Evaluation of Two Amendments (Biochar and Acid Mine Drainage Sludge) on Arsenic Contaminated Soil Using Chemical, Biological, and Ecological Assessments
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Soil and Amendments
2.2. Analysis of Soil Physico-Chemical Properties and Amendments
2.3. Phytotoxicity Test and Enzyme Activity Test for Biological Assessment
2.4. Microbial Analysis for Simplified Ecological Assessment
2.4.1. Microbial Counts
2.4.2. DNA Extraction, Sequencing, and Microbial Community Analysis
2.5. Data Analysis
3. Results and Discussion
3.1. Basic Soil Characteristics
3.2. Effect of Amendment Materials on Soil Characteristics
3.3. Effect of Amendments on Lettuce Growth and Biological Properties
3.4. Effect of Amendments on Microbial Activity and Diversity
3.5. Principal Component Analysis and Major Soil Factors
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mench, M.; Bussière, S.; Boisson, J.; Castaing, E.; Vangronsveld, J.; Rutten, A.; Koe, T.D.; Bleeker, P.; Assunção, A.; Manceau, A. Progress in remediation and revegetation of the barren Jales gold mine spoil after in situ treatments. Plant Soil 2003, 249, 187–202. [Google Scholar] [CrossRef]
- Mench, M.; Vangronsveld, J.; Beckx, C.; Ruttens, A. Progress in assisted natural remediation of an arsenic contaminated agricultural soil. Environ. Pollut. 2006, 144, 51–61. [Google Scholar] [CrossRef]
- Miretzky, P.; Cirelli, A.F. Remediation of Arsenic-Contaminated Soils by Iron Amendments: A Review. Crit. Rev. Environ. Sci. Technol. 2010, 40, 93–115. [Google Scholar] [CrossRef]
- Jain, A.; Raven, A.K.P.; Loeppert, R.H. Arsenite and Arsenate Adsorption on Ferrihydrite: Surface Charge Reduction and Net OH- Release Stoichiometry. Environ. Sci. Technol. 1999, 33, 1179–1184. [Google Scholar] [CrossRef]
- Fuller, C.; Davis, J.A.; Waychunas, G.A. Surface chemistry of ferrihydrite: Part 2. Kinetics of arsenate adsorption and coprecipitation. Geochim. Cosmochim. Acta 1993, 57, 2271–2282. [Google Scholar] [CrossRef]
- Koo, N.; Kim, M.-S.; Hyun, S.; Kim, J.-G. Effects of the Incorporation of Phosphorus and Iron into Arsenic-Spiked Artificial Soils on Root Growth of Lettuce using Response Surface Methodology. Commun. Soil Sci. Plant Anal. 2013, 44, 1259–1271. [Google Scholar] [CrossRef]
- Suda, A.; Yamaguchi, N.; Taniguchi, H.; Makino, T. Arsenic immobilization in anaerobic soils by the application of by-product iron materials obtained from the casting industry. Soil Sci. Plant Nutr. 2018, 64, 210–217. [Google Scholar] [CrossRef]
- Lee, S.-H.; Kim, E.Y.; Park, H.; Yun, J.; Kim, J.-G. In situ stabilization of arsenic and metal-contaminated agricultural soil using industrial by-products. Geoderma 2011, 161, 1–7. [Google Scholar] [CrossRef]
- Kim, M.-S.; Min, H.; Kim, J.-G.; Koo, N.; Park, J.S.; Bak, G.I. Effects of Various Amendments on Heavy Metal Stabilization in Acid and Alkali Soils. Korean J. Environ. Agric. 2014, 33, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Byambaa, E.; Seon, J.; Kim, T.-H.; Kim, S.D.; Ji, W.H.; Hwang, Y. Arsenic (V) Removal by an Adsorbent Material Derived from Acid Mine Drainage Sludge. Appl. Sci. 2020, 11, 47. [Google Scholar] [CrossRef]
- Guo, M.; Xiao, P.; Li, H. Valorization of agricultural byproducts through conversion to biochar and bio-oil. In Byproducts from Agriculture and Fisheries: Adding Value for Food, Feed, Pharma, and Fuels; Simpson, B.K., Aryee, A.N.A., Toldrá, F., Eds.; John Wiley & Sons Ltd.: Hoboken, NY, USA, 2020; pp. 501–522. [Google Scholar]
- Li, L.; Zou, D.; Xiao, Z.; Zeng, X.; Zhang, L.; Jiang, L.; Wang, A.; Ge, D.; Zhang, G.; Liu, F. Biochar as a sorbent for emerging contaminants enables improvements in waste management and sustainable resource use. J. Clean. Prod. 2019, 210, 1324–1342. [Google Scholar] [CrossRef]
