Iron-Based Water Treatment Residuals: Phase, Physicochemical Characterization, and Textural Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. GWTRs
2.2. Method of Analysis
3. Results and Discussion
3.1. XRD Analysis
3.2. Chemical Composition
3.3. FTIR Analysis
3.4. TG/DTA
3.5. BET Analysis
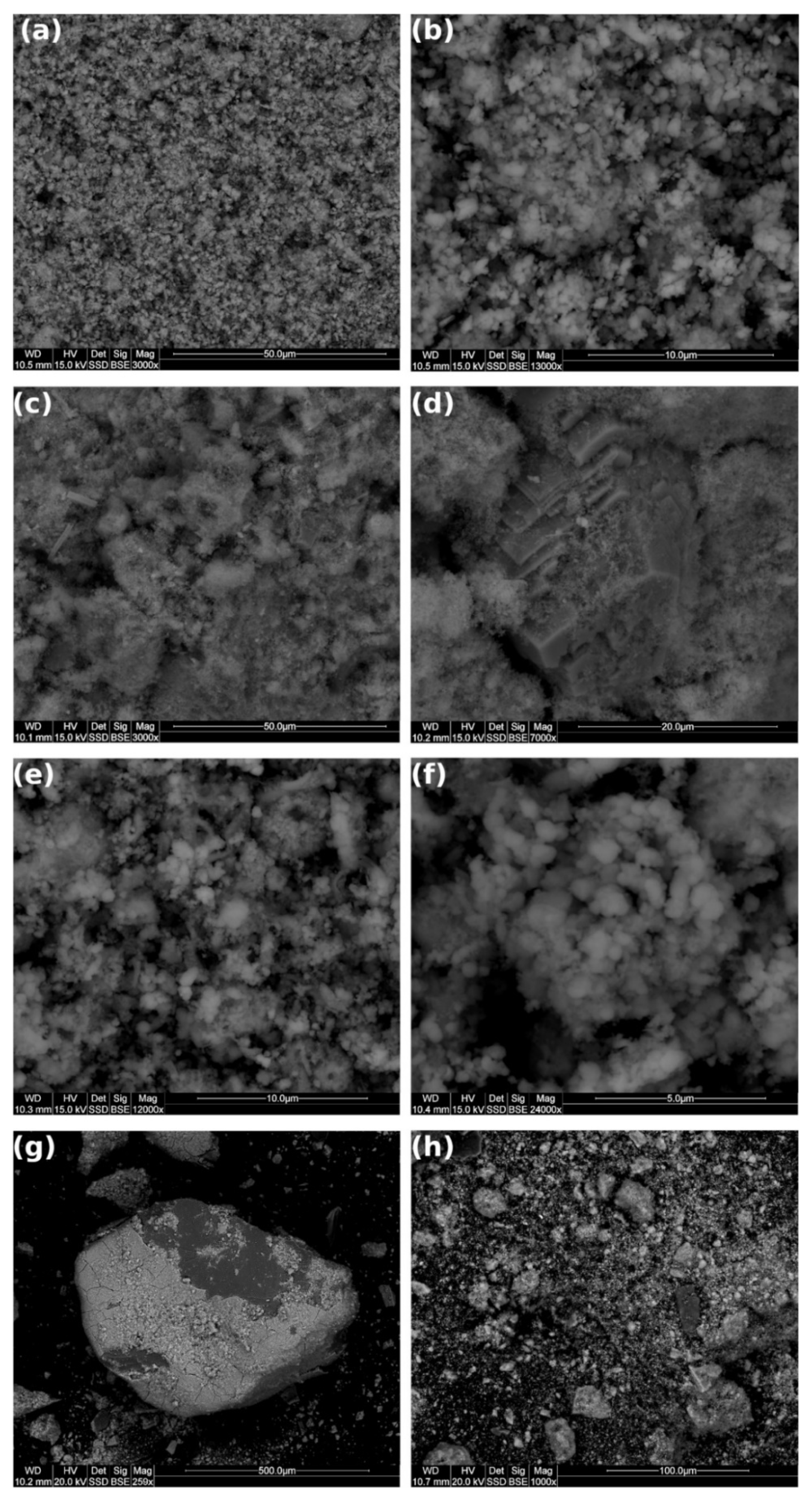
3.6. SEM Analysis
3.7. Isoelectric Point
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ippolito, J.A.; Barbarick, K.A.; Elliott, H.A. Drinking Water Treatment Residuals: A Review of Recent Uses. J. Environ. Qual. 2011, 40, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Kyncl, M.; Cihalova, S.; Jurokova, M.; Langarova, S. Disposal and Reuse of the Water Processing Sludge. Inz. Miner. J. Pol. Miner. Eng. Soc. 2012, 13, 11–20. [Google Scholar]
- Gibbons, M.K.; Gagnon, G.A. Adsorption of arsenic from a Nova Scotia groundwater onto water treatment residual solids. Water Res. 2010, 44, 5740–5749. [Google Scholar] [CrossRef]
- Zhao, Y.Q.; Doherty, L.P.; Doyle, D. Fate of water treatment resiudal: An entire profile of Ireland regarding beneficial reuse. Int. J. Environ. Stud. 2011, 68, 161–170. [Google Scholar] [CrossRef]
- Ren, B.M.; Lyczko, N.; Zhao, Y.Q.; Nzihou, A. Alum sludge as an efficient sorbent for hydrogen sulfide removal: Experimental, mechanisms and modeling studies. Chemosphere 2020, 248, 13. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, T.; Ahmad, K.; Alam, M. Sustainable management of water treatment sludge through 3′R′ concept. J. Clean. Prod. 2016, 124, 1–13. [Google Scholar] [CrossRef]
- Nimwinya, E.; Arjharn, W.; Horpibulsuk, S.; Phoo-Ngernkham, T.; Poowancum, A. A sustainable calcined water treatment sludge and rice husk ash geopolymer. J. Clean. Prod. 2016, 119, 128–134. [Google Scholar] [CrossRef]
- Jiao, J.; Zhao, J.B.; Pei, Y.S. Adsorption of Co(II) from aqueous solutions by water treatment residuals. J. Environ. Sci. 2017, 52, 232–239. [Google Scholar] [CrossRef] [PubMed]
- Van Houtte, E.; Verbauwhede, J. Operational experience with indirect potable reuse at the Flemish Coast. Desalination 2008, 218, 198–207. [Google Scholar] [CrossRef]
- Wang, C.H.; Liang, J.C.; Pei, Y.S.; Wendling, L.A. A method for determining the treatment dosage of drinking water treatment residuals for effective phosphorus immobilization in sediments. Ecol. Eng. 2013, 60, 421–427. [Google Scholar] [CrossRef]
