Effect of Transition Elements on the Thermal Stability of Glassy Alloys 82Al–16Fe–2TM (TM: Ti, Ni, Cu) Prepared by Mechanical Alloying
Abstract
:1. Introduction
2. Materials and Methods
3. Results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- He, Y.; Poon, S.J.; Shiflet, G.J. Synthesis and Properties of Metallic Glasses That Contain Aluminum. Science 1988, 241, 1640. [Google Scholar] [CrossRef]
- He, Y.; Shiflet, G.J.; Poon, S.J. Synthesis and properties of aluminum-based metallic glasses containing rare earths. J. Alloys Compd. 1994, 207–208, 349–354. [Google Scholar] [CrossRef]
- Miller, M.K.; Liaw, P. Bulk Metallic Glasses; Springer: New York, NY, USA, 2008; p. 256. [Google Scholar]
- Suryanarayana, C.; Inoue, A. Metallic Glasses. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH Verlag GmbH: Weinheim, Germany, 2012. [Google Scholar] [CrossRef]
- Eckert, J.; Calin, M.; Yu, P.; Zhang, L.C.; Scudino, S.; Duhamel, C. Al-based alloys containing amorphous and nanostructured phases. Rev. Adv. Mater. Sci. 2008, 18, 169–172. [Google Scholar]
- Seikh, A.H.; Baig, M.; Singh, J.K.; Mohammed, J.A.; Luqman, M.; Abdo, H.S.; Khan, A.R.; Alharthi, N.H. Microstructural and Corrosion Characteristics of Al-Fe Alloys Produced by High-Frequency Induction-Sintering Process. Coatings 2019, 9, 686. [Google Scholar] [CrossRef] [Green Version]
- Krasnowski, M.; Kulik, T. Nanocrystalline and amorphous Al–Fe alloys containing 60–85% of Al synthesised by mechanical alloying and phase transformations induced by heating of milling products. Mater. Chem. Phys. 2009, 116, 631–637. [Google Scholar] [CrossRef]
- Inoue, A.; Kimura, H. Fabrications and mechanical properties of bulk amorphous, nanocrystalline, nanoquasicrystalline alloys in aluminum-based system. J. Light Met. 2001, 1, 31–41. [Google Scholar] [CrossRef]