- Jung, C.; Boateng, L.K.; Flora, J.R.; Oh, J.; Braswell, M.C.; Son, A.; Yoon, Y. Competitive adsorption of selected non-steroidal anti-inflammatory drugs on activated biochars: Experimental and molecular modeling study. Chem. Eng. J. 2015, 264, 1–9. [Google Scholar] [CrossRef]
- Vithanage, M.; Herath, I.; Joseph, S.; Bundschuh, J.; Bolan, N.; Ok, Y.S.; Kirkham, M.; Rinklebe, J. Interaction of arsenic with biochar in soil and water: A critical review. Carbon 2017, 113, 219–230. [Google Scholar] [CrossRef]
- Namgay, T.; Singh, B.; Singh, B.P. Influence of biochar application to soil on the availability of As, Cd, Cu, Pb, and Zn to maize (Zea mays L.) 2010. Aust. J. Soil Res. 2010, 48, 638–647. [Google Scholar] [CrossRef]
- Kim, M.-S.; Min, H.-G.; Lee, S.-H.; Kim, J.-G. The Effects of Various Amendments on Trace Element Stabilization in Acidic, Neutral, and Alkali Soil with Similar Pollution Index. PLoS ONE 2016, 11, e0166335. [Google Scholar] [CrossRef] [Green Version]
- Wuana, R.A.; Okieimen, F.E. Heavy Metals in Contaminated Soils: A Review of Sources, Chemistry, Risks and Best Available Strategies for Remediation. ISRN Ecol. 2011, 2011, 402647. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.-S.; Min, H.; Lee, B.; Chang, S.; Kim, J.-G.; Koo, N.; Park, J.-S.; Bak, G.-I. The Applicability of the Acid Mine Drainage Sludge in the Heavy Metal Stabilization in Soils. Korean J. Environ. Agric. 2014, 33, 78–85. [Google Scholar] [CrossRef] [Green Version]
- Koo, N.; Lee, S.H.; Kim, J.G. Arsenic mobility in the amended mine tailings and tis impact on soil enzyme activity. Environ. Geochem. Health 2012, 34, 337–348. [Google Scholar]
- Ghosh, A.; Bhattacharyya, P.; Pal, R. Effect of arsenic contamination on microbial biomass and its activities in arsenic contaminated soils of Gangetic West Bengal, India. Environ. Int. 2004, 30, 491–499. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhang, H.; Li, J.; Liu, Y.; Shi, W.; Hu, H. The toxic factor of copper should be adjusted during the ecological risk assessment for soil bacterial community. Ecol. Indic. 2020, 111, 106072. [Google Scholar] [CrossRef]
- Son, J.; Kim, J.-G.; Hyun, S.; Cho, K. Screening level ecological risk assessment of abandoned metal mines using chemical and ecotoxicological lines of evidence. Environ. Pollut. 2019, 249, 1081–1090. [Google Scholar] [CrossRef]
- Ministry of Environment. Korean Standard Method for Waste; Ministry of Environment: Sejong, Korea, 2016. [Google Scholar]
- Nelson, D.W.; Sommers, L.E. Total carbon, organic carbon and organic matter. In Methods of Soil Analysis. Part 3—Chemical Methods; Spark, D.L., Page, A.L., Helmke, P.A., Loeppert, R.H., Soltanpour, P.N., Tabatabai, M.A., Johnson, C.T., Sommer, M.E., Eds.; Soil Science Society of America: Madison, WI, USA, 1996; pp. 961–1010. [Google Scholar]
- National Institute of Agricultural Science and Technology. Method of Soil and Plant Analysis; Rural Development Administration: Suwon, Korea, 2008. [Google Scholar]
- ISO. Soil Quality–Extraction of Trace Element Soluble in Aqua Regia; ISO 11466; International Organization for Standardization: Geneva, Switzerland, 1995. [Google Scholar]
- Esnaola, M.V.; Millán, E. Evaluation of heavy metal lability in polluted soils by a cation exchange bath procedure. Environ. Pollut. 1998, 99, 79–86. [Google Scholar] [CrossRef]
- Wenzel, W.W.; Kirchbaumer, N.; Prohaska, T.; Stingeder, G.; Lombi, E.; Adriano, D.C. Arsenic fractionation in soils using an improved sequential extraction procedure. Anal. Chim. Acta 2001, 436, 309–323. [Google Scholar] [CrossRef]
- Sharmistha, P.A.L.; Marschner, P. Soil respiration, microbial biomass C and N availability in a sandy soil amended with clay and residue mixtures. Pedosphere 2016, 26, 643–651. [Google Scholar]
