Effects of Zinc Addition on the Corrosion Behavior of Pre-Filmed Alloy 690 in Borated and Lithiated Water at 330 °C
Abstract
:1. Introduction
2. Experimental Methods
2.1. Test Material and Conditions
2.2. Oxide Characterization
2.3. Descaling of Corrosion Coupons
2.4. Calculation of Corrosion and Release Rates
3. Results and Discussion
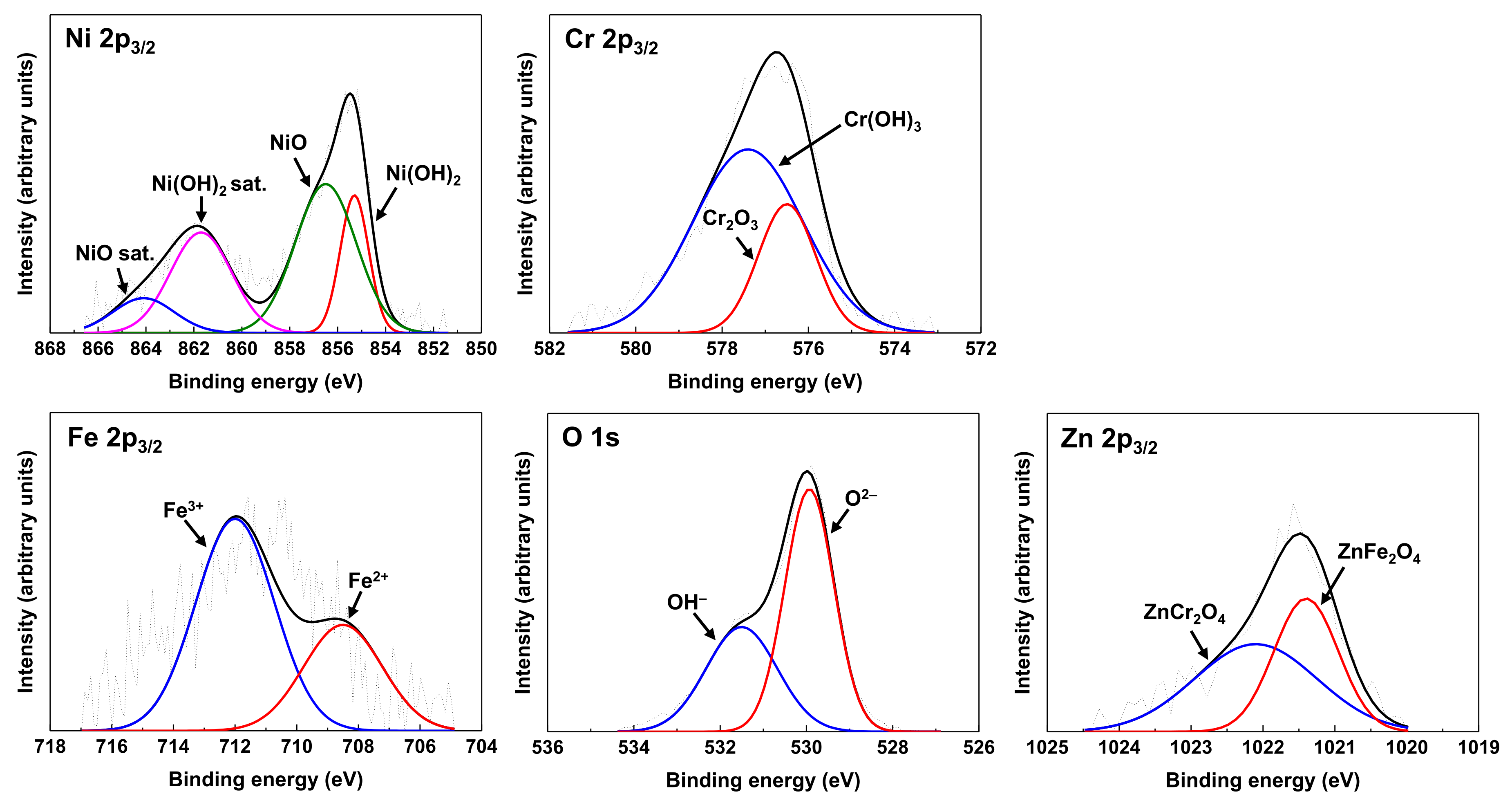