- Wolowiec, M.; Pruss, A.; Komorowska-Kaufman, M.; Lasocka-Gomula, I.; Rzepa, G.; Bajda, T. The properties of sludge formed as a result of coagulation of backwash water from filters removing iron and manganese from groundwater. SN Appl. Sci. 2019, 1, 10. [Google Scholar] [CrossRef] [Green Version]
- Ellis, D.; Bouchard, C.; Lantagne, G. Removal of iron and manganese from groundwater by oxidation and microfiltration. Desalination 2000, 130, 255–264. [Google Scholar] [CrossRef]
- Lewinska, K.; Karczewska, A.; Siepak, M.; Galka, B. Potential of Fe-Mn wastes produced by a water treatment plant for arsenic immobilization in contaminated soils. J. Geochem. Explor. 2018, 184, 226–231. [Google Scholar] [CrossRef]
- Ministry of Health. The Regulation of the Minister of Health of March 29, 2007, as Amended on December 7, 2017 Changing Regulation on the Quality of Drinking Water for Man; Government Publishing Service: Warsaw, Poland, 2017.
- Directive (EU) 2020/2184 of the European Parliament and of the Council of 16 December 2020 on the Quality of Water Intended for Human Consumption; European Union: Brussels, Belgium, 2020.
- Makris, K.C.; Harris, W.G.; O’Connor, G.A.; Obreza, T.A. Phosphorus immobilization in micropores of drinking-water treatment residuals: Implications for long-term stability. Environ. Sci. Technol. 2004, 38, 6590–6596. [Google Scholar] [CrossRef] [PubMed]
- Wolowiec, M.; Komorowska-Kaufman, M.; Pruss, A.; Rzepa, G.; Bajda, T. Removal of Heavy Metals and Metalloids from Water Using Drinking Water Treatment Residuals as Adsorbents: A Review. Minerals 2019, 9, 487. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.H.; Yuan, N.N.; Pei, Y.S. Effect of pH on Metal Lability in Drinking Water Treatment Residuals. J. Environ. Qual. 2014, 43, 389–397. [Google Scholar] [CrossRef]
- Wang, C.H.; Yuan, N.N.; Pei, Y.S. An anaerobic incubation study of metal lability in drinking water treatment residue with implications for practical reuse. J. Hazard. Mater. 2014, 274, 342–348. [Google Scholar] [CrossRef]
- Elliott, H.A.; Dempsey, B.A.; Maille, P.J. Content and fractionation of heavy-metals in water-treatment sludges. J. Environ. Qual. 1990, 19, 330–334. [Google Scholar] [CrossRef] [Green Version]
- Siswoyo, E.; Mihara, Y.; Tanaka, S. Determination of key components and adsorption capacity of a low cost adsorbent based on sludge of drinking water treatment plant to adsorb cadmium ion in water. Appl. Clay Sci. 2014, 97-98, 146–152. [Google Scholar] [CrossRef]
- Kan, C.C.; Aganon, M.C.; Futalan, C.M.; Dalida, M.L.P. Adsorption of Mn2+ from aqueous solution using Fe and Mn oxide-coated sand. J. Environ. Sci. 2013, 25, 1483–1491. [Google Scholar] [CrossRef]
