The Study of Magnetic Properties for Non-Magnetic Ions Doped BiFeO3
Abstract
:1. Introduction
2. Experiments
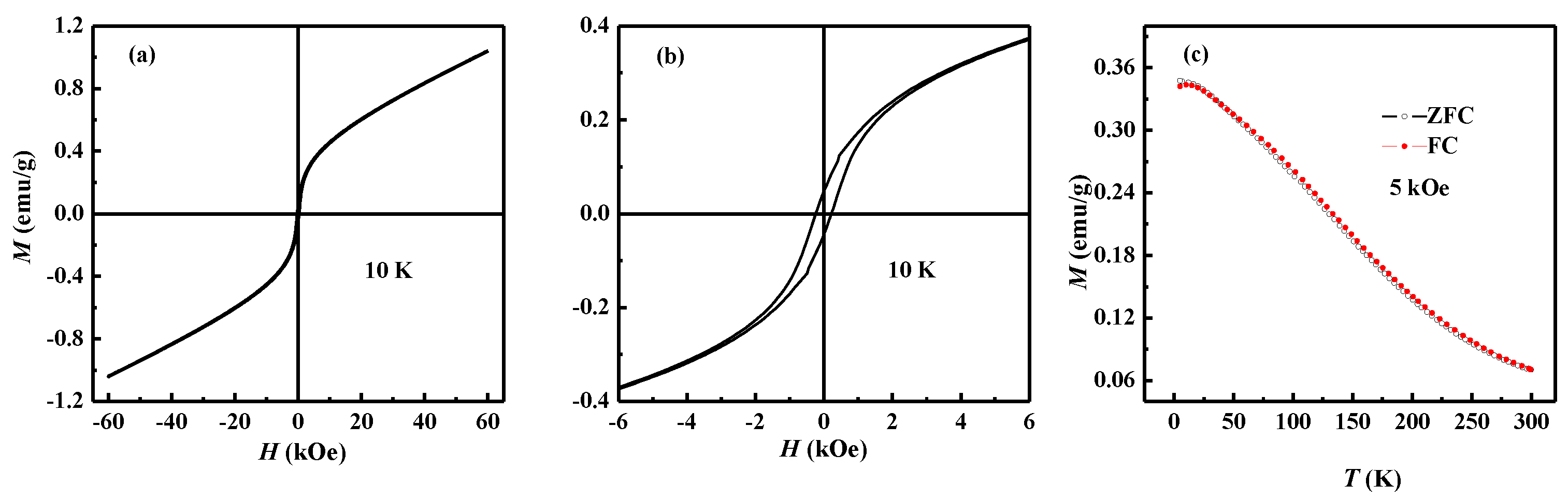
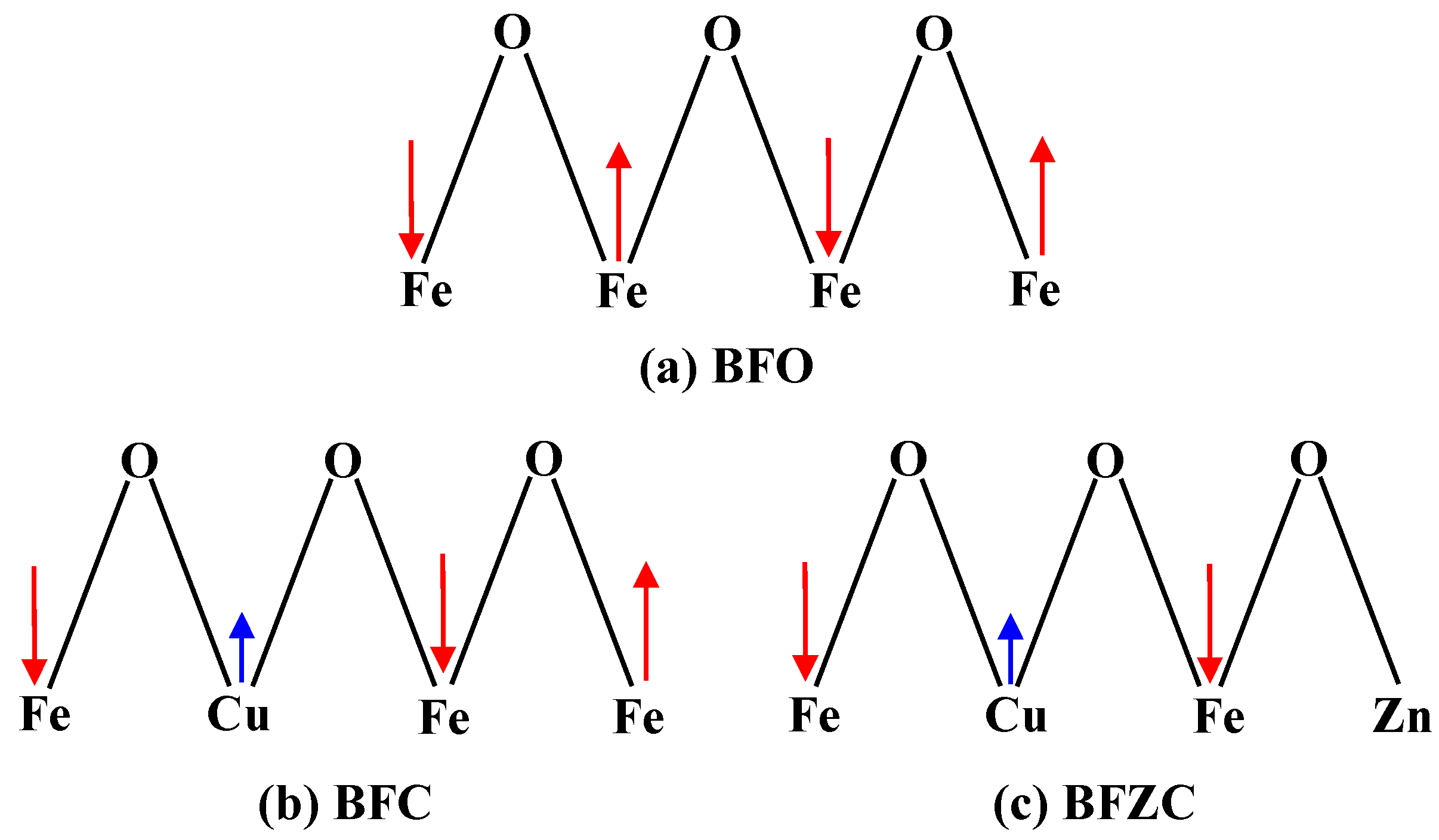
3. Results and Discussions
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wu, J.G.; Fan, Z.; Xiao, D.Q.; Zhu, J.G.; Wang, J. Multiferroic bismuth ferrite-based materials for multifunctional applications: Ceramic bulks, thin films and nanostructures. Prog. Mater. Sci. 2016, 84, 335–402. [Google Scholar] [CrossRef] [Green Version]
- Kuo, C.-Y.; Hu, Z.; Yang, J.C.; Liao, S.-C.; Huang, Y.L.; Vasudevan, R.K.; Okatan, M.B.; Jesse, S.; Kalinin, S.V.; Li, L.; et al. Single-domain multiferroic BiFeO3 films. Nat. Commun. 2016, 7, 12712. [Google Scholar] [CrossRef] [PubMed]
- Sosnowska, I.; Neumaier, T.P.; Steichele, E. Spiral magnetic ordering in bismuth ferrite. J. Phys. C Solid State Phys. 1982, 15, 4835. [Google Scholar] [CrossRef]
- Przenioslo, R.; Palewicz, A.; Regulski, M.; Sosnowska, I.; Ibberson, R.M.; Knight, K.S. Does the modulated magnetic structure of BiFeO3 change at low temperatures? J. Phys. Condens. Matter 2006, 18, 2069–2075. [Google Scholar] [CrossRef]
- Sosnowska, I.; Loewenhaupt, M.; David, W.I.F.; Ibberson, R.M. Investigation of the unusual magnetic spiral arrangement in BiFeO3. Phys. B 1992, 180, 117–118. [Google Scholar] [CrossRef]
- Dhanalakshmi, B.; Kollu, P.; Sekhar, B.C.; Parvatheeswara Rao, B.; Subba Rao, P.S.V. Enhanced magnetic and magnetoelectric properties of Mn doped multiferroic ceramics. Ceram. Int. 2017, 43, 9272–9275. [Google Scholar] [CrossRef]
- Singh, V.; Sharma, S.; Dwivedi, R.K. Improved dielectric, magnetic and optical properties of Pr and Ti co-substituted BFO ceramics. J. Alloys Compd. 2018, 747, 611–620. [Google Scholar] [CrossRef]