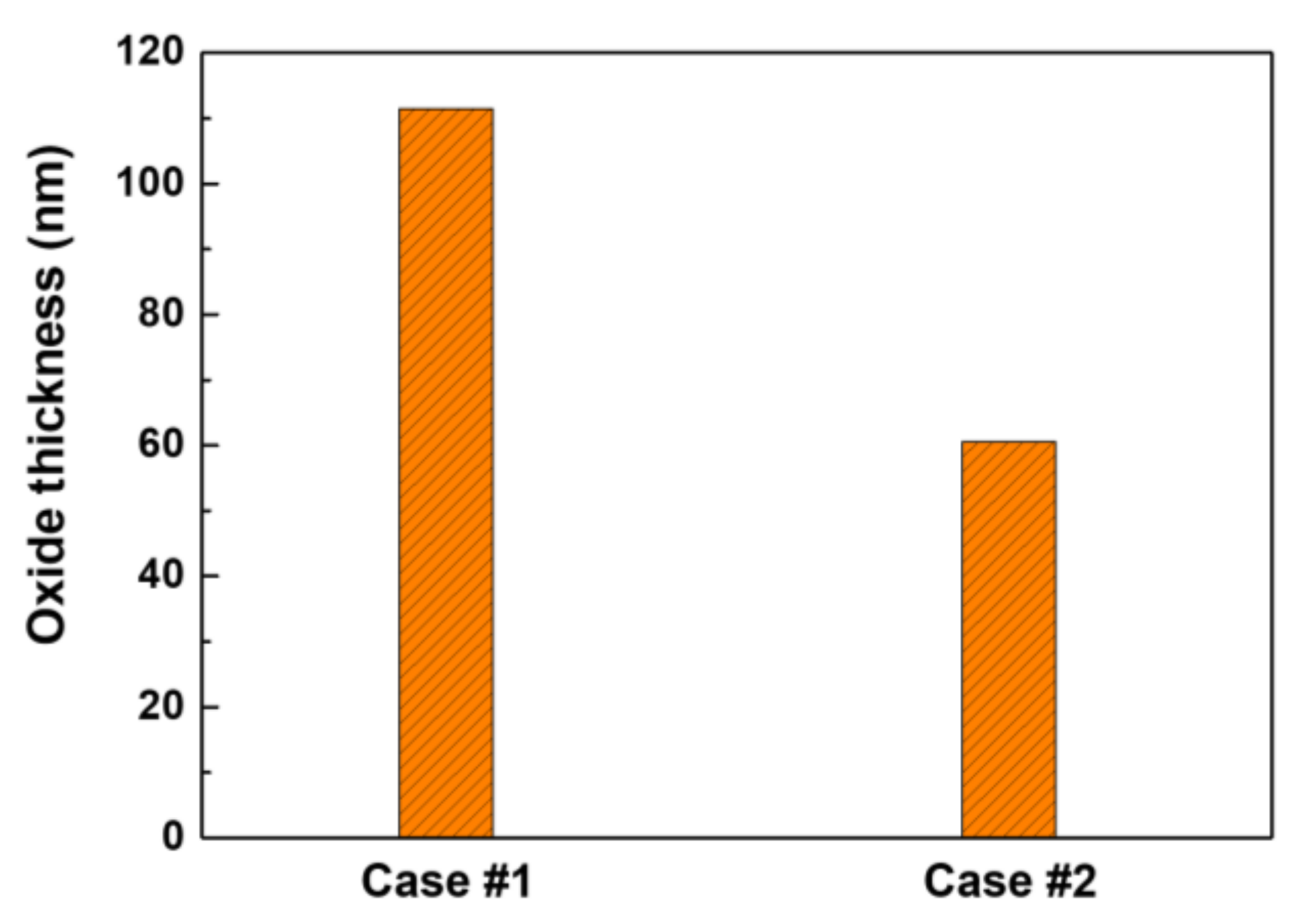
3.1. Oxide Characteristics
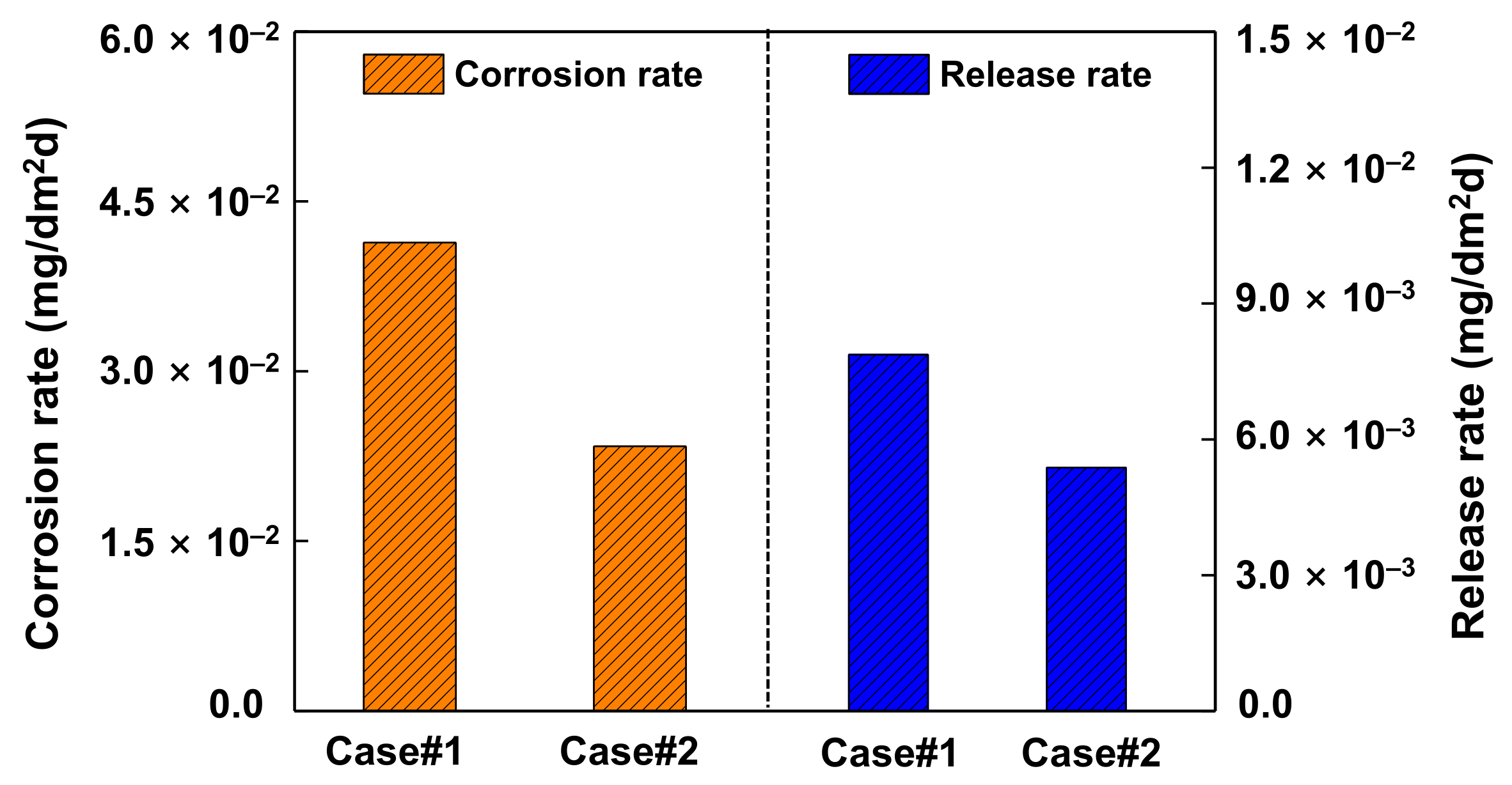
3.2. Corrosion and Release Behavior
4. Conclusions
- (1)
- Zinc was incorporated and nickel content increased in the oxides when the pre-oxidized coupons were subsequently exposed to water containing zinc, indicating a mitigation effect against selective nickel dissolution by zinc addition.