- Friedel, J.K.; Fischer, W.R. Comparison and improvement of methods for determining soil dehydrogenase activity by using triphenyltetrazolium chloride and iodonitrotetrazolium chloride. Biol. Fertil. Soils 1994, 18, 291–296. [Google Scholar] [CrossRef]
- Kandeler, E.; Gerber, H. Short term assay of soil urease activity using colormetric determination of ammonium. Biol. Fert. Soil 1988, 6, 68–72. [Google Scholar] [CrossRef]
- Chen, H.; Liang, Q.; Gong, Y.; Kuzyakov, Y.; Fan, M.; Plante, A.F. Reduced tillage and increased residue retention increase enzyme activity and carbon and nitrogen concentrations in soil particle size fractions in a long-term field experiment on Loess Plateau in China. Soil Tillage Res. 2019, 194, 104296. [Google Scholar] [CrossRef]
- Tanaka, Y.; Takahashi, H.; Simidu, U.; Kimura, B. Design of a New Universal Real-Time PCR System Targeting the tuf Gene for the Enumeration of Bacterial Counts in Food. J. Food Prot. 2010, 73, 670–679. [Google Scholar] [CrossRef] [PubMed]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef]
- Callahan, B.J.; Mcmurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef] [Green Version]
- Rognes, T.; Flouri, T.; Nichols, B.; Quince, C.; Mahé, F. VSEARCH: A versatile open source tool for metagenimics. Peer J. 2016, 4, e2584. [Google Scholar] [CrossRef] [PubMed]
- Bokulich, N.A.; Kaehler, B.D.; Rideout, J.R.; Dillon, M.; Bolyen, E.; Knight, R.; Huttley, G.A.; Caporaso, J.G. Optimizing taxonomic classification of marker-gene amplicon sequences with QIIME 2’s q2-feature-classifier plugin. Microbiome 2018, 6, 90. [Google Scholar] [CrossRef] [PubMed]
- McMurdie, P.J.; Holmes, S. Phyloseq: An R Package for Reproducible Interactive Analysis and Graphics of Microbiome Census Data. PLoS ONE 2013, 8, e61217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, M.-S.; Lee, S.-H.; Kim, J.-G. Assessment of Fraction and Mobility of Arsenic in Soil Near the Mine Waste Dam. Sustainability 2020, 12, 1480. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.-S.; Kim, M.-S.; Wee, J.; Min, H.-G.; Kim, J.-G.; Cho, K. Effect of bioavailable arsenic fractions on the collembolan community in an old abandoned mine waste. Environ. Geochem. Heal. 2021. [Google Scholar] [CrossRef]
- Chintala, R.; Mollinedo, J.; Schumacher, T.E.; Malo, D.D.; Julson, J.L. Effect of biochar on chemical properties of acidic soil. Arch. Agron. Soil Sci. 2014, 60, 393–404. [Google Scholar] [CrossRef]
- Shi, R.-Y.; Hong, Z.-N.; Li, J.-Y.; Jiang, J.; Baquy, M.A.-A.; Xu, R.-K.; Qian, W. Mechanisms for Increasing the pH Buffering Capacity of an Acidic Ultisol by Crop Residue-Derived Biochars. J. Agric. Food Chem. 2017, 65, 8111–8119. [Google Scholar] [CrossRef]
- Hameed, R.; Cheng, L.; Yang, K.; Fang, J.; Lin, D. Endogenous release of metals with dissolved organic carbon from biochar: Effects of pyrolysis temperature, particle size, and solution chemistry. Environ. Pollut. 2019, 255, 113253. [Google Scholar] [CrossRef]
- Tang, J.; Cao, C.; Gao, F.; Wang, W. Effects of biochar amendment on the availability of trace elements and the properties of dissolved organic matter in contaminated soils. Environ. Technol. Innov. 2019, 16, 100492. [Google Scholar] [CrossRef]
- Kim, M.-S.; Min, H.-G.; Kim, J.-G. Integrating Amendment and Liquid Fertilizer for Aided-Phytostabilization and Its Impacts on Soil Microbiological Properties in Arsenic-Contaminated Soil. Appl. Sci. 2020, 10, 3985. [Google Scholar] [CrossRef]
- Ko, M.-S.; Kim, J.-Y.; Lee, J.-S.; Ko, J.-I.; Kim, K.-W. Arsenic immobilization in water and soil using acid mine drainage sludge. Appl. Geochem. 2013, 35, 1–6. [Google Scholar] [CrossRef]
- Chen, M.; Alim, N.; Zhang, Y.; Xu, N.; Cao, X. Contrasting effects of biochar nanoparticles on the retention and transport of phosphorus in acidic and alkaline soils. Environ. Pollut. 2018, 239, 562–570. [Google Scholar] [CrossRef]