- Suryanarayana, C. Mechanical Alloying: A Novel Technique to Synthesize Advanced Materials. Research 2019, 2019, 4219812. [Google Scholar] [CrossRef] [Green Version]
- Inoue, A.; Kong, F.; Zhu, S.; Liu, C.; Al-Marzouki, F. Development and Applications of Highly Functional Al-based Materials by Use of Metastable Phases. Mater. Res. 2015, 18. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Witkin, D.; Lavernia, E.J. Crystallization behavior of a gas atomized Al85Ni10La5 amorphous alloy. J. Non-Cryst. Solids 2005, 351, 1646–1652. [Google Scholar] [CrossRef] [Green Version]
- Viet, N.H.; Oanh, N.T.; Kim, J.-S.; Jorge, A.M. Crystallization Kinetics and Consolidation of Al82La10Fe4Ni4 Glassy Alloy Powder by Spark Plasma Sintering. Metals 2018, 8, 812. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.S.; Hong, S.I.; Kato, H.; Inoue, A. Strengthening Mechanisms in Al-Based and Zr-Based Amorphous Nanocomposites. Mater. Trans. 2002, 43, 2026–2030. [Google Scholar] [CrossRef] [Green Version]
- Roy, D.; Chakravarty, D.; Mitra, R.; Manna, I. Effect of sintering on microstructure and mechanical properties of nano-TiO2 dispersed Al65Cu20Ti15 amorphous/nanocrystalline matrix composite. J. Alloys Compd. 2008, 460, 320–325. [Google Scholar] [CrossRef]
- Bassim, N.; Kiminami, C.S.; Kaufman, M.J.; Oliveira, M.F.; Perdigao, M.N.R.V.; Botta Filho, W.J. Crystallization behavior of amorphous Al84Y9Ni5CO2 alloy. Mater. Sci. Eng. A 2001, 304–306, 332–337. [Google Scholar] [CrossRef]
- Wang, J.Q.; Chang, X.C.; Hou, W.L.; Hu, Z.Q. Crystallization behaviour of Al-based amorphous alloy and nanocomposites by rapid quenching. Philos. Mag. Lett. 2000, 80, 349–357. [Google Scholar] [CrossRef]