- (2)
- Subsequent exposure of the pre-filmed specimens to zinc water resulted in a significant reduction in the corrosion rate, the release rate, and the oxide thickness, respectively. The beneficial effects are attributed to the formation of a zinc-incorporated chromium-rich inner oxide layer, having a relatively low solubility and defect density.
- (3)
- Zinc addition reduced the amount of released base metal and suppressed selective dissolution of nickel, thereby contributing to a reduction of the radiation source term.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ocken, H. Reducing the Cobalt Inventory in Light Water Reactors. Nucl. Technol. 1985, 68, 18–28. [Google Scholar] [CrossRef]
- McElrath, J. Pressurized Water Reactor Primary Water Chemistry Guidelines; Revision 7; 3002000505; Electric Power Research Institute: Palo Alto, CA, USA, 2014; Volume 1. [Google Scholar]
- Wood, C.J. Zinc Injection to Control Radiation Buildup at BWRs: Plant Demonstrations; NP-6168; Electric Power Research Institute: Palo Alto, CA, USA, 1989. [Google Scholar]
- Gold, R.E.; Kormuth, J.W.; Bergmann, C.A.; Perock, J.D.; Corpora, G.J.; Miller, R.S.; Springer, G.; Jenkins, S.L.; Roesmer, J. Evaluation of Zinc Addition to Primary Coolant of Farley-2 PWR; TR-106358-V1; Electric Power Research Institute: Palo Alto, CA, USA, 1996. [Google Scholar]
- Wells, D.M.; Fruzzetti, K.; Garcia, S.; McElrath, J. Chemistry control to meet the demands of modern nuclear power plant operation. In Proceedings of the International Conference on Water Chemistry in Nuclear Power Plants, Brighton, UK, 2–7 October 2016. Paper No. 02. [Google Scholar]
- Zhang, L.; Chen, K.; Wang, J.; Guo, X.; Dua, D.; Andresen, P.L. Effects of zinc injection on stress corrosion cracking of cold worked austenitic stainless steel in high-temperature water environments. Scr. Mater. 2017, 140, 50–54. [Google Scholar] [CrossRef]
- Kawamura, H.; Hirano, H.; Shirai, S.; Takamatsu, H.; Matsunaga, T.; Yamaoka, K.; Oshinden, K.; Takiguchi, H. Inhibitory effect of zinc addition to high-temperature hydrogenated water on mill-annealed and prefilmed Alloy 600. Corrosion 2000, 56, 623–637. [Google Scholar] [CrossRef]
- Angeliu, T.M.; Andresen, P.L. Effect of zinc additions on oxide rupture strain and repassivation kinetics of iron-based alloys in 288 °C water. Corrosion 1996, 52, 28–35. [Google Scholar] [CrossRef]
- Kim, H.S.; Lee, H.B.; Chen, J.; Jang, C.; Kim, T.S.; Stevens, G.L.; Ahluwalia, K. Effect of zinc on the environmentally-assisted fatigue behavior of 316 stainless steels in simulated PWR primary environment. Corros. Sci. 2019, 151, 97–107. [Google Scholar] [CrossRef]
- Gold, R.E.; Byers, W.A.; Jacko, R.J. Zinc-oxide corrosion film interactions in PWR primary coolant. In Proceedings of the International Symposium Fontevraud III, SFEN, Fontevraud, France, 12–16 September 1994; pp. 300–309. [Google Scholar]
