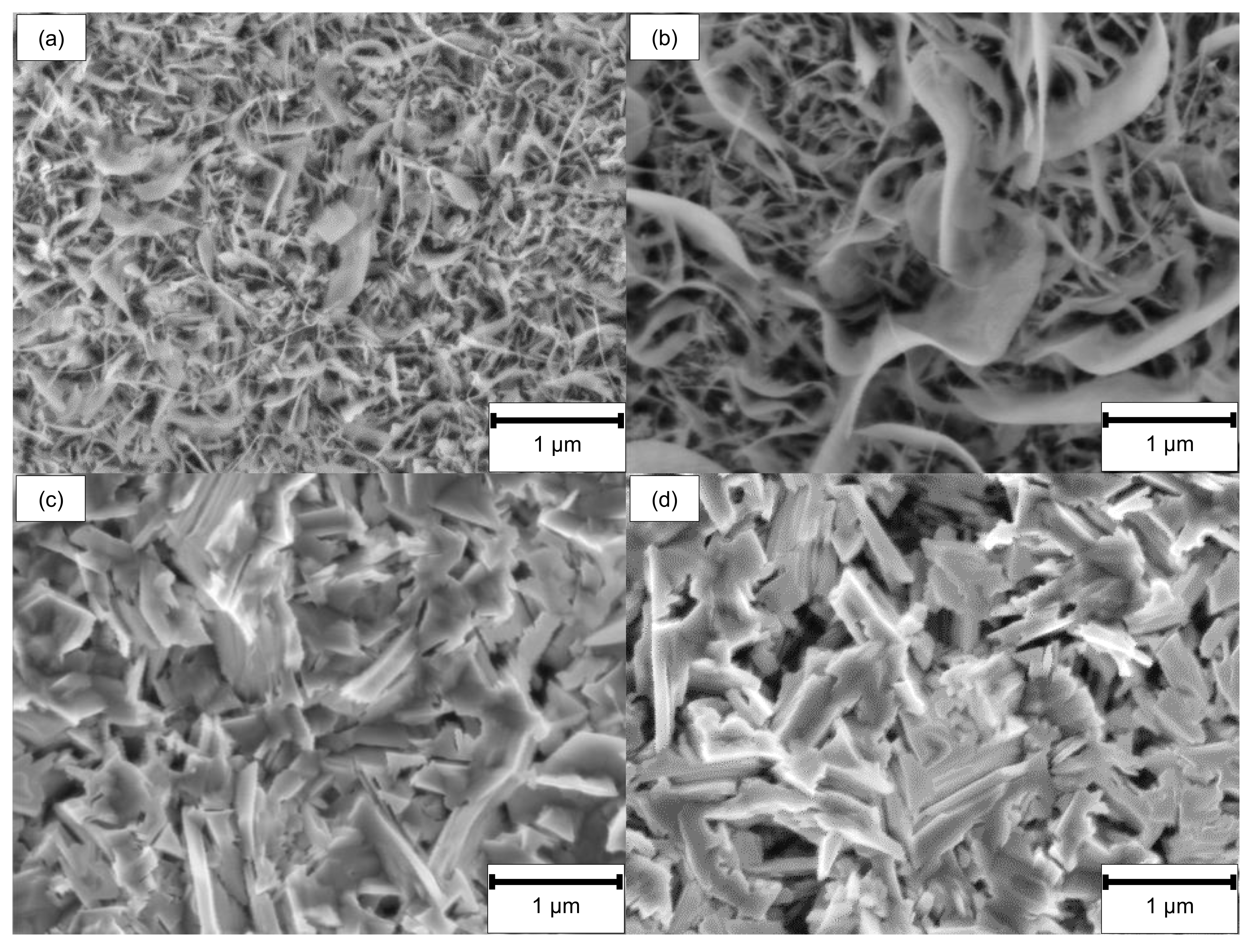
3.1. Surface and Rim Zone Analysis before Oxidation
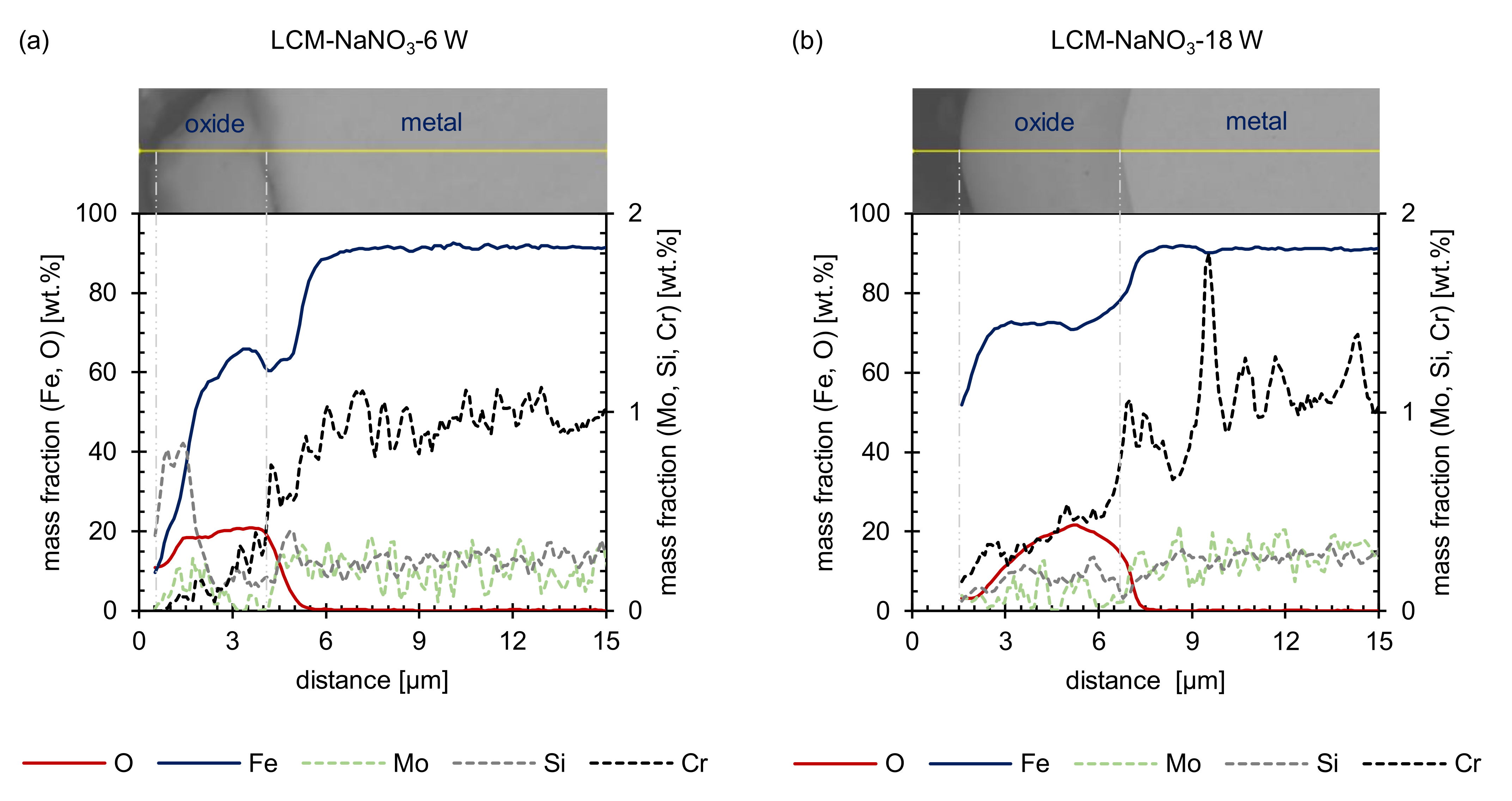
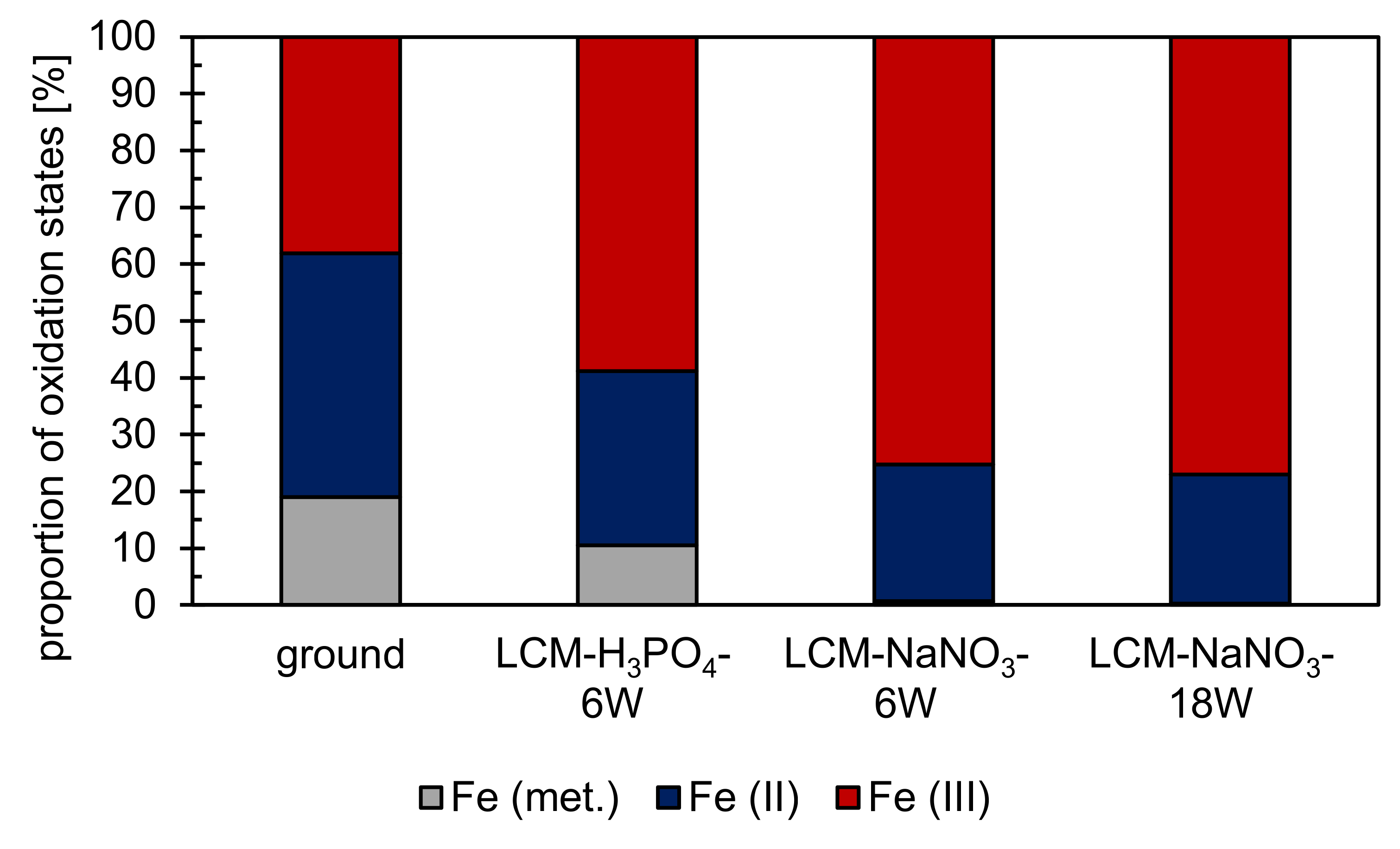
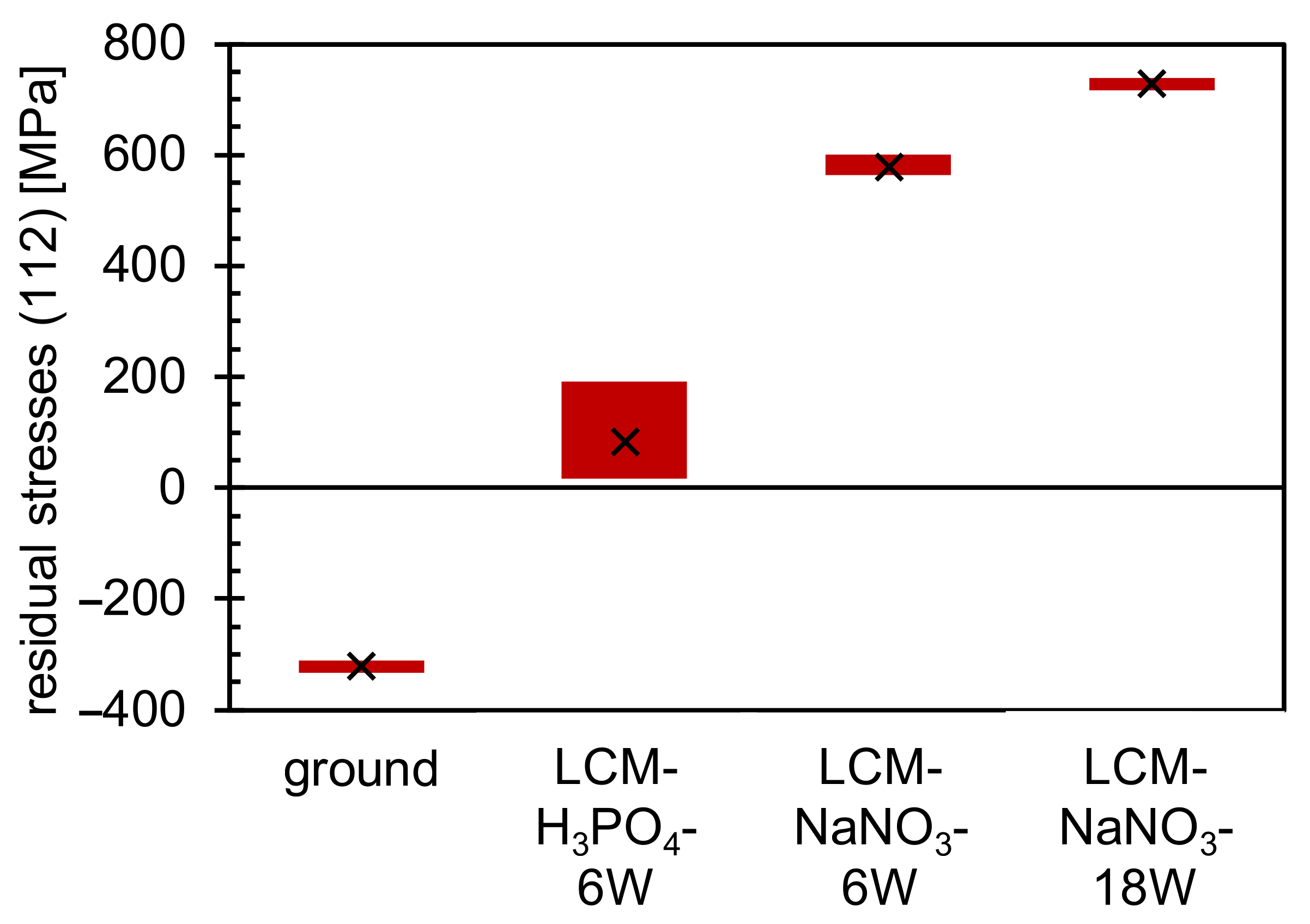
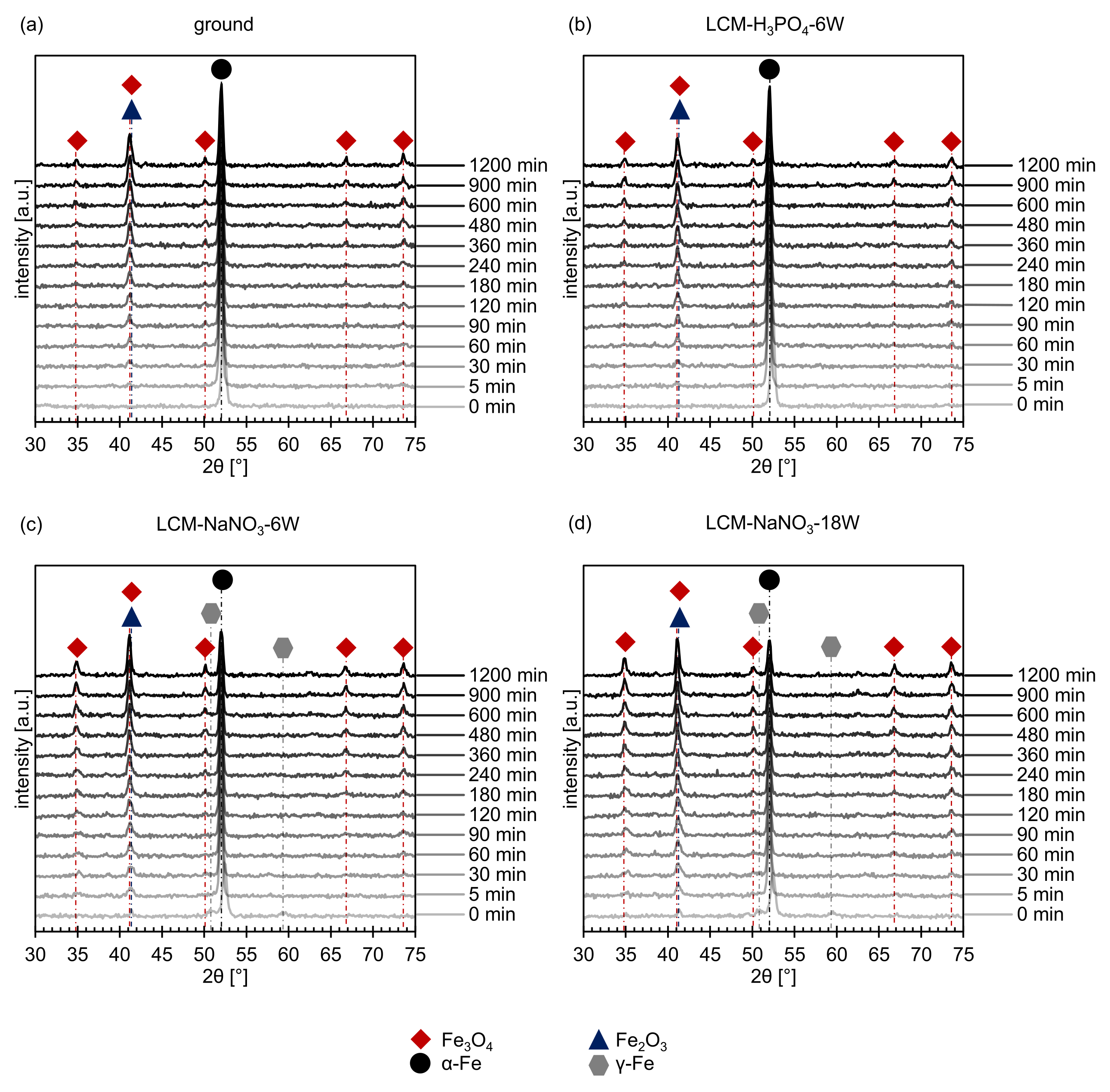
3.2. Oxide Formation at 500 °C
4. Discussion
4.1. LCM Rim Zone before Oxidation
4.2. Oxidation Mechanism in Air at 500 °C
5. Conclusions
- The thickness of the oxide layer is larger for LCM-NaNO3-6W and LCM-NaNO3-18W than for ground and LCM-H3PO4-6W surfaces. This is assumed to be predominantly attributed to the presence of Fe3O4-type oxides from the LCM process, which serve as oxidation nucleation sites at the beginning of the oxidation and thus accelerate the oxide layer growth.
- For all surfaces examined, an inner oxide layer of (Fe,Cr,Mo,Si)3O4 and an outer oxide layer of Fe3O4 could be detected. For the ground and LCM-H3PO4-6W surfaces, an additional outer Fe2O3 layer was identified. This layer is non-existent on LCM-NaNO3-6W and LCM-NaNO3-18W. This is attributed to the effect that iron diffusion is faster through the oxide layer for LCM-NaNO3-6W and LCM-NaNO3-18W than in LCM-H3PO4-6W 42CrMo4 steel and the ground surfaces. Since Fe3O4 preferentially converts to Fe2O3 in the presence of an iron deficit, rapid iron diffusion in the oxide can delay the formation of such iron deficiencies and thus the formation of Fe2O3.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zander, D.; Klink, A.; Harst, S.; Klocke, F.; Altenbach, C. Influence of machining processes on rim zone properties and high temperature oxidation behavior of 42CrMo4. Mater. Corros. 2019, 70, 2190–2204. [Google Scholar] [CrossRef]