- Krishna, K.C.B.; Aryal, A.; Jansen, T. Comparative study of ground water treatment plants sludges to remove phosphorus from wastewater. J. Environ. Manag. 2016, 180, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Ocinski, D.; Jacukowicz-Sobala, I.; Mazur, P.; Raczyk, J.; Kociolek-Balawejder, E. Water treatment residuals containing iron and manganese oxides for arsenic removal from water—Characterization of physicochemical properties and adsorption studies. Chem. Eng. J. 2016, 294, 210–221. [Google Scholar] [CrossRef]
- Ong, D.C.; Kan, C.C.; Pingul-Ong, S.M.B.; de Luna, M.D.G. Utilization of groundwater treatment plant (GWTP) sludge for nickel removal from aqueous solutions: Isotherm and kinetic studies. J. Environ. Chem. Eng. 2017, 5, 5746–5753. [Google Scholar] [CrossRef]
- APHA. Standard Methods for the Examination of Water and Wastewater, 23rd ed.; American Public Health Association/American Water Works Association/Water Environment Federation: Washington, DC, USA, 2012. [Google Scholar]
- Zhu, S.Y.; Fang, S.; Huo, M.X.; Yu, Y.; Chen, Y.; Yang, X.; Geng, Z.; Wang, Y.; Bian, D.J.; Huo, H.L. A novel conversion of the groundwater treatment sludge to magnetic particles for the adsorption of methylene blue. J. Hazard. Mater. 2015, 292, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Adegoke, H.I.; Adekola, F.A.; Fatoki, O.S.; Ximba, B.J. Sorptive Interaction of Oxyanions with Iron Oxides: A Review. Pol. J. Environ. Stud. 2013, 22, 7–24. [Google Scholar]
- Houben, G.J. Iron oxide incrustations in wells. Part 1: Genesis, mineralogy and geochemistry. Appl. Geochem. 2003, 18, 927–939. [Google Scholar] [CrossRef]
- Drits, V.A.; Sakharov, B.A.; Manceau, A. Structure of feroxyhite as determined by simulation of X-ray diffraction curves. Clay Miner. 1993, 28, 209–222. [Google Scholar] [CrossRef]
- Schwertmann, U.; Cornell, R.M. Iron Oxides in the Laboratory: Preparation and Characterization; VCH: Weinheim, Germany, 2000; p. 204. [Google Scholar]
- Zhang, R.C.; Leiviska, T.; Tanskanen, J.; Gao, B.Y.; Yue, Q.Y. Utilization of ferric groundwater treatment residuals for inorganic-organic hybrid biosorbent preparation and its use for vanadium removal. Chem. Eng. J. 2019, 361, 680–689. [Google Scholar] [CrossRef]
- Cui, X.L.; Liu, X.T.; Lin, C.Y.; He, M.C.; Ouyang, W. Activation of peroxymonosulfate using drinking water treatment residuals modified by hydrothermal treatment for imidacloprid degradation. Chemosphere 2020, 254, 12. [Google Scholar] [CrossRef]