- Ziemniak, S.E.; Hanson, M. Zinc treatment effects on corrosion behavior of 304 stainless steel in high temperature, hydrogenated water. Corros. Sci. 2006, 48, 2525–2546. [Google Scholar] [CrossRef] [Green Version]
- Ziemniak, S.E.; Hanson, M. Zinc treatment effects on corrosion behavior of Alloy 600 in high temperature, hydrogenated water. Corros. Sci. 2006, 48, 3330–3348. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Wu, X.; Han, E.-H. Effect of zinc injection on established surface oxide films on 316L stainless steel in borated and lithiated high temperature water. Corros. Sci. 2012, 65, 136–144. [Google Scholar] [CrossRef]
- Esposito, J.N.; Economy, G.; Byers, W.A.; Esposito, J.B.; Pement, F.W.; Jacko, R.J.; Bergmann, C.A. The addition of zinc to primary reactor coolant for enhanced PWSCC resistance. In Proceedings of the Fifth International Symposium on Environmental Degradation of Materials in Nuclear Power Systems–Water Reactors, Monterey, CA, USA, 25–29 August 1991; American Nuclear Society: La Grange Park, IL, USA; pp. 495–501. [Google Scholar]
- Lister, D.H.; Godin, M.S. The Effect of Dissolved Zinc on the Transport of Corrosion Products in PWR; NP-6975-D; Electric Power Research Institute: Palo Alto, CA, USA, 1990. [Google Scholar]
- Byers, W.A.; Jacko, R.J. The influence of zinc additions and PWR primary water chemistry on surface films that form on nickel based alloys and stainless steels. In Proceedings of the Sixth International Symposium on Environmental Degradation of Materials in Nuclear Power Systems—Water Reactors, San Diego, CA, USA, 1–5 August 1993; pp. 837–844. [Google Scholar]
- Liu, X.; Wu, X.; Han, E.-H. Influence of Zn injection on characteristics of oxide film on 304 stainless steel in borated and lithiated high temperature water. Corros. Sci. 2011, 53, 3337–3345. [Google Scholar] [CrossRef]
- Huang, J.; Liu, X.; Han, E.-H.; Wu, X. Influence of Zn on oxide films on Alloy 690 in borated and lithiated high temperature water. Corros. Sci. 2011, 53, 3254–3261. [Google Scholar] [CrossRef]
- Liu, X.; Han, E.-H.; Wu, X. Effects of pH value on characteristics of oxide films on 316L stainless steel in Zn-injected borated and lithiated high temperature water. Corros. Sci. 2014, 78, 200–207. [Google Scholar] [CrossRef]
- Liu, X.; Wu, X.; Han, E.-H. Electrochemical and surface analytical investigation of the effects of Zn concentrations on characteristics of oxide films on 304 stainless steel in borated and lithiated high temperature water. Electrochim. Acta 2013, 108, 554–565. [Google Scholar] [CrossRef]
- Bennett, P.J.; Gunnerud, P.; Loner, H.; Pettersen, J.K.; Harper, A. The effects of zinc addition on cobalt deposition in PWRs. In Water Chemistry of Nuclear Reactor Systems 7; BNES: Bournemouth, UK, 1996. [Google Scholar]