- Eckert, S.; Vollertsen, F. Mechanisms and processing limits of surface finish using laser-thermochemical polishing. Cirp Ann. 2018, 67, 201–204. [Google Scholar] [CrossRef]
- Eckert, S. Multi-Cycle Process Signature of Laser-Induced Thermochemical Polishing. J. Manuf. Mater. Process. 2019, 3, 90. [Google Scholar] [CrossRef] [Green Version]
- Messaoudi, H.; Mikulewitsch, M.; Brand, D.; von Freyberg, A.; Fischer, A. Removal behavior and output quality for laser chemical machining of tool steels. Manuf. Rev. 2019, 6, 13. [Google Scholar] [CrossRef]
- Eckert, S.; Vollertsen, F.; Schupp, A.; Zander, D.; Rommes, B.; Klink, A. Understanding the influence of chemical and thermal loads on the productivity for processing 42CrMo4 steel and titanium via LCM. Proc. CIRP 2021. accepted. [Google Scholar]
- Manjaiah, M.; Narendranath, S.; Basavarajappa, S. Review on non-conventional machining of shape memory alloys. Trans. Nonferrous Met. Soc. China 2014, 24, 12–21. [Google Scholar] [CrossRef]
- Stephen, A.; Vollertsen, F. Mechanisms and processing limits in laser thermochemical machining. Cirp Ann. 2010, 59, 251–254. [Google Scholar] [CrossRef]
- Bäuerle, D. Laser Processing and Chemistry, 4th ed.; Springer: Berlin/Heidelberg, Germany, 2011. [Google Scholar] [CrossRef]