- Soltani, T.; Lee, B.-K. Novel and facile synthesis of Ba-doped BiFeO3 nanoparticles and enhancement of their magnetic and photocatalytic activities for complete degradation of benzene in aqueous solution. J. Hazard. Mater. 2016, 316, 122–133. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.Y.; Shi, L.; Zhao, J.Y.; Zhou, S.M.Y.; Li, Y.; Xie, C.Z.; Guo, J.H. Sr and Pb co-doping effect on the crystal structure, dielectric and magnetic properties of BiFeO3 multiferroic compounds. J. Alloys Compd. 2017, 708, 93–98. [Google Scholar] [CrossRef] [Green Version]
- Ederer, C.; Spaldin, N.A. Influence of strain and oxygen vacancies on the magnetoelectric properties of multiferroic bismuth ferrite. Phys. Rev. B 2005, 71, 224103. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.T.; Pang, L.H.; Zhang, Y.; Lu, M.H.; Chen, Y.F. Preparation, structures, and multiferroic properties of single phase Bi1-xLaxFeO3 (x = 0 − 0.40) ceramics. J. Appl. Phys. 2006, 100, 114108. [Google Scholar] [CrossRef]
- Vijayasundaram, S.V.; Suresh, G.; Mondal, R.A.; Kanagadurai, R. Substitution-driven enhanced magnetic and ferroelectric properties of BiFeO3 nanoparticles. J. Alloys Compd. 2016, 658, 726–731. [Google Scholar] [CrossRef]
- Liu, Y.Q.; Qi, J.; Zhang, Y.L.; Wang, Y.H.; Feng, M.; Zhang, J.K.; Wei, M.B.; Yang, J.H. Surface agglomeration is beneficial for release of magnetic property via research of rare earth (RE) element-substitution. Appl. Surf. Sci. 2018, 427, 745–752. [Google Scholar] [CrossRef]
- Dhanalakshmi, B.; Pratap, K.; Parvatheeswara Rao, B.; Subba Rao, P.S.V. Effects of Mn doping on structural, dielectric and multiferroic properties of BiFeO3 nanoceramics. J. Alloys Compd. 2016, 676, 193–201. [Google Scholar] [CrossRef]
- Mukherjee, A.; Basu, S.; Green, L.A.W.; Thanh, N.T.K.; Pal, M. Enhanced multiferroic properties of Y and Mn codoped multiferroic BiFeO3 nanoparticles. J. Mater. Sci. 2015, 50, 1891–1900. [Google Scholar] [CrossRef]
- Li, Y.T.; Zhang, H.G.; Liu, H.; Li, Q.; Li, X.A.; Mao, W.W.; Wang, X.F.; Xu, Q.Y.; Wei, S.Q. The study of local atomic and electronic structure with magnetic properties of Bi(Fe0.95Co0.05)O-3 ceramics. Solid State Commun. 2013, 153, 13–16. [Google Scholar] [CrossRef]