- Riess, R.; Stellwag, B. Effect of zinc on the contamination and structure of oxide layers. In Water Chemistry of Nuclear Reactor Systems 7; BNES: Bournemouth, UK, 1996. [Google Scholar]
- Beverskog, B.; Makela, K. Activity pickup in zinc doped PWR oxides. Proceedings of JAIF International Conference on Water Chemistry in Nuclear Power Plants, Kashiwazaki, Japan, 13–16 October 1998; Japanese Nuclear Society: Tokyo, Japan, 1998. [Google Scholar]
- Hussey, D. Source Term Reduction: Impact of Plant Design and Chemistry on PWR Shutdown Releases and Dose Rates; 1013507; Electric Power Research Institute: Palo Alto, CA, USA, 2006. [Google Scholar]
- Little, M. Advanced Nuclear Technology: Alloy 690 Steam Generator Tubing Specification Sourcebook; Report No. 3002003124; Electric Power Research Institute: Palo Alto, CA, USA, 2014. [Google Scholar]
- Ziemniak, S.E.; Hanson, M. Corrosion behavior of 304 stainless steel in high temperature, hydrogenated water. Corros. Sci. 2002, 44, 2209–2230. [Google Scholar] [CrossRef] [Green Version]
- Ziemniak, S.E.; Hanson, M.; Sander, P.C. Electropolishing effects on corrosion behavior of 304 stainless steel in high temperature, hydrogenated water. Corros. Sci. 2008, 50, 2465–2477. [Google Scholar] [CrossRef]
- Shim, H.-S.; Seo, M.J.; Hur, D.H. Effect of electropolishing on general corrosion of Alloy 690TT tubes in simulated primary coolant of pressurized water. Appl. Surf. Sci. 2019, 467–468, 467–476. [Google Scholar] [CrossRef]
- Marble, W.J. Control of Radiation-Field Buildup in BWRs; NP-4072; Electric Power Research Institute: Palo Alto, CA, USA, 1985. [Google Scholar]
- Mansur, A.N. Characterization of NiO by XPS. Surf. Sci. Spectra 1994, 3, 231–238. [Google Scholar] [CrossRef]
- Shalvoy, R.B.; Reucroft, P.J.; Davis, B.H. Characterization of coprecipitated nickel on silica methanation catalysts by X-ray photoelectron spectroscopy. J. Catal. 1979, 56, 336–348. [Google Scholar] [CrossRef]
- Mansur, A.N. Characterization of β-Ni(OH)2 by XPS. Surf. Sci. Spectra 1994, 3, 239–246. [Google Scholar] [CrossRef]
- Paparazzo, E. XPS and auger spectroscopy studies on mixtures of the oxides SiO2, Al2O3, Fe2O3 and Cr2O3. J. Electron Spectrosc. Relat. Phenom. 1987, 43, 97–112. [Google Scholar] [CrossRef]
- Paparazzo, E. XPS analysis of oxide. Surf. Interface Anal. 1988, 12, 115–118. [Google Scholar] [CrossRef]
- Halada, G.P.; Clayton, C.R. Photoreduction of hexavalent chromium during X-ray photoelectron spectroscopy analysis of electrochemical and thermal films. J. Electrochem. Soc. 1991, 138, 2921–2927. [Google Scholar] [CrossRef]
- Shuttleworth, D. Preparation of metal-polymer dispersions by plasma techniques. An ESCA investigation. J. Phys. Chem. 1980, 84, 1629–1634. [Google Scholar] [CrossRef]
- Freire, L.; Nóvoa, R.; Montemor, M.F.; Carmezim, M.J. Study of passive films formed on mild steel in alkaline media by the application of anodic potentials. Mater. Chem. Phys. 2009, 114, 962–972. [Google Scholar] [CrossRef]