- Li, L.; Achara, C. Chemical assisted laser machining for the minimisation of recast and heat affected zone. Cirp Ann. 2004, 53, 175–178. [Google Scholar] [CrossRef]
- Messaoudi, H. Thermische Bedingungen der Laserchemischen Mikrobearbeitung von Metallen. Doctoral Dissertation, Universität Bremen, Bremen, Germany, 2020. [CrossRef]
- Simons, M.; Radel, T.; Shanta, V.; Vollertsen, F. Comparison of boiling bubble behavior during laser chemical machining under superatmospheric pressure. Procedia Cirp 2020, 94, 561–564. [Google Scholar] [CrossRef]
- Mehrafsun, S.; Harst, S.; Hauser, O.; Eckert, S.; Klink, A.; Klocke, F.; Vollertsen, F. Energy-based analysis of material dissolution behavior for laser-chemical and electrochemical machining. Proc. Cirp 2016, 45, 347–350. [Google Scholar] [CrossRef] [Green Version]
- Brinksmeier, E.; Reese, S.; Klink, A.; Langenhorst, L.; Lübben, T.; Meinke, M.; Meyer, D.; Riemer, O.; Sölter, J. Underlying mechanisms for developing process signatures in manufacturing. Nanomanuf. Metrol. 2018, 1, 193–208. [Google Scholar] [CrossRef] [Green Version]
- Von Gutfeld, R.J.; Vigliotti, D.R.; Datta, M. Laser chemical etching of metals in sodium nitrate solution. J. Appl. Phys. 1988, 64, 5197–5200. [Google Scholar] [CrossRef]
- Chang, Y.N.; Wei, F.I. High temperature oxidation of low alloy steels. J. Mater. Sci. 1989, 24, 14–22. [Google Scholar] [CrossRef]
- Chen, R.Y.; Yeun, W.Y.D. Review of the high-temperature oxidation of iron and carbon steels in air or oxygen. Oxid. Met. 2003, 59, 433–468. [Google Scholar] [CrossRef]