- Pei, D.J.; Li, Y.; Cang, D.Q. In situ XRD study on sintering mechanism of SiO2-Al2O3-CaO-MgO ceramics from red mud. Mater. Lett. 2019, 240, 229–232. [Google Scholar] [CrossRef]
- Jacukowicz-Sobala, I.; Ocinski, D.; Kociolek-Balawejder, E. Iron and aluminium oxides containing industrial wastes as adsorbents of heavy metals: Application possibilities and limitations. Waste Manag. Res. 2015, 33, 612–629. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.P.; Qiao, T.D.; Zhao, Y.X.; Yu, Y.P.; Zhang, J.; Li, D. Characterization and Arsenic Adsorption Behaviors of Water Treatment Residuals from Waterworks for Iron and Manganese Removal. Int. J. Environ. Res. Public Health 2019, 16, 4912. [Google Scholar] [CrossRef] [Green Version]
- Jambor, J.L.; Dutrizac, J.E. Occurrence and constitution of natural and synthetic ferrihydrite, a widespread iron oxyhydroxide. Chem. Rev. 1998, 98, 2549–2585. [Google Scholar] [CrossRef] [PubMed]
- Pieczara, G.; Manecki, M.; Rzepa, G.; Borkiewicz, O.; Gawel, A. Thermal Stability and Decomposition Products of P-Doped Ferrihydrite. Materials 2020, 13, 4113. [Google Scholar] [CrossRef] [PubMed]
- Rzepa, G.; Pieczara, G.; Gawel, A.; Tomczyk, A.; Zalecki, R. The influence of silicate on transformation pathways of synthetic 2-line ferrihydrite. J. Therm. Anal. Calorim. 2016, 125, 407–421. [Google Scholar] [CrossRef] [Green Version]
- Michel, F.M.; Ehm, L.; Antao, S.M.; Lee, P.L.; Chupas, P.J.; Liu, G.; Strongin, D.R.; Schoonen, M.A.A.; Phillips, B.L.; Parise, J.B. The structure of ferrihydrite, a nanocrystalline material. Science 2007, 316, 1726–1729. [Google Scholar] [CrossRef] [Green Version]
- Michel, F.M.; Barron, V.; Torrent, J.; Morales, M.P.; Serna, C.J.; Boily, J.F.; Liu, Q.S.; Ambrosini, A.; Cismasu, A.C.; Brown, G.E. Ordered ferrimagnetic form of ferrihydrite reveals links among structure, composition, and magnetism. Proc. Natl. Acad. Sci. USA 2010, 107, 2787–2792. [Google Scholar] [CrossRef] [Green Version]
- Park, J.H.; Han, Y.S.; Ahn, J.S. Comparison of arsenic co-precipitation and adsorption by iron minerals and the mechanism of arsenic natural attenuation in a mine stream. Water Res. 2016, 106, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Asmel, N.K.; Yusoff, A.R.M.; Lakkaboyana, S.K.; Majid, Z.A.; Salmiati, S. High concentration arsenic removal from aqueous solution using nano-iron ion enrich material (NIIEM) super adsorbent. Chem. Eng. J. 2017, 317, 343–355. [Google Scholar] [CrossRef]