- Xu, W.; Daub, K.; Zhang, X.; Noel, J.J.; Shoesmith, D.W.; Wren, J.C. Oxide formation and conversion on carbon steel in mildly basic solutions. Electrochim. Acta 2009, 54, 5727–5738. [Google Scholar] [CrossRef]
- Wang, J.; Li, X.; Huang, F.; Zhang, Z.; Wang, J.; Staehle, R.W. Comparison of corrosion resistance of UNS N06690TT and UNS N08800SN in simulated primary water with various concentration of dissolved oxygen. Corrosion 2009, 70, 598–614. [Google Scholar] [CrossRef]
- Duan, Z.; Arjmand, F.; Zhang, L.; Meng, F.; Abe, H. Electrochemical and XPS investigation of the corrosion behavior of Alloy 690 at high-temperature water. J. Solid State Electrochem. 2015, 19, 2265–2273. [Google Scholar] [CrossRef]
- Sun, H.; Wu, X.; Han, E.H.; Wei, Y. Effects of pH and dissolved oxygen on electrochemical behavior and oxide films of 304SS in borated and lithiated high temperature water. Corros. Sci. 2012, 59, 334–342. [Google Scholar] [CrossRef]
- Dake, L.S.; Baer, D.R.; Zachara, J.M. Auger parameter measurement of zinc compounds relevant to zinc transport in the environment. Surf. Interface Anal. 1989, 14, 71–75. [Google Scholar] [CrossRef]
- Battistoni, C.; Dormann, J.L.; Fiorani, D.; Paparazzo, E.; Viticoli, S. An XPS and Mössbauer study of the electronic properties of ZnCrxGa2-xO4 spinel solid solutions. Solid State Commun. 1981, 39, 581–585. [Google Scholar] [CrossRef]
- Goldstein, J.I.; Choi, S.K.; van Loo, F.J.J.; Heuligers, H.J.M.; Bastin, G.F.; Sloof, W.G. The influence of oxide surface layers on bulk electron probe microanalysis of oxygen-application to Ti-Si-O compounds. Scanning 1993, 15, 165–170. [Google Scholar] [CrossRef] [Green Version]
- Panter, J.; Viguier, B.; Cloue, J.-M.; Foucault, M.; Combrade, P.; Andrieu, E. Influence of oxide films on primary water stress corrosion cracking initiation of alloy 600. J. Nucl. Mater. 2006, 348, 213–221. [Google Scholar] [CrossRef] [Green Version]
- Sennour, M.; Marchetti, L.; Martin, F.; Perrin, S.; Molins, R.; Pijolat, M. A detailed TEM and SEM study of Ni-base alloys oxide scales formed in primary conditions of pressurized water reactor. J. Nucl. Mater. 2010, 402, 147–156. [Google Scholar] [CrossRef] [Green Version]
- Kuang, W.; Song, M.; Wang, P.; Was, G.S. The oxidation of Alloy 690 in simulated pressurized water reactor primary water. Corros. Sci. 2017, 126, 227–237. [Google Scholar] [CrossRef]
- Stellwag, B. The mechanism of oxide film formation on austenitic stainless steels in high temperature water. Corros. Sci. 1998, 40, 337–370. [Google Scholar] [CrossRef]
- Robertson, J. The mechanism of high temperature aqueous corrosion of steel. Corros. Sci. 1989, 29, 1275–1291. [Google Scholar] [CrossRef]
- Ma, C.; Tschauner, O.; Beckett, J.R.; Liu, Y.; Greenberg, E.; Prakapenka, V.B. Chenmingite, FeCr2O4 in the CaFe2O4-type structure, a shock-induced, high-pressure mineral in the Tissint martian meteorite. Am. Mineral. 2019, 104, 1521–1525. [Google Scholar] [CrossRef]