- Hidayat, T.; Shishin, D.; Jak, E.; Decterov, S.A. Thermodynamic reevaluation of the Fe–O system. Calphad 2015, 48, 131–144. [Google Scholar] [CrossRef]
- Trindade, V.B.; Borin, R.; Hanjari, B.Z.; Yang, S.; Krupp, U.; Christ, H.J. High-temperature oxidation of pure Fe and the ferritic steel 2.25 Cr1Mo. Mater. Res. 2005, 8, 365–369. [Google Scholar] [CrossRef]
- Pujilaksono, B.; Jonsson, T.; Halvarsson, M.; Svensson, J.E.; Johansson, L.G. Oxidation of iron at 400–600 C in dry and wet O2. Corr. Sci. 2010, 52, 1560–1569. [Google Scholar] [CrossRef]
- Al-Mazrouee, A.; Singh Raman, R.K. High temperature oxidation of Cr-Mo steels in the context of accelerated rupture testing for creep life prediction. J. Pressure Vessel Technol. 2007, 129, 454–459. [Google Scholar] [CrossRef]
- Folkeson, N.; Jonsson, T.; Halvarsson, M.; Johansson, L.G.; Svensson, J.E. The influence of small amounts of KCl (s) on the high temperature corrosion of a Fe-2.25 Cr-1Mo steel at 400 and 500 °C. Mater. Corros. 2011, 62, 606–615. [Google Scholar] [CrossRef]
- Bae, S.Y.; Kang, H.G.; Yun, H.S.; Kim, C.W.; Lee, D.B.; Lim, B.S. Oxidation and fatigue crack propagation in the range of low stress intensity factor in relation to the microstructure in P122 Cr–Mo steel. Mater. Sci. Eng. A 2009, 499, 262–266. [Google Scholar] [CrossRef]
- Raceanu, L.; Optasanu, V.; Montesin, T.; Montay, G.; François, M. Shot-peening of pre-oxidized plates of zirconium: Influence of residual stress on oxidation. Oxid. Met. 2013, 79, 135–145. [Google Scholar] [CrossRef]