- Pruss, A.; Jeż-Walkowiak, J.; Sozański, M.M. Arsenie in intake water at Poland and possibilities of its removal. In Zaopatrzenie w Wodę, Jakość i Ochrona Wód: Zagadnienia Współczesne; Sozański, M.M., Ed.; Polskie Zrzeszenie Inżynierów i Techników Sanitarnych: Poznań, Poland, 2010; pp. 495–504. [Google Scholar]
- Harper, T.R.; Kingham, N.W. Removal of arsenic from wastewater using chemicalm precipitation methods. Water Environ. Res. 1992, 64, 200–203. [Google Scholar] [CrossRef]
- Sobesto, J.; Stover, T. Arsenic removal from potable water by means of flocculation filtration. In Proceedings of the Municipal and Rural Water Supply and Water Quality III International Conference, Poznań, Poland, 11–13 September 1998. [Google Scholar]
- Elliott, H.A.; Dempsey, B.A. Agronomic effects of land application of water-treatment sludges. J. Am. Water Work. Assoc. 1991, 83, 126–131. [Google Scholar] [CrossRef]
- Sidhu, V.; Barrett, K.; Park, D.Y.; Deng, Y.; Datta, R.; Sarkar, D. Wood mulch coated with iron-based water treatment residuals for the abatement of metals and phosphorus in simulated stormwater runoff. Environ. Technol. Innov. 2021, 21, 11. [Google Scholar] [CrossRef]
- Schmitt, J.; Flemming, H.C. FTIR-spectroscopy in microbial and material analysis. Int. Biodeterior. Biodegrad. 1998, 41, 1–11. [Google Scholar] [CrossRef]
- Krishnan, D.; Raidongia, K.; Shao, J.J.; Huang, J.X. Graphene Oxide Assisted Hydrothermal Carbonization of Carbon Hydrates. ACS Nano 2014, 8, 449–457. [Google Scholar] [CrossRef]
- Wang, C.H.; Jiang, H.L.; Yuan, N.N.; Pei, Y.S.; Yan, Z.S. Tuning the adsorptive properties of drinking water treatment residue via oxygen-limited heat treatment for environmental recycle. Chem. Eng. J. 2016, 284, 571–581. [Google Scholar] [CrossRef]
- Vempati, R.K.; Loeppert, R.H. Influence of structural and adsorbed Si on the transformation of synthetic ferrihydrite. Clays Clay Miner. 1989, 37, 273–279. [Google Scholar] [CrossRef]
- Russell, J.D.; Fraser, A.R. Infrared Methods; Chapman & Hall: London, UK, 1994. [Google Scholar]
- Wang, Q.; Shaheen, S.M.; Jiang, Y.H.; Li, R.H.; Slany, M.; Abdelrahman, H.; Kwon, E.; Bolan, N.; Rinklebe, J.; Zhang, Z.Q. Fe/Mn- and P-modified drinking water treatment residuals reduced Cu and Pb phytoavailability and uptake in a mining soil. J. Hazard. Mater. 2021, 403, 9. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.G.; Li, X.Y.; Liu, B.J.; Zhao, Q.D.; Chen, G.H. Hexagonal microspindle of NH2-MIL-101(Fe) metal-organic frameworks with visible-light-induced photocatalytic activity for the degradation of toluene. RSC Adv. 2016, 6, 4289–4295. [Google Scholar] [CrossRef]