- Ishii, T.; Kojitani, H.; Tsukamoto, S.; Fujino, K.; Mori, D.; Inaguma, Y.; Tsujino, N.; Yoshino, T.; Yamazaki, D.; Higo, Y.; et al. High-pressure phase transitions in FeCr2O4 and structure analysis of new post-spinel FeCr2O4 and Fe2Cr2O5 phases with meteoritical and petrological implications. Am. Mineral. 2014, 99, 1788–1797. [Google Scholar] [CrossRef] [Green Version]
- Kubaschewski, O.; Hopkins, B.E. Oxidation of Metals and Alloys; Butterworths: London, UK, 1967. [Google Scholar]
- Holdsworth, S.; Scenini, F.; Burke, M.G.; Bertali, G.; Ito, T.; Wada, Y.; Hosokawa, H.; Ota, N.; Nagase, M. The effect of high-temperature water chemistry and dissolved zinc on the cobalt incorporation on type 316 stainless steel oxide. Corros. Sci. 2018, 140, 241–251. [Google Scholar] [CrossRef] [Green Version]
- Navrotsky, A.; Kleppa, O.J. The thermodynamics of cation distribution in simple spinels. J. Inorg. Nucl. Chem. 1967, 29, 2701–2714. [Google Scholar] [CrossRef]
- Lister, D.H. Activity transport and corrosion processes in PWRs. In Water Chemistry of Nuclear Reactor System 6; Bournemouth, British Nuclear Energy Society: London, UK, 1992; Volume 2, pp. 49–60. [Google Scholar]
- Korb, J.; Stellwag, B. Thermodynamics of zinc chemistry in PWRS–Effects and alternatives to zinc. In Water Chemistry of Nuclear Reactor Systems 7; BNES: Bournemouth, UK, 1996. [Google Scholar]
- Prince, E. Structure of nickel chromite. J. Appl. Phys. 1961, 32, 68S–69S. [Google Scholar] [CrossRef]
- Hastings, J.M.; Corliss, L.M. Magnetic structure of manganese chromite. Phys. Rev. 1962, 126, 556–565. [Google Scholar] [CrossRef]
- Maignan, A.; Martin, C.; Singh, K.; Simon, C.; Lebedev, O.I.; Turner, S. From spin induced ferroelectricity to dipolar glasses: Spinel chromites and mixed delafossites. J. Solid State Chem. 2012, 195, 41–49. [Google Scholar] [CrossRef]
- Chen, X.H.; Zhang, H.T.; Wang, C.H.; Luo, X.G.; Li, P.H. Effect of particle size on magnetic properties of zinc chromite synthesized by sol–gel method. Appl. Phys. Lett. 2002, 81, 4419–4421. [Google Scholar] [CrossRef]
- Lister, D.H.; Davidson, R.D.; McAlpine, E. The mechanism and kinetics of corrosion product release from stainless steel in lithiated high temperature water. Corros. Sci. 1987, 27, 113–1411. [Google Scholar] [CrossRef]
- Marchetti, L.; Perrin, S.; Jambon, F.; Pijolat, M. Corrosion of nickel-base alloys in primary medium of pressurized water reactors: New insights on the oxide growth mechanisms and kinetic modelling. Corros. Sci. 2016, 102, 24–35. [Google Scholar] [CrossRef]
- Miyajima, K.; Hirano, H. Thermodynamic Consideration on the Effect of Zinc Injection into PWR Primary Coolant for the Reduction of Radiation Buildup and Corrosion Control; Corrosion 2001; NACE: Houston, TX, USA, 2001; Paper No. 01143. [Google Scholar]