- Brito, P.; Pinto, H.; Kostka, A. The crystallographic template effect assisting the formation of stable α-Al2O3 during low temperature oxidation of Fe–Al alloys. Corr. Sci. 2016, 105, 100–108. [Google Scholar] [CrossRef]
- Seal, S.; Bose, S.K.; Roy, S.K. Improvement in the oxidation behavior of austenitic stainless steels by superficially applied, cerium oxide coatings. Oxid. Met. 1994, 41, 139–178. [Google Scholar] [CrossRef]
- Moon, D.P.; Bennett, M.J. The effects of reactive element oxide coatings on the oxidation behaviour of metals and alloys at high temperatures. Mater. Sci. Forum 1989, 43, 269–298. [Google Scholar] [CrossRef]
- Buscail, H.; Larpin, J.P. The influence of cerium surface addition on low-pressure oxidation of pure iron at high temperatures. Solid State Ion. 1996, 92, 243–251. [Google Scholar] [CrossRef]
- Borchers, F.; Clausen, B.; Eckert, S.; Ehle, L.; Epp, J.; Harst, S.; Hettig, M.; Klink, A.; Kohls, E.; Meyer, H.; et al. Comparison of Different Manufacturing Processes of AISI 4140 Steel with Regard to Surface Modification and Its Influencing Depth. Metals 2020, 10, 895. [Google Scholar] [CrossRef]
- Zander, D.; Schupp, A.; Beyss, O.; Rommes, B.; Klink, A. Oxide Formation during Transpassive Material Removal of Martensitic 42CrMo4 Steel by Electrochemical Machining. Materials 2021, 14, 402. [Google Scholar] [CrossRef]