- Nguyen, V.H.; Nguyen, O.T.H.; Dudina, D.V.; Le, V.V.; Kim, J.-S. Crystallization Kinetics of Al-Fe and Al-Fe-Y Amorphous Alloys Produced by Mechanical Milling. J. Nanomater. 2016, 2016, 1909108. [Google Scholar] [CrossRef] [Green Version]
- Weeber, A.W.; Bakker, H. Amorphization by ball milling. A review. Phys. B Condens. Matter 1988, 153, 93–135. [Google Scholar] [CrossRef]
- Lü, L.; Lai, M.O. Mechanical Alloying, 1st ed.; Springer: Boston, MA, USA, 1998; p. 276. [Google Scholar] [CrossRef]
- Suryanarayana, C. Mechanical Alloying and Milling, 1st ed.; CRC Press: Boca Raton, FL, USA, 2004; p. 488. [Google Scholar] [CrossRef]
- Surreddi, K.B.; Scudino, S.; Nguyen, H.V.; Nikolowski, K.; Stoica, M.; Sakaliyska, M.; Kim, J.S.; Gemming, T.; Vierke, J.; Wollgarten, M.; et al. Spark plasma sintering of gas atomized Al87Ni8La5 amorphous powder. J. Phys. Conf. Ser. 2009, 144, 012079. [Google Scholar] [CrossRef]
- Nguyen, H.-V.; Kim, J.-S.; Kwon, Y.-S.; Kim, J.-C. Amorphous Ti–Cu–Ni–Al alloys prepared by mechanical alloying. J. Mater. Sci. 2009, 44, 2700–2704. [Google Scholar] [CrossRef]
- Liu, X.; Wang, X.; Si, Y.; Han, F. Glass-Forming Ability and Thermal Properties of Al70Fe12.5V12.5X5(X = Zr, Nb, Ni) Amorphous Alloys via Minor Alloying Additions. Nanomaterials 2021, 11, 488. [Google Scholar] [CrossRef] [PubMed]
- Babilas, R.; Spilka, M.; Młynarek, K.; Łoński, W.; Łukowiec, D.; Radoń, A.; Kądziołka-Gaweł, M.; Gębara, P. Glass-Forming Ability and Corrosion Resistance of Al88Y8−xFe4+x (x = 0, 1, 2 at.%) Alloys. Materials 2021, 14, 1581. [Google Scholar] [CrossRef]