- Li, Q.; Xu, X.T.; Cui, H.; Pang, J.F.; Wei, Z.B.; Sun, Z.Q.; Zhai, J.P. Comparison of two adsorbents for the removal of pentavalent arsenic from aqueous solutions. J. Environ. Manag. 2012, 98, 98–106. [Google Scholar] [CrossRef]
- Basu, T.; Gupta, K.; Ghosh, U.C. Performances of As(V) adsorption of calcined (250 degrees C) synthetic iron(III)-aluminum(III) mixed oxide in the presence of some groundwater occurring ions. Chem. Eng. J. 2012, 183, 303–314. [Google Scholar] [CrossRef]
- Gastaldini, A.L.G.; Hengen, M.F.; Gastaldini, M.C.C.; do Amaral, F.D.; Antolini, M.B.; Coletto, T. The use of water treatment plant sludge ash as a mineral addition. Constr. Build. Mater. 2015, 94, 513–520. [Google Scholar] [CrossRef]
- Carlson, L.; Schwertmann, U. Natural ferrihydrites in surface deposits from Finland and their application with silica. Geochim. Et Cosmochim. Acta 1981, 45, 421–429. [Google Scholar] [CrossRef]
- Rout, K.; Mohapatra, M.; Anand, S. 2-Line ferrihydrite: Synthesis, characterization and its adsorption behaviour for removal of Pb(II), Cd(II), Cu(II) and Zn(II) from aqueous solutions. Dalton Trans. 2012, 41, 3302–3312. [Google Scholar] [CrossRef]
- Tresintsi, S.; Simeonidis, K.; Georgiou, I.; Vourlias, G.; Stavropoulos, G.; Mitrakas, M. Effect of precipitation pH on arsenic removal by single-Fe and binary-Fe/Mn oxyhydroxides. In Proceedings of the International Water Association Speclialist Conferences, Valladolid, Spain, 1–4 May 2011; pp. 1–9. [Google Scholar]
- Schwertmann, U.; Fischer, W.R. Natural amorphous ferric hydroxide. Geoderma 1973, 10, 237–247. [Google Scholar] [CrossRef]
- Bakardjieva, S.; Stengl, V.; Subrt, J.; Vecernikova, E. Characteristic of hydrous iron (III) oxides prepared by homogeneous precipitation of iron (III) sulphate with urea. Solid State Sci. 2005, 7, 367–374. [Google Scholar] [CrossRef]
- Sing, K.S.W.; Everett, D.H.; Haul, R.A.W.; Moscou, L.; Pierotti, R.A.; Rouquerol, J.; Siemieniewska, T. Reporting physisorption data for gas solid systems with special reference to the determination of surface-area and porosity. Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Rouquerol, F.; Rouquerol, J.; Sing, K. Adsorption by Powders and Porous Solids. Principles, Methodology and Applications; Academic Press: London, UK, 1999; pp. 93–115. [Google Scholar]