- Schupp, A.; Beyss, O.; Rommes, B.; Klink, A.; Zander, D. Insights on the Influence of Surface Chemistry and Rim Zone Microstructure of 42CrMo4 on the Efficiency of ECM. Materials 2021, 14, 2132. [Google Scholar] [CrossRef]
- Czech, N.; Juez-Lorenzo, M.; Kolarik, V.; Stamm, W. Influence of the surface roughness on the oxide scale formation on MCrAlY coatings studied in situ by high temperature X-ray diffraction. Surf. Coat. Technol. 1998, 108, 36–42. [Google Scholar] [CrossRef]
- Grosvenor, A.P.; Kobe, B.A.; Biesinger, M.C.; McIntyre, N.S. Investigation of multiplet splitting of Fe 2p XPS spectra and bonding in iron compounds. Surf. Interface Anal. 2004, 36, 1564–1574. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Payne, B.P.; Grosvenor, A.P.; Lau, L.W.; Gerson, A.R.; Smart, R.S.C. Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Cr, Mn, Fe, Co and Ni. Appl. Surf. Sci. 2011, 257, 2717–2730. [Google Scholar] [CrossRef]
- Kurp, P.; Mucha, Z.; Mulczyk, K.; Gradoń, R.; Trela, P. The influence of surface preparation on the absorption coefficient of laser radiation. Proc. SPIE 2016, 10159, 101590M. [Google Scholar] [CrossRef]
- Mehrafsun, S.; Messaoudi, H.; Vollertsen, F. Influence of material and surface roughness on gas bubble formation and adhesion in laser-chemical machining. In Proceedings of the 5th International Conference Nanomanufacturing, 2016, Macao, China, 15–17 August 2016; pp. 1–10. [Google Scholar]
- Ellingham, H.J.T. Reducibility of oxides and sulphides in metallurgical processes. J. Soc. Chem. Ind. 1944, 63, 125–133. [Google Scholar]