- Oleszak, D. Mechanical alloying—A novel method for synthesis and processing of materials. Acta Phys. Pol. A 1999, 96, 101–112. [Google Scholar] [CrossRef]
- Shan, L.; Wang, X.; Wang, Y. Extension of Solid Solubility and Structural Evolution in Nano-Structured Cu-Cr Solid Solution Induced by High-Energy Milling. Materials 2020, 13, 5532. [Google Scholar] [CrossRef] [PubMed]
- Suñol, J.-J. Mechanical Alloying: Processing and Materials. Metals 2021, 11, 798. [Google Scholar] [CrossRef]
- Oanh, N.T.H.; Viet, N.H.; Dudina, D.V.; Jorge, A.M.; Kim, J.-S. Structural characterization and magnetic properties of Al82Fe16TM2 (TM: Ti, Ni, Cu) alloys prepared by mechanical alloying. J. Non-Cryst. Solids 2017, 468, 67–73. [Google Scholar] [CrossRef]
- Doebelin, N.; Kleeberg, R. Profex: A graphical user interface for the Rietveld refinement program BGMN. J. Appl. Cryst. 2015, 48, 1573–1580. [Google Scholar] [CrossRef] [Green Version]
- Takeuchi, A.; Inoue, A. Classification of Bulk Metallic Glasses by Atomic Size Difference, Heat of Mixing and Period of Constituent Elements and Its Application to Characterization of the Main Alloying Element. Mater. Trans. 2005, 46, 2817–2829. [Google Scholar] [CrossRef] [Green Version]
- Takeuchi, A.; Inoue, A. Analyses of characteristics of atomic pairs in ferrous bulk metallic glasses using classification of bulk metallic glasses and pettifor map. J. Optoelectron. Adv. Mater. 2006, 8, 1679–1684. [Google Scholar]
- Suryanarayana, C.; Inoue, A. Iron-based bulk metallic glasses. Int. Mater. Rev. 2013, 58, 131–166. [Google Scholar] [CrossRef]
- Urban, P.; Cuevas, F.G.; Montes, J.M.; Cintas, J. Solid state amorphization in the Al-Fe binary system during high energy milling. AIP Conf. Proc. 2013, 1569, 476–479. [Google Scholar] [CrossRef]