- Chao, C.Y.; Lin, L.F.; Macdonald, D.D. A point defect model for anodic passive films. J. Electrochem. Soc. 1981, 128, 1187–1194. [Google Scholar] [CrossRef]
- Macdonald, D.D. The history of the point defect model for the passive state: A brief review of film growth aspects. Electrochim. Acta 2011, 56, 1761–1772. [Google Scholar] [CrossRef]

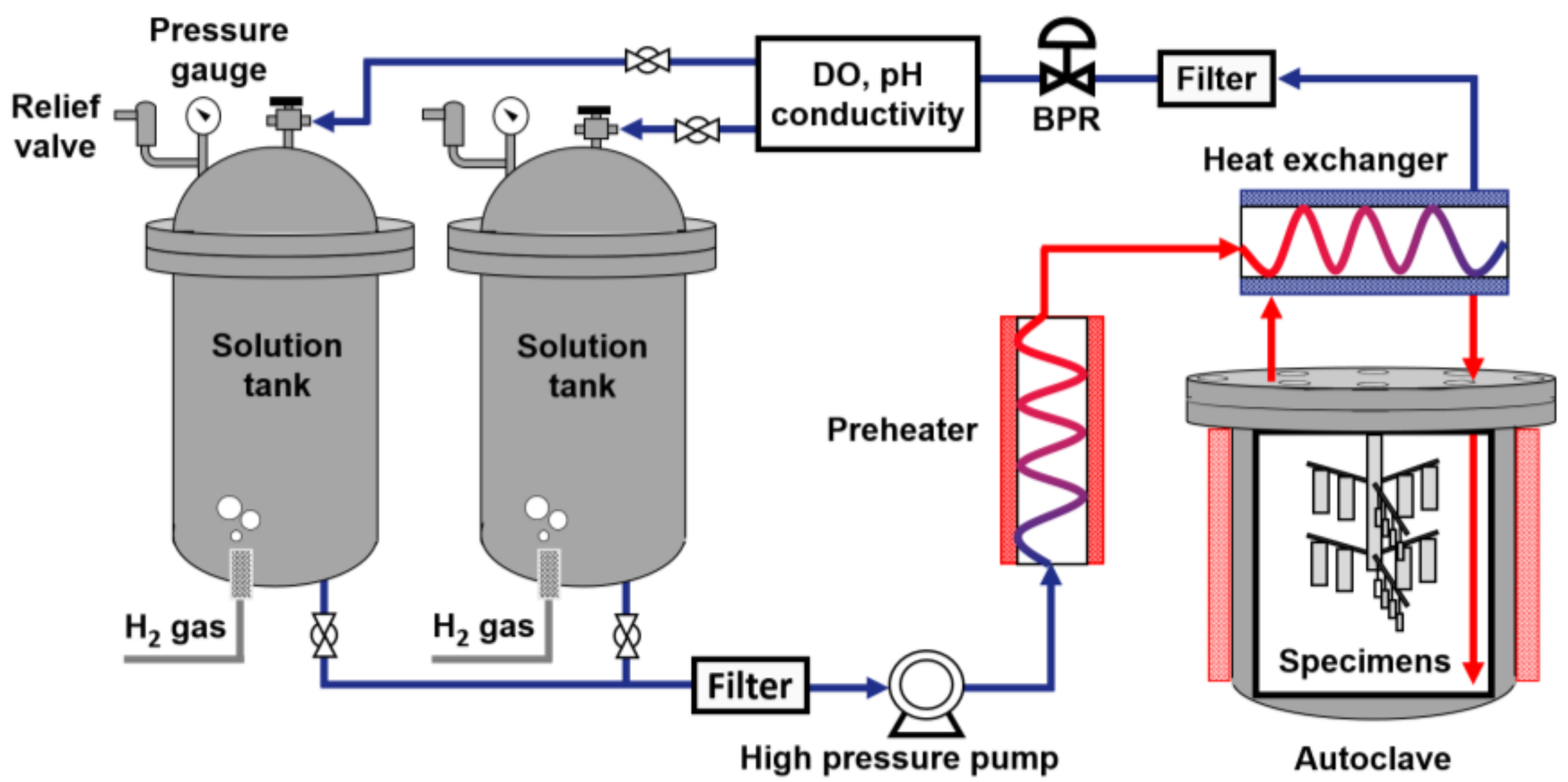


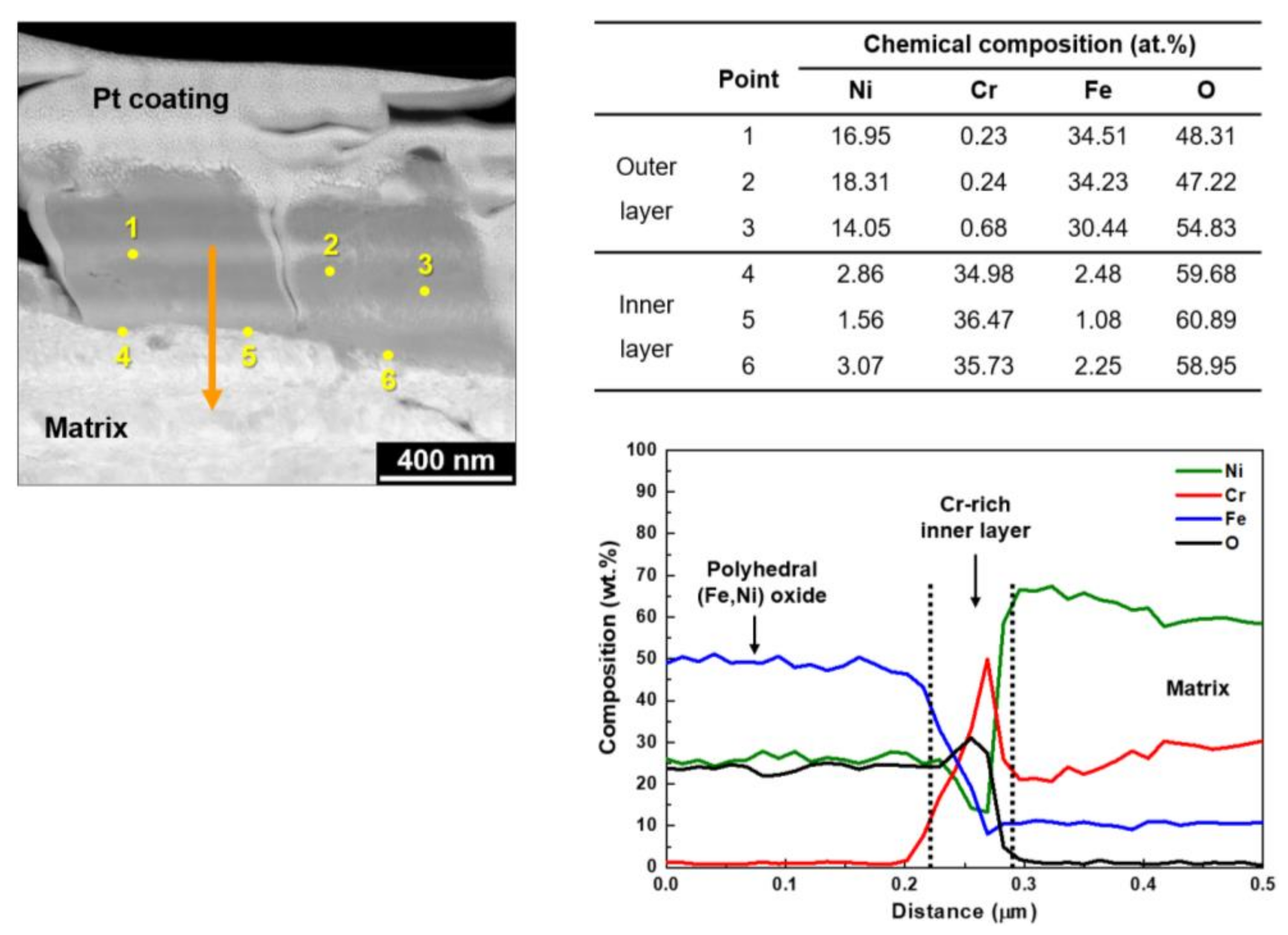
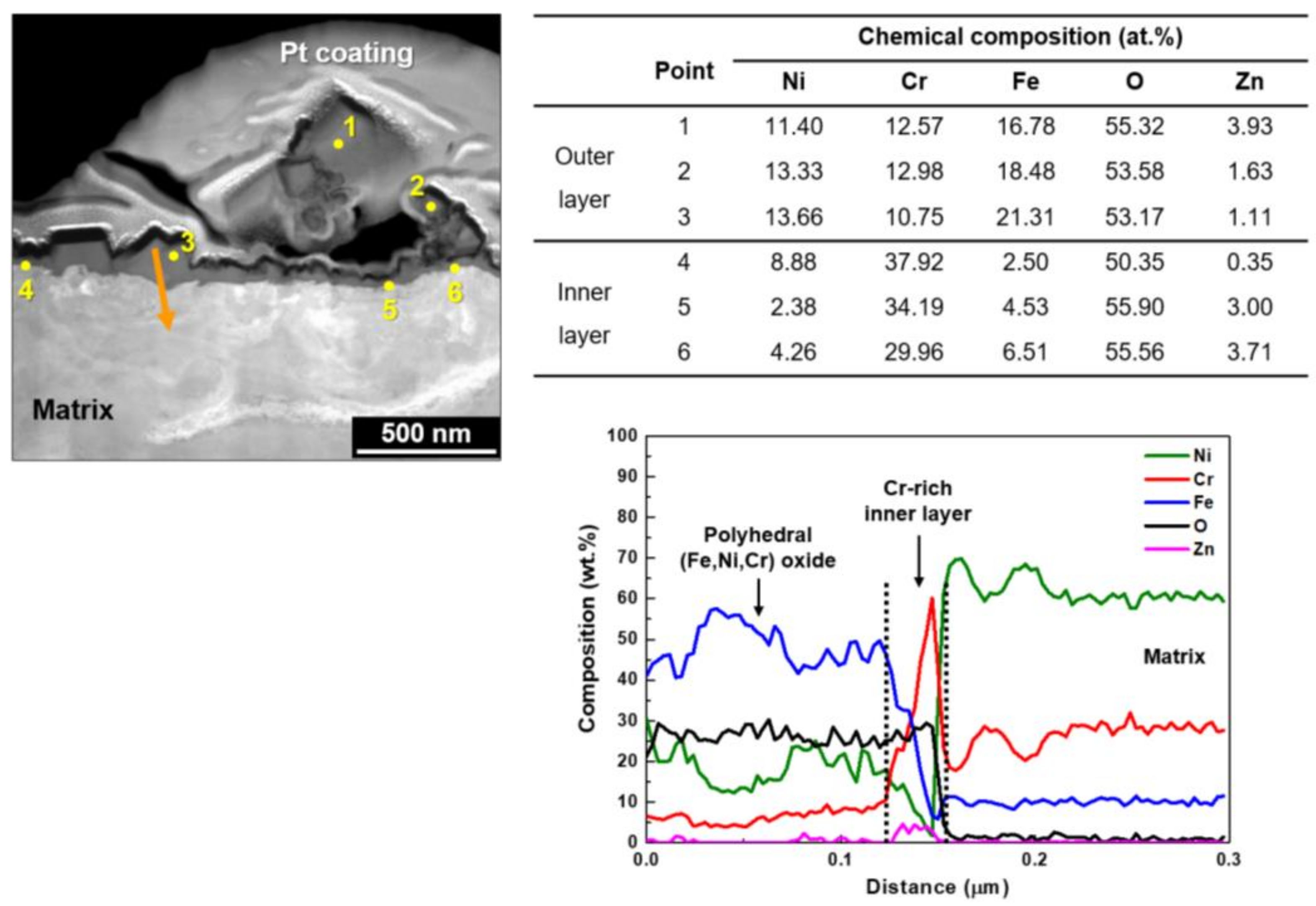
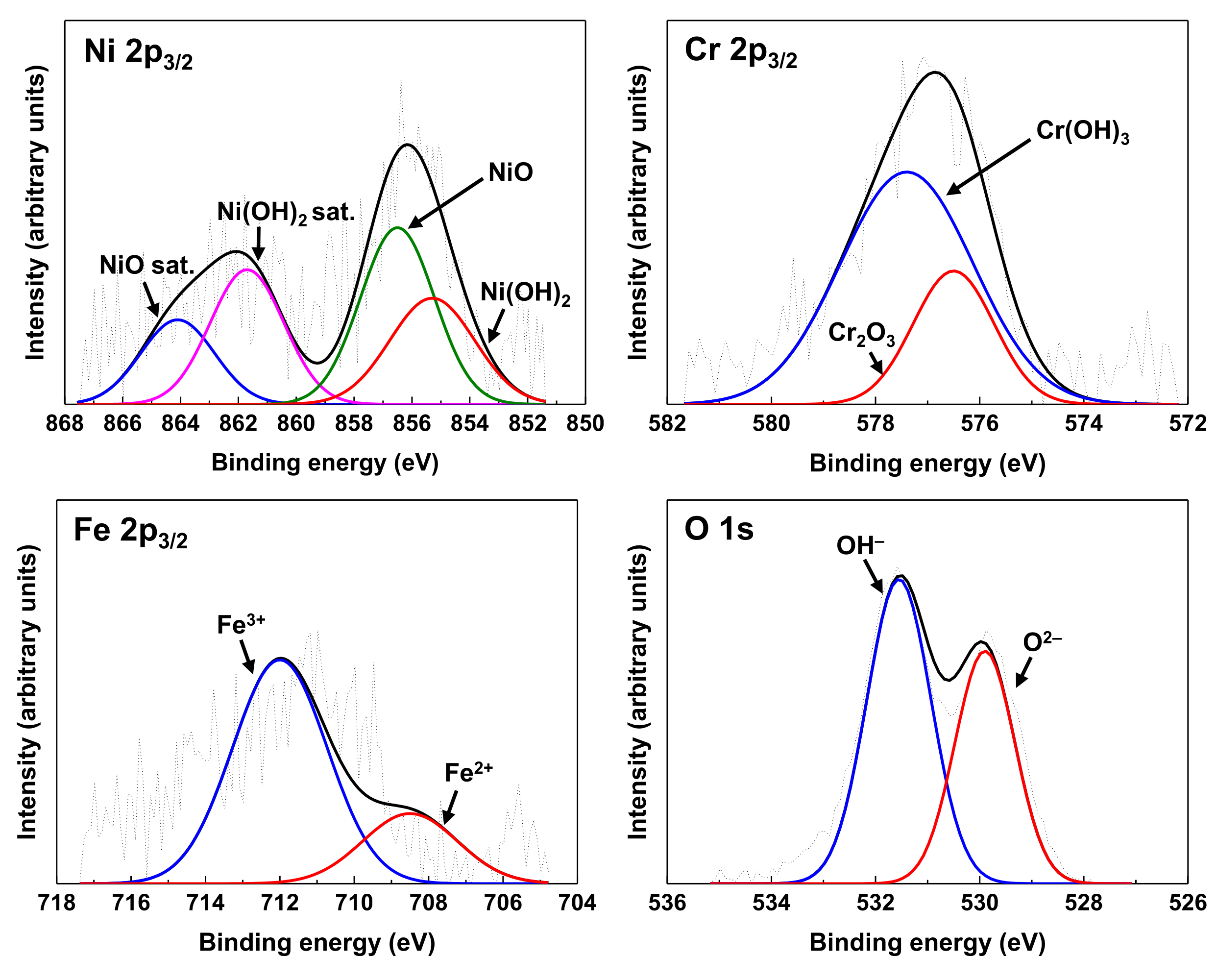

| Ni | Cr | Fe | C | Si | Mn | Ti | Al | N | Cu | Co |
|---|---|---|---|---|---|---|---|---|---|---|
| 59.12 | 29.28 | 10.40 | 0.019 | 0.32 | 0.33 | 0.33 | 0.16 | 0.024 | 0.01 | 0.007 |
| Exposure Method | Solution Chemistry | Common | |
|---|---|---|---|
| Case #1 | 1500 h without Zn→1500 h without Zn | 2 ppm Li + 1000 ppm B | DO < 5 ppb DH = 3.12 ppm 330 °C |
| Case #2 | 1500 h without Zn→1500 h with 60 ppb Zn | 2 ppm Li + 1000 ppm B (+ 60 ppb Zn) | |
| Chemical Species | Binding Energy (eV) | Chemical Species | Binding Energy (eV) |
|---|---|---|---|
| NiO 2p3/2 | 856.5 [30] | Fe2+ 2p3/2 | 708.5 [37,38,39] |
| NiO sat. 2p3/2 | 864.1 [30] | Fe3+ 2p3/2 | 712.0 [37,38,39] |
| Ni(OH)2 2p3/2 | 855.3 [31] | O2– 1s | 530.0 [40,41] |
| Ni(OH)2 sat. 2p3/2 | 861.7 [32] | OH– 1s | 531.5 [40,41] |
| Cr2O3 2p3/2 | 576.5 [33,34,35] | ZnFe2O4 2p3/2 | 1021.4 [42] |
| Cr(OH)3 2p3/2 | 577.4 [36] | ZnCr2O4 2p3/2 | 1022.1 [43] |
| Chemical Composition (wt.%) | Average Stoichiometry | Weight Fraction of Ni, Cr and Fe in the Oxides | |||||
|---|---|---|---|---|---|---|---|
| Ni | Cr | Fe | Zn | O | |||
| Case #1 | 21.8 | 28.6 | 20.9 | - | 28.7 | Ni0.86Fe0.87Cr1.27O4 | 0.71 |
| Case #2 | 25.9 | 31.4 | 13.3 | 3.9 | 25.5 | Zn0.13Ni0.99Fe0.53Cr1.35O4 | 0.71 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeon, S.-H.; Lim, D.-S.; Choi, J.; Song, K.-M.; Lee, J.-H.; Hur, D.-H. Effects of Zinc Addition on the Corrosion Behavior of Pre-Filmed Alloy 690 in Borated and Lithiated Water at 330 °C. Materials 2021, 14, 4105. https://doi.org/10.3390/ma14154105
Jeon S-H, Lim D-S, Choi J, Song K-M, Lee J-H, Hur D-H. Effects of Zinc Addition on the Corrosion Behavior of Pre-Filmed Alloy 690 in Borated and Lithiated Water at 330 °C. Materials. 2021; 14(15):4105. https://doi.org/10.3390/ma14154105
Chicago/Turabian StyleJeon, Soon-Hyeok, Dong-Seok Lim, Jinsoo Choi, Kyu-Min Song, Jong-Hyeon Lee, and Do-Haeng Hur. 2021. "Effects of Zinc Addition on the Corrosion Behavior of Pre-Filmed Alloy 690 in Borated and Lithiated Water at 330 °C" Materials 14, no. 15: 4105. https://doi.org/10.3390/ma14154105
APA StyleJeon, S.-H., Lim, D.-S., Choi, J., Song, K.-M., Lee, J.-H., & Hur, D.-H. (2021). Effects of Zinc Addition on the Corrosion Behavior of Pre-Filmed Alloy 690 in Borated and Lithiated Water at 330 °C. Materials, 14(15), 4105. https://doi.org/10.3390/ma14154105