- Prabakaran, K.; Rajeswari, S. Electrochemical, SEM and XPS investigations on phosphoric acid treated surgical grade type 316L SS for biomedical applications. J. Appl. Electrochem. 2009, 39, 887–897. [Google Scholar] [CrossRef]
- Trindade, V.; Christ, H.J.; Krupp, U. Grain-size effects on the high-temperature oxidation behaviour of chromium steels. Oxid. Met. 2010, 73, 551–563. [Google Scholar] [CrossRef]
- Gokhale, K.V.G.K. Studies on the oxidation of magnetite. Econ. Geol. 1961, 56, 963–971. [Google Scholar] [CrossRef]
- Colombo, U.; Fagherazzi, G.; Gazzarrini, F.; Lanzavecchia, G.; Sironi, G. Mechanisms in the first stage of oxidation of magnetites. Nature 1964, 202, 175–176. [Google Scholar] [CrossRef]
- Kuroda, K.; Labun, P.A.; Welsch, G.; Mitchell, T.E. Oxide-formation characteristics in the early stages of oxidation of Fe and Fe-Cr alloys. Oxid. Met. 1983, 19, 117–127. [Google Scholar] [CrossRef]
- Khanna, A.S.; Gnanamoorthy, J.B. Effect of cold work on the oxidation resistance of 2 1/4 Cr-1 Mo steel. Oxid. Met. 1985, 23, 17–33. [Google Scholar] [CrossRef]
- Vannerberg, N.; Svedung, I. The influence of small amounts of P on the oxidation properties of Fe. Corros. Sci. 1971, 11, 915–927. [Google Scholar] [CrossRef]






| Element | Cr | Mo | Mn | C | Si | Fe |
|---|---|---|---|---|---|---|
| wt.% | 1.09 | 0.24 | 0.74 | 0.45 | 0.26 | bal. |
| Process | Electrolyte | Electrolyte Volume [L] | Electrolyte Flow Rate [L/min] | Laser Power [W] |
|---|---|---|---|---|