- Nguyen, H.V.; Oanh, N.T.H.; Quynh, P.N.D.; Lap, T.Q.; Kim, J.S. Thermal Stability of Amorphous Al-Fe-Y Prepared by Mechanical Alloying. Mater. Sci. Forum 2015, 804, 271–274. [Google Scholar] [CrossRef]
- Takeuchi, A.; Inoue, A. Calculations of Mixing Enthalpy and Mismatch Entropy for Ternary Amorphous Alloys. Mater. Trans. JIM 2000, 41, 1372–1378. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Xiong, X.Z.; Zhou, W.; Li, J.F. Influence of substitution of La by Ce on the glass forming ability and crystallization behavior of Al–Ni–La alloys. J. Alloys Compd. 2013, 576, 181–186. [Google Scholar] [CrossRef]
- Zhang, R.F.; Zhang, S.H.; He, Z.J.; Jing, J.; Sheng, S.H. Miedema Calculator: A thermodynamic platform for predicting formation enthalpies of alloys within framework of Miedema’s Theory. Comput. Phys. Commun. 2016, 209, 58–69. [Google Scholar] [CrossRef]
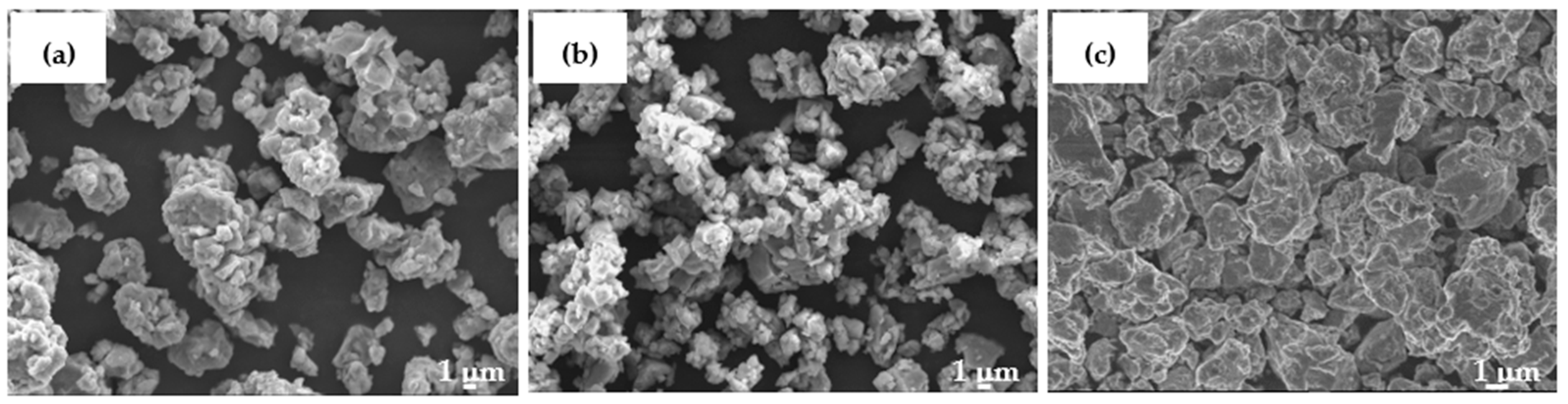
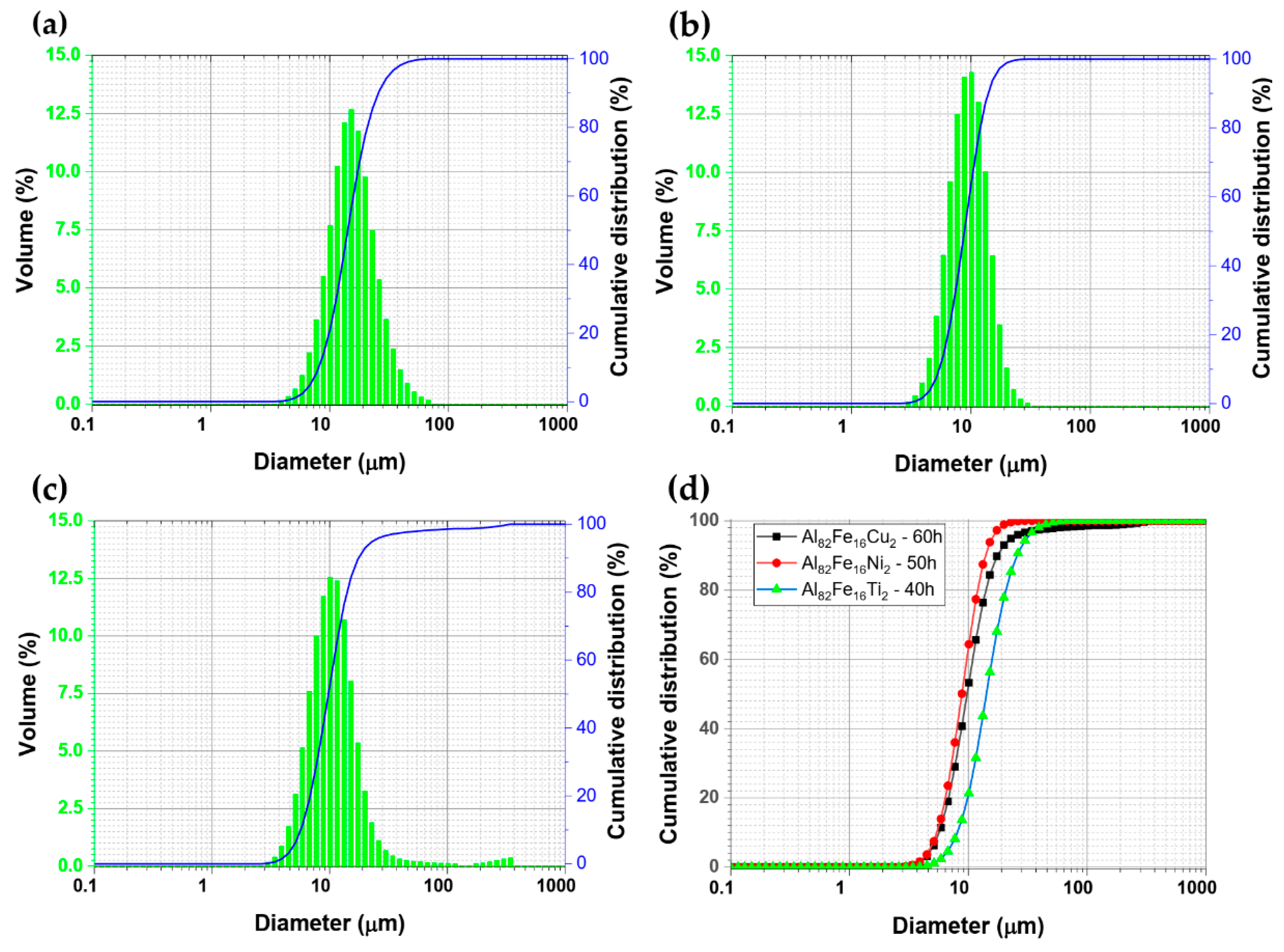
| Element | Al | Fe | Ti | Ni | Cu | Y | La |
|---|---|---|---|---|---|---|---|
| Al | - | 13 (%) | 2.7 (%) | 12.5 (%) | 10 (%) | 21.4 (%) | 23.9 (%) |
| Fe | −11 (kJ/mole) | - | 15.6 (%) | 0.8 (%) | 3.1 (%) | 31.8 (%) | 34 (%) |
| Ti | −30 (kJ/mole) | −17 (kJ/mole) | - | 14.9 (%) | 12.9 (%) | 19.2 (%) | 21.8 (%) |
| Ni | −22 (kJ/mole) | −2 (kJ/mole) | −35 (kJ/mole) | - | 2.3 (%) | 31.3 (%) | 33 (%) |
| Cu | −1 (kJ/mole) | +4 (kJ/mole) | −9 (kJ/mole) | +4 (kJ/mole) | - | 29.6 (%) | 31.9 (%) |
| Y | −38 (kJ/mole) | −1 (kJ/mole) | +15 (kJ/mole) | −31 (kJ/mole) | −22 (kJ/mole) | - | 3 (%) |
| La | −38 (kJ/mole) | +5 (kJ/mole) | +20 (kJ/mole) | −27 (kJ/mole) | −21 (kJ/mole) | +20 (kJ/mole) | - |
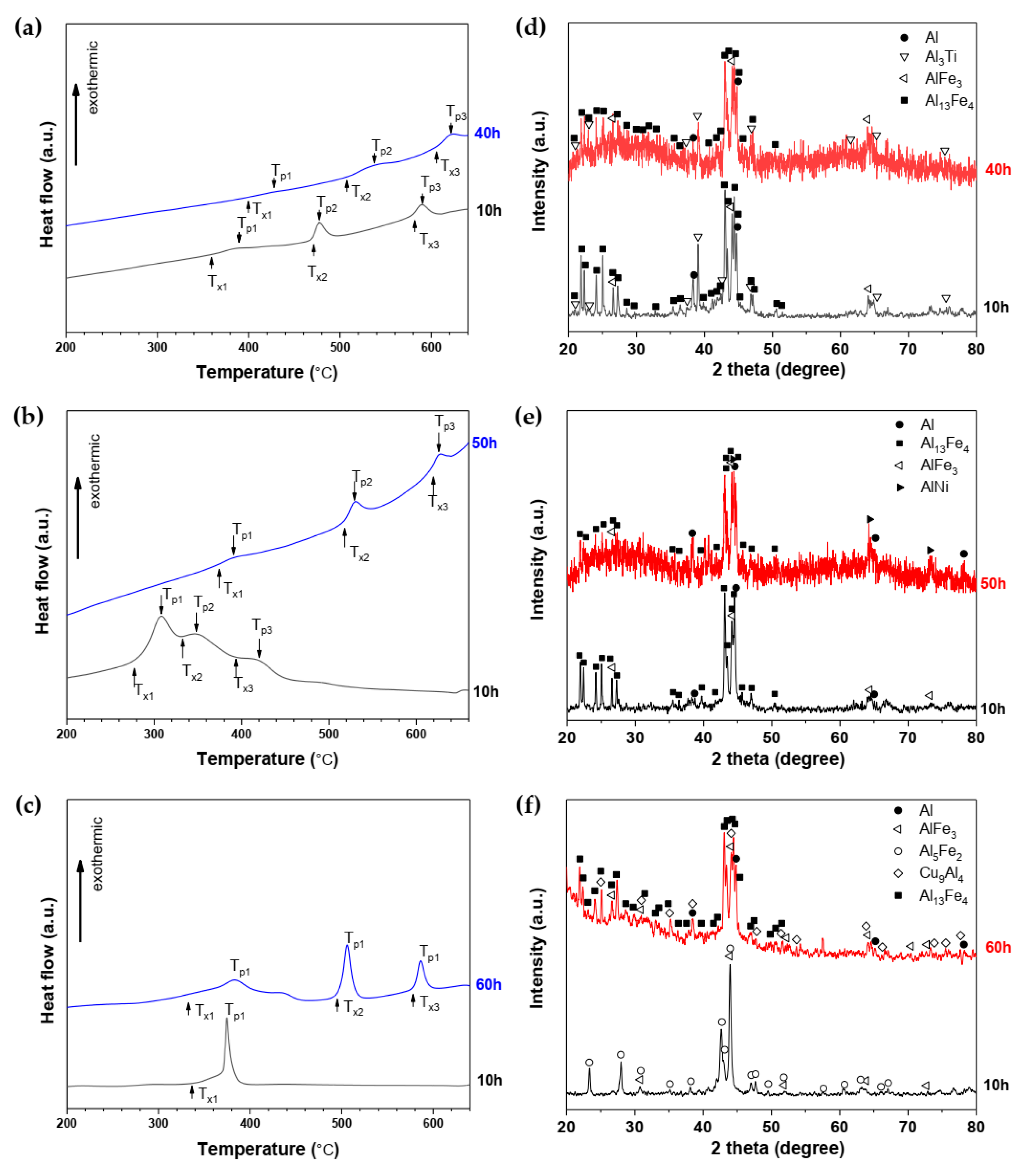