- Wei, J.; Haumont, R.; Jarrier, R.; Berhtet, P.; Dkhil, B. Nonmagnetic Fe-site doping of BiFeO3 multiferroic ceramics. Appl. Phys. Lett. 2010, 96, 102509. [Google Scholar] [CrossRef]
- Xu, J.L.; Xie, D.; Yin, C.; Feng, T.T.; Zhang, X.W.; Li, G.; Zhao, H.M.; Zhao, Y.F.; Ma, S.; Ren, T.L.; et al. Enhanced dielectric and multiferroic properties of single-phase Y and Zr co-doped BiFeO3 ceramics. J. Appl. Phys. 2013, 114, 154103. [Google Scholar] [CrossRef]
- Wu, J.G.; Wang, J. Multiferroic behavior of Sn-modified BiFeO3 films. Electrochem. Solid State Lett. 2010, 13, G83–G85. [Google Scholar] [CrossRef]
- Xu, Q.Y.; Zai, H.F.; Wu, D.; Tang, Y.K.; Xu, M.X. The magnetic properties of BiFeO3 and Bi(Fe0.95Zn0.05)O-3. J. Alloys Compd. 2009, 485, 13–16. [Google Scholar] [CrossRef]
- Chaudhari, Y.A.; Singh, A.; Abuassaj, E.M.; Chatterjee, R.; Bendre, S.T. Multiferroic properties in BiFe1-xZnxO3 (x = 0.1 − 0.2) ceramics by solution combustion method (SCM). J. Alloys Compd. 2012, 518, 51–57. [Google Scholar] [CrossRef]
- Rong, Q.Y.; Xiao, W.Z.; Xiao, G.; Hu, A.M.; Wang, L.L. Magnetic properties in BiFeO3 doped with Cu and Zn first-principles investigation. J. Alloys Compd. 2016, 674, 463–469. [Google Scholar] [CrossRef]
- Reddy, V.R.; Kothari, D.; Gupta, A.; Gupta, S.M. Study of weak ferromagnetism in polycrystalline multiferroic Eu doped bismuth ferrite. Appl. Phys. Lett. 2009, 94, 082505. [Google Scholar] [CrossRef]
- Lee, D.; Kim, M.G.; Ryu, S.; Jang, H.M.; Lee, S.G. Epitaxially grown La-modified BiFeO3 magnetoferroelectric thin films. Appl. Phys. Lett. 2005, 86, 222903. [Google Scholar] [CrossRef] [Green Version]
- Koningsberger, D.C.; Mojet, B.L.; van Dorssen, G.E.; Ramaker, D.E. XAFS spectroscopy; fundamental principles and data analysis. Top. Catal. 2000, 10, 143–155. [Google Scholar] [CrossRef]