| LCM-NaNO3-6W | 2.5 M NaNO3 | 2 | 4 | 6 |
| LCM-NaNO3-18W | 2.5 M NaNO3 | 2 | 4 | 18 |
| LCM-H3PO4-6W | 5 M H3PO4 | 2 | 4 | 6 |
| Element [wt.%] | Fe | O | Cr | Mo | C | P | N |
|---|---|---|---|---|---|---|---|
| ground [30] | 52.0 | 31.5 | 1.0 | <0.5 | 11.0 | n.e. | n.e. |
| LCM-H3PO4-6W | 34.5 | 42.0 | 1.5 | 3.5 | 12.5 | 6.0 | n.e. |
| LCM-NaNO3-6W | 33.5 | 40.0 | 6.0 | <0.5 | 14.0 | n.e. | <1.0 |
| LCM-NaNO3-18W | 35.5 | 42.5 | 3.0 | <0.5 | 11.5 | n.e. | <1.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schupp, A.; Pütz, R.D.; Beyss, O.; Beste, L.-H.; Radel, T.; Zander, D. Change of Oxidation Mechanisms by Laser Chemical Machined Rim Zone Modifications of 42CrMo4 Steel. Materials 2021, 14, 3910. https://doi.org/10.3390/ma14143910
Schupp A, Pütz RD, Beyss O, Beste L-H, Radel T, Zander D. Change of Oxidation Mechanisms by Laser Chemical Machined Rim Zone Modifications of 42CrMo4 Steel. Materials. 2021; 14(14):3910. https://doi.org/10.3390/ma14143910
Chicago/Turabian StyleSchupp, Alexander, René Daniel Pütz, Oliver Beyss, Lucas-Hermann Beste, Tim Radel, and Daniela Zander. 2021. "Change of Oxidation Mechanisms by Laser Chemical Machined Rim Zone Modifications of 42CrMo4 Steel" Materials 14, no. 14: 3910. https://doi.org/10.3390/ma14143910
APA StyleSchupp, A., Pütz, R. D., Beyss, O., Beste, L.-H., Radel, T., & Zander, D. (2021). Change of Oxidation Mechanisms by Laser Chemical Machined Rim Zone Modifications of 42CrMo4 Steel. Materials, 14(14), 3910. https://doi.org/10.3390/ma14143910