- Nagar, R.; Sarkar, D.; Makris, K.C.; Datta, R. Effect of solution chemistry on arsenic sorption by Fe- and Al-based drinking-water treatment residuals. Chemosphere 2010, 78, 1028–1035. [Google Scholar] [CrossRef]
- Wang, C.H.; Guo, W.; Tian, B.H.; Pei, Y.S.; Zhang, K.J. Characteristics and kinetics of phosphate adsorption on dewatered ferric-alum residuals. J. Environ. Sci. Health Part. A-Toxic/Hazard. Subst. Environ. Eng. 2011, 46, 1632–1639. [Google Scholar] [CrossRef]
- Li, J.H.; Zheng, L.R.; Wang, S.L.; Wu, Z.P.; Wu, W.D.; Niazi, N.K.; Shaheen, S.M.; Rinklebe, J.; Bolan, N.; Ok, Y.S.; et al. Sorption mechanisms of lead on silicon-rich biochar in aqueous solution: Spectroscopic investigation. Sci. Total Environ. 2019, 672, 572–582. [Google Scholar] [CrossRef] [PubMed]
- Zohar, I.; Ippolito, J.A.; Massey, M.S.; Litaor, I.M. Innovative approach for recycling phosphorus from agro-wastewaters using water treatment residuals (WTR). Chemosphere 2017, 168, 234–243. [Google Scholar] [CrossRef]
- Irawan, C.; Liu, J.C.; Wu, C.C. Removal of boron using aluminum-based water treatment residuals (Al-WTRs). Desalination 2011, 276, 322–327. [Google Scholar] [CrossRef]
- Zhang, H.; Xue, G.; Chen, H.; Li, X. Magnetic biochar catalyst derived from biological sludge and ferric sludge using hydrothermal carbonization: Preparation, characterization and its circulation in Fenton process for dyeing wastewater treatment. Chemosphere 2018, 191, 64–71. [Google Scholar] [CrossRef] [PubMed]
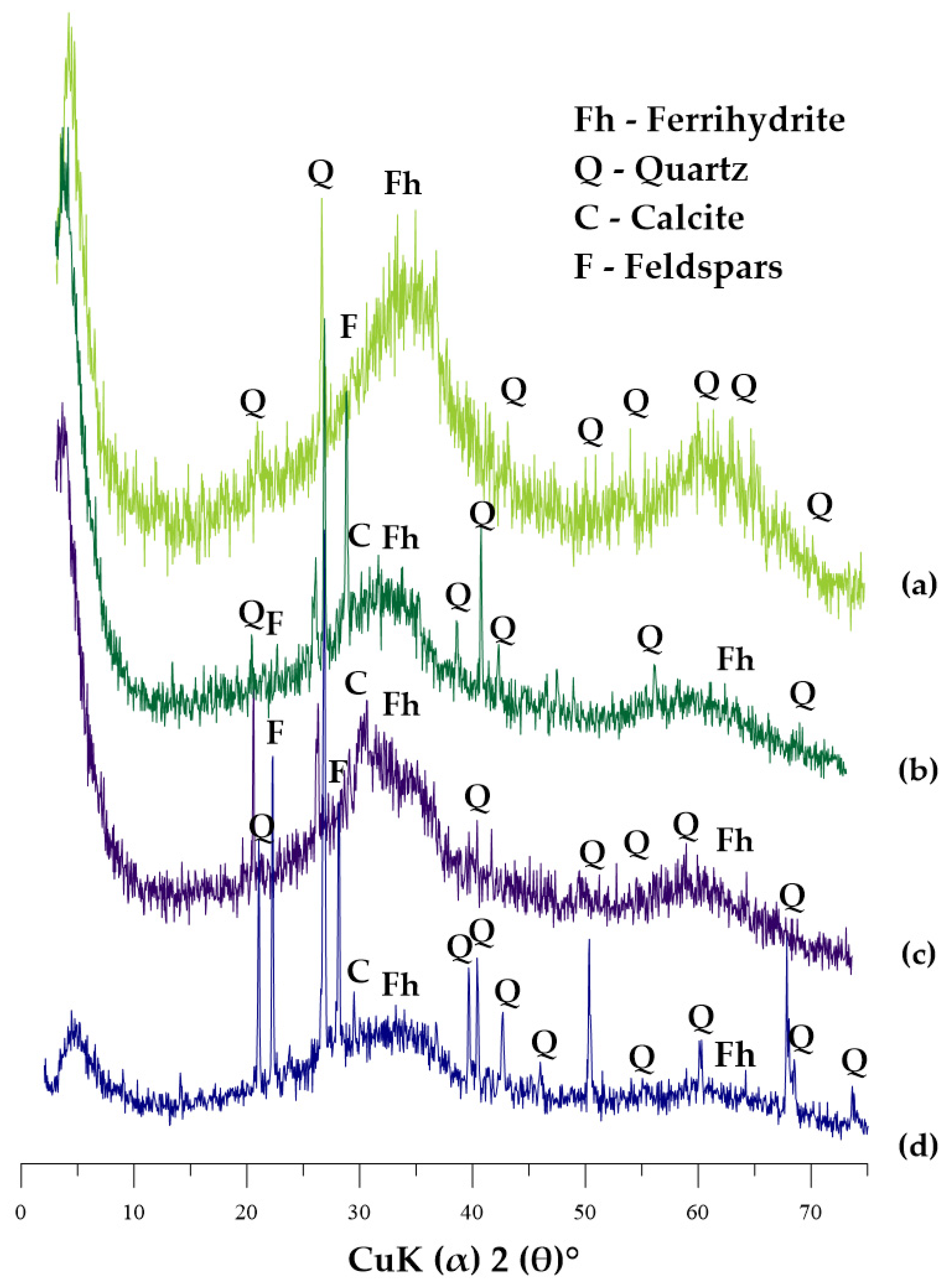
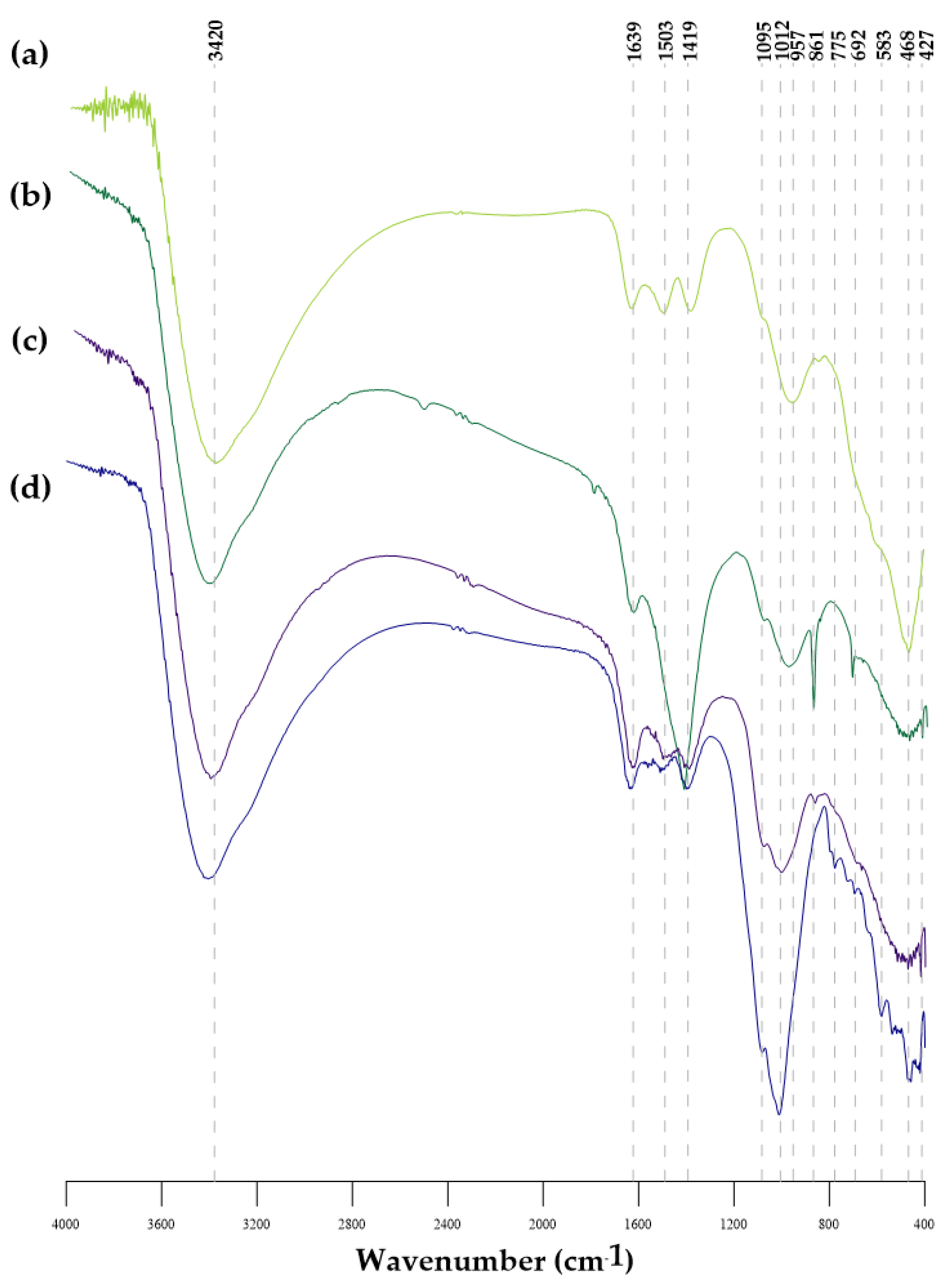
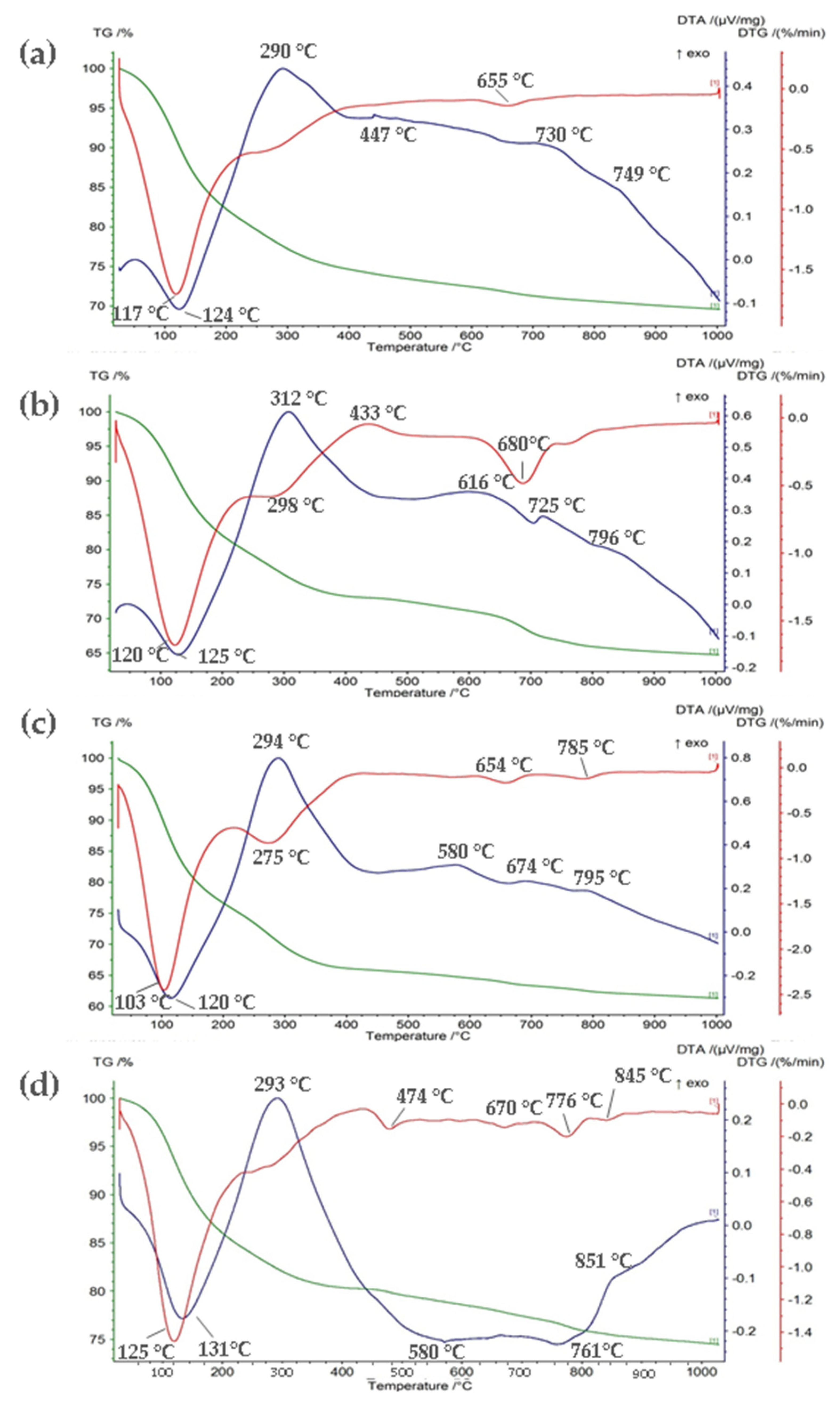
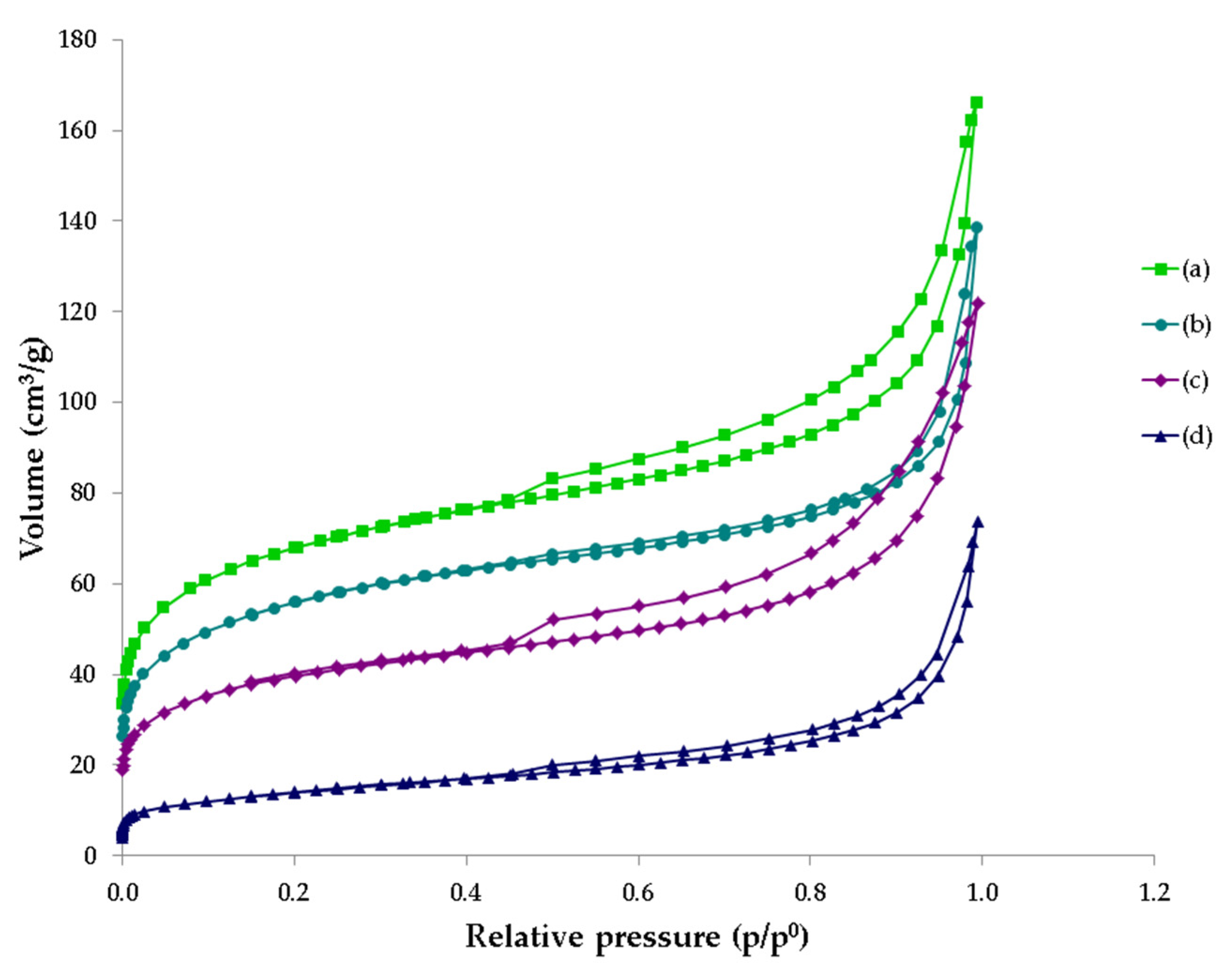
| Parameters | Unit | GWTP-1 | GWTP-2 | GWTP-3 | GWTP-4 | ||||
|---|---|---|---|---|---|---|---|---|---|
| Raw | Treated | Raw | Treated | Raw | Treated | Raw | Treated | ||
| Color | mg Pt/L | 10.0 | 5.0 | 33.0 | 2.5 | 10.0 | 5.0 | 200.0 | 5.0 |
| Turbidity | NTU | 27.0 | 0.5 | 15.3 | 0.5 | 6.4 | 0.2 | 12.4 | 0.5 |
| Hardness | mg CaCO3/L | 352 | 352 | 290 | 290 | NA * | NA | 235 | 229 |
| pH | — | 7.0 | 7.4 | 7.3 | 7.5 | 7.3 | 7.2 | 7.3 | 7.5 |
| Fe | mg Fe/L | 2.490 | 0.044 | 3.620 | 0.020 | 1.000 | 0.060 | 2.900 | 0.116 |
| Mn | mg Mn/L | 0.382 | 0.007 | <0.150 | <0.005 | 0.130 | 0.004 | 0.130 | <0.025 |
| TOC | mg C/L | 2.62 | 2.10 | 3.53 | 3.09 | 1.20 | NA | 4.70 | 4.70 |
| Element | Unit | GWTR-1 | GWTR-2 | GWTR-3 | GWTR-4 |
|---|---|---|---|---|---|
| SiO2 | % | 8.09 | 4.11 | 6.15 | 28.68 |
| TiO2 | % | 0.03 | 0.02 | 0.03 | 0.05 |
| MnO | % | 4.33 | 0.29 | 2.29 | 1.36 |
| Al2O3 | % | 0.28 | 0.58 | 0.60 | 2.20 |
| Fe2O3 | % | 55.76 | 32.17 | 44.88 | 36.51 |