- Yoneda, Y.; Kitanaka, Y.; Noguchi, Y.; Miyayama, M. Electronic and local structures of Mn-doped BiFeO3 crystals. Phys. Rev. B 2012, 86, 184112. [Google Scholar] [CrossRef]
- Park, T.J.; Papaefthymiou, G.C.; Viescas, A.J.; Moodenbaugh, A.R.; Wong, S.S. Size-dependent magnetic properties of single-crystalline multiferroic BiFeO3 nanoparticles. Nano Lett. 2007, 7, 766–772. [Google Scholar] [CrossRef] [PubMed]
- Alikhanov, N.M.R.; Rabadanov, M.K.; Orudzhev, F.F.; Gadzhimagomedov, S.K.; Emirov, R.M.; Sadykov, S.A.; Kallaev, S.N.; Ramazanov, S.M.; Abdulvakhidov, K.G.; Sobola, D. Size-dependent structural parameters, optical, and magnetic properties of facile synthesized pure-phase BiFeO3. J. Mater. Sci. Mater. 2021, 32, 13323–13335. [Google Scholar] [CrossRef]
- Irandoust, R.; Gholizadeh, A. A comparative study of the effect of the non-magnetic and magnetic trivalent rare-earth ion substitutions on bismuth ferrite properties: Correlation between the crystal structure and physical properties. Solid State Sci. 2020, 101, 106142. [Google Scholar] [CrossRef]



| BFO | BFC | BFZC | |
|---|---|---|---|
| a(Å) | 5.58092 ± 0.00018 | 5.58143 ± 0.00026 | 5.58602 ± 0.0007 |
| c(Å) | 13.87368 ± 0.00084 | 13.86918 ± 0.00128 | 13.88424 ± 0.00035 |
| R(Bi-O1) (Å) | 1.92675 ± 0.00006 | 1.95260 ± 0.00008 | 2.01782 ± 0.00002 |
| r(Bi-O) (Å) | 1.94194 ± 0.02 | 1.95234 ± 0.02 | 1.97262 ± 0.02 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Liu, L.; Wang, D.; Zhang, H.; He, X.; Li, Q. The Study of Magnetic Properties for Non-Magnetic Ions Doped BiFeO3. Materials 2021, 14, 4061. https://doi.org/10.3390/ma14154061
Li Y, Liu L, Wang D, Zhang H, He X, Li Q. The Study of Magnetic Properties for Non-Magnetic Ions Doped BiFeO3. Materials. 2021; 14(15):4061. https://doi.org/10.3390/ma14154061
Chicago/Turabian StyleLi, Yongtao, Liqing Liu, Dehao Wang, Hongguang Zhang, Xuemin He, and Qi Li. 2021. "The Study of Magnetic Properties for Non-Magnetic Ions Doped BiFeO3" Materials 14, no. 15: 4061. https://doi.org/10.3390/ma14154061