| Alloys | Phase after MA | Crystallization Temperatures, (°C) | Crystallization Phases | Ref | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Tx1 | Tp1 | Tx2 | Tp2 | Tx3 | Tp3 | Tx4 | ||||
| Al84Fe16 | amorphous | 353 | - | 450 | - | 511 | - | 590 | Al, Al13Fe4, Al6Fe | [34] |
| Al82Fe18 | amorphous | 380 | - | 491 | - | 579 | - | - | Al, Al13Fe4 | [34] |
| Al82Fe16Y2 | amorphous | 382 | - | 486 | - | 584 | - | - | Al, Al6Fe, Fe4Y, Al13Fe4 | [34] |
| Al82Fe16Ti2 | amorphous | 398 | 424 | 507 | 535 | 605 | 623 | - | Al, Al3Ti, AlFe3, Al13Fe4 | This work |
| Al82Fe16Ni2 | amorphous | 365 | 393 | 516 | 530 | 617 | 627 | - | Al, Al13Fe4, AlFe3, AlNi | This work |
| Al82Fe16Cu2 | Partly amorphous | 334 | 382 | 495 | 506 | 576 | 586 | - | Al, Al13Fe4, AlFe3, Al5Fe2, Cu9Al4 | This work |
| Sample | Phase | ICDD/JCPDS ID * | Lattice Parameters (nm) | CIF ID ** | Refined Lattice Parameters (nm) | Formation Enthalpy, kJ/mol | |
|---|---|---|---|---|---|---|---|
| Al82Fe16Ti2 | MA 10 h | MA 40 h | |||||
| Cubic, Fm−3m (225) | Al | 04-0787 | a = 0.40494 | a = 0.40584 | a = 0.4049 | ||
| Monoclinic C2/m (12) | Al13Fe4 | 29-0042 | a = 1.5489 b = 0.8083 c = 1.2476 β = 107.7 | ICSD_151129 | a = 1.5498 b = 0.8089 c = 1.2501 β = 107.93 | a = 1.5511 b = 0.8092 c = 1.2527 β = 108.15 | −18.052 |
| Cubic Fm-3m (225) | AlFe3 | 45-1203 | a = 0.57934 | mp-2018 | a = 0.5765 | a = 0.5803 | −22.078 |
| Tetragonal I4/mmm (139) | Al3Ti | 37-1449 | a = 0.38537 c = 0.85839 | mp-542915 | a = 0.3851 c = 0.8602 | a = 0.3852 c = 0.8609 | −39.020 |
| Al82Fe16Ni2 | MA 10 h | MA 50 h | - | ||||
| Cubic Fm-3m (225) | Al | - | a = 0.40494 | - | a = 0.4053 | a = 0.4049 | - |