| CaO | % | 4.11 | 17.72 | 5.85 | 3.97 |
| MgO | % | 0.10 | 0.37 | 0.28 | 0.10 |
| BaO | % | 0.13 | 0.16 | 0.27 | b.d. |
| K2O | % | 0.06 | 0.27 | 0.12 | 0.49 |
| Na2O | % | 0.04 | b.d. | 0.13 | 0.51 |
| SO3 | % | 0.22 | 0.41 | 0.02 | 0.07 |
| SrO | % | 0.01 | b.d. | 0.03 | b.d. |
| P2O5 | % | 1.69 | 3.28 | 8.69 | 4.85 |
| As2O3 | % | 0.03 | b.d. | 0.03 | b.d. |
| LOI | % | 25.12 | 40.62 | 30.63 | 21.21 |
| Se | ppm | b.d. | b.d. | b.d. | 0.013 |
| As | ppm | 0.710 | 0.997 | 0.869 | 4.217 |
| Zn | ppm | 1.86 | 0.17 | 0.89 | 2.25 |
| Sr | ppm | 0.50 | 3.91 | 1.09 | 2.06 |
| Ba | ppm | 5.46 | 6.60 | 10.84 | 15.83 |
| Cu | ppm | 0.341 | 0.040 | 0.216 | 0.092 |
| Ni | ppm | b.d. | 0.084 | b.d. | b.d. |
| Cd | ppm | 0.021 | 0.040 | 0.060 | 0.041 |
| Cr | ppm | 0.990 | 0.694 | 0.783 | 0.935 |
| Hg | ppm | b.d. | b.d. | b.d. | b.d. |
| V | ppm | 0.261 | 0.310 | 0.310 | 0.254 |
| Parameters | GWTR-1 | GWTR-2 | GWTR-3 | GWTR-4 |
|---|---|---|---|---|
| SBET (m2/g) | 246 | 203 | 144 | 49 |
| Vtot0.99 (cm3/g) | 0.249 | 0.202 | 0.181 | 0.105 |
| VmicT (cm3/g) | 0.091 | 0.073 | 0.053 | 0.017 |
| VmicT/Vtot0.99 | 0.365 | 0.361 | 0.293 | 0.162 |
| Vmes (cm3/g) | 0.121 | 0.084 | 0.098 | 0.058 |
| VmesBJH/Vtot0.99 | 0.486 | 0.416 | 0.541 | 0.552 |
| Vmac (cm3/g) | 0.037 | 0.045 | 0.03 | 0.030 |
| VmacBJH/Vtot0.99 | 0.149 | 0.223 | 0.166 | 0.286 |
| Parameter | GWTR-1 | GWTR-2 | GWTR-3 | GWTR-4 |
|---|---|---|---|---|
| pHIEP | 4.5 | 4.4 | 4.0 | 4.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Likus, M.; Komorowska-Kaufman, M.; Pruss, A.; Zych, Ł.; Bajda, T. Iron-Based Water Treatment Residuals: Phase, Physicochemical Characterization, and Textural Properties. Materials 2021, 14, 3938. https://doi.org/10.3390/ma14143938
Likus M, Komorowska-Kaufman M, Pruss A, Zych Ł, Bajda T. Iron-Based Water Treatment Residuals: Phase, Physicochemical Characterization, and Textural Properties. Materials. 2021; 14(14):3938. https://doi.org/10.3390/ma14143938
Chicago/Turabian StyleLikus, Magdalena, Małgorzata Komorowska-Kaufman, Alina Pruss, Łukasz Zych, and Tomasz Bajda. 2021. "Iron-Based Water Treatment Residuals: Phase, Physicochemical Characterization, and Textural Properties" Materials 14, no. 14: 3938. https://doi.org/10.3390/ma14143938
APA StyleLikus, M., Komorowska-Kaufman, M., Pruss, A., Zych, Ł., & Bajda, T. (2021). Iron-Based Water Treatment Residuals: Phase, Physicochemical Characterization, and Textural Properties. Materials, 14(14), 3938. https://doi.org/10.3390/ma14143938