| Monoclinic C2/m (12) | Al13Fe4 | 29-0042 | a = 1.5489 b = 0.8083 c = 1.2476 β = 107.7 | ICSD_151129 | a = 1.5462 b = 0.8118 c = 1.2489 β = 107.81 | a = 1.5495 b = 0.8084 c = 1.2491 β = 107.89 | - |
| Cubic Fm-3m (225) | AlFe3 | 50-0955 | a = 0.58152 | mp-2018 | a = 0.5747 | a = 0.5803 | - |
| Al82Fe16Cu2 | MA 10 h | MA 60 h | - | ||||
| Cubic Fm-3m (225) | Al | - | a = 0.40494 | - | - | a = 0.4050 | - |
| Monoclinic C2/m (12) | Al13Fe4 | 29-0042 | a = 1.5489 b = 0.8083 c = 1.2476 β = 107.7 | ICSD_151129 | - | a = 1.5515 b = 0.8094 c = 1.2521 β = 107.86 | - |
| Cubic Fm-3m (225) | AlFe3 | 50-0955 | a = 0.58152 | mp-2018 | a = 0.5803 | a = 0.5771 | - |
| Orthorhombic Cmcm (63) | Al5Fe2 | 47-1435 | a = 0.76486 b = 0.64131 c = 0.42165 | COD_2101159 | a = 0.7620 b = 0.6424 c = 0.4204 | - | −21.855 |
| Cubic P-43m (215) | Cu9Al4 | 24-0003 | a = 0.87027 | mp-593 | - | a = 0.8789 | −13.104 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oanh, N.T.H.; Binh, D.N.; Dang Duc, D.; Hoang Thi Ngoc, Q.; Viet, N.H. Effect of Transition Elements on the Thermal Stability of Glassy Alloys 82Al–16Fe–2TM (TM: Ti, Ni, Cu) Prepared by Mechanical Alloying. Materials 2021, 14, 3978. https://doi.org/10.3390/ma14143978
Oanh NTH, Binh DN, Dang Duc D, Hoang Thi Ngoc Q, Viet NH. Effect of Transition Elements on the Thermal Stability of Glassy Alloys 82Al–16Fe–2TM (TM: Ti, Ni, Cu) Prepared by Mechanical Alloying. Materials. 2021; 14(14):3978. https://doi.org/10.3390/ma14143978
Chicago/Turabian StyleOanh, Nguyen Thi Hoang, Do Nam Binh, Dung Dang Duc, Quyen Hoang Thi Ngoc, and Nguyen Hoang Viet. 2021. "Effect of Transition Elements on the Thermal Stability of Glassy Alloys 82Al–16Fe–2TM (TM: Ti, Ni, Cu) Prepared by Mechanical Alloying" Materials 14, no. 14: 3978. https://doi.org/10.3390/ma14143978