- Singh, N.; Ma, L.Q.; Srivastava, M.; Rathinasabapathi, B. Metabolic adaptations to arsenic-induced oxidative stress in Pteris vittata L and Pteris ensiformis L. Plant Sci. 2006, 170, 274–282. [Google Scholar] [CrossRef]
- Fayiga, A.O.; Ma, L.Q.; Zhou, Q. Effects of plant arsenic uptake and heavy emtals on arsenic distribution in an arsenic-contaminated soil. Environ. Pollut. 2007, 147, 737–742. [Google Scholar] [CrossRef]
- Anghinoni, I.; Magalhãs, J.R.; Barber, S.A. Enzyme activity, nitrogen uptake and corn trowth as affected by ammonium concentration in soil solution. J. Plant Nutr. 2008, 11, 131–144. [Google Scholar] [CrossRef]
- Eivazi, F.; Tabatabai, M. Factros affecting glucosidase and galactosidase in soils. Soil Biol. Biochem. 1990, 22, 891–897. [Google Scholar] [CrossRef]
- Nannipieri, P.; Giagnoni, L.; Landi, L.; Renella, G. Role of phosphatase enzymes in soil. In Phosphorus in Action; Bunemann, E.K., Obreson, A., Frrpssard, E., Eds.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 215–243. [Google Scholar]
- Rejsek, K.; Vranova, V.; Pavelka, M.; Formanek, P. Acid phosphomonoeseterase (E.C. 3.1.3.2.) location in soil. J. Plant Nutr. 2012, 175, 196–211. [Google Scholar] [CrossRef]
- Aanderud, Z.T.; Jones, S.E.; Schoolmaster, D.R., Jr.; Fierer, N.; Lennon, J.T. Sensitivity of soil respiration and microbial communities to altered snowfall. Soil Biol. Biochem. 2013, 57, 217–227. [Google Scholar] [CrossRef]
- Lin, Y.T.; Whitman, W.B.; Coleman, D.C.; Chiu, C.Y. Comparison of soil bacterial communities between coastal and inland forests in a subtropical area. Appl. Soil Ecol. 2012, 60, 49–55. [Google Scholar] [CrossRef]
- Kim, Y.; Lee, Y.; Weon, H.Y.; Sang, M.K.; Song, J. Comparative analysis of soil microbial communities between conventional and organic farming systems in pepper cultivation. Korea J. Org. Agric. 2020, 28, 235–250. [Google Scholar]
- Khan, M.; Williams, S. Studies on the ecology of actinomycetes in soil—VIII: Distribution and characteristics of acidophilic actinomycetes. Soil Biol. Biochem. 1975, 7, 345–348. [Google Scholar] [CrossRef]
- Brandt, K.K.; Frandsen, R.J.; Holm, P.; Nybroe, O. Development of pollution-induced community tolerance is linked to structural and functional resilience of a soil bacterial community following a five-year field exposure to copper. Soil Biol. Biochem. 2010, 42, 748–757. [Google Scholar] [CrossRef]
- Malik, A.; Parvaiz, A.; Mushtaq, N.; Hussain, I.; Javed, T.; Rehman, H.U.; Farooqi, A. Characterization and role of derived dissolved organic matter on arsenic mobilization in alluvial aquifers of Punjab, Pakistan. Chemosphere 2020, 251, 126374. [Google Scholar] [CrossRef] [PubMed]
- Koo, N.; Jo, H.-J.; Lee, S.-H.; Kim, J.-G. Using response surface methodology to assess the effects of iron and spent mushroom substrate on arsenic phytotoxicity in lettuce (Lactuca sativa L.). J. Hazard. Mater. 2011, 192, 381–387. [Google Scholar] [CrossRef]
- Covey, A.K.; Furbish, D.J.; Savage, K.S. Earthworms agents for arsenic transport and transformation in roxarsone-impacted soil mesocosms: A μXANES and modeling study. Geoderma 2010, 156, 99–111. [Google Scholar] [CrossRef]
- Kravchenko, A.; Guber, A.; Gunina, A.; Dippold, M.; Kuzyakov, Y. Pore-scale view of microbial turnover: Combining 14 C imaging, μCT and zymography after adding soluble carbon to soil pores of specific sizes. Eur. J. Soil Sci. 2021, 72, 593–607. [Google Scholar] [CrossRef]
- Rezanezhad, F.; Kleimeier, C.; Milojevic, T.; Liu, H.; Weber, T.K.D.; Van Cappellen, P.; Lennartz, B. The Role of Pore Structure on Nitrate Reduction in Peat Soil: A Physical Characterization of Pore Distribution and Solute Transport. Wetlands 2017, 37, 951–960. [Google Scholar] [CrossRef]
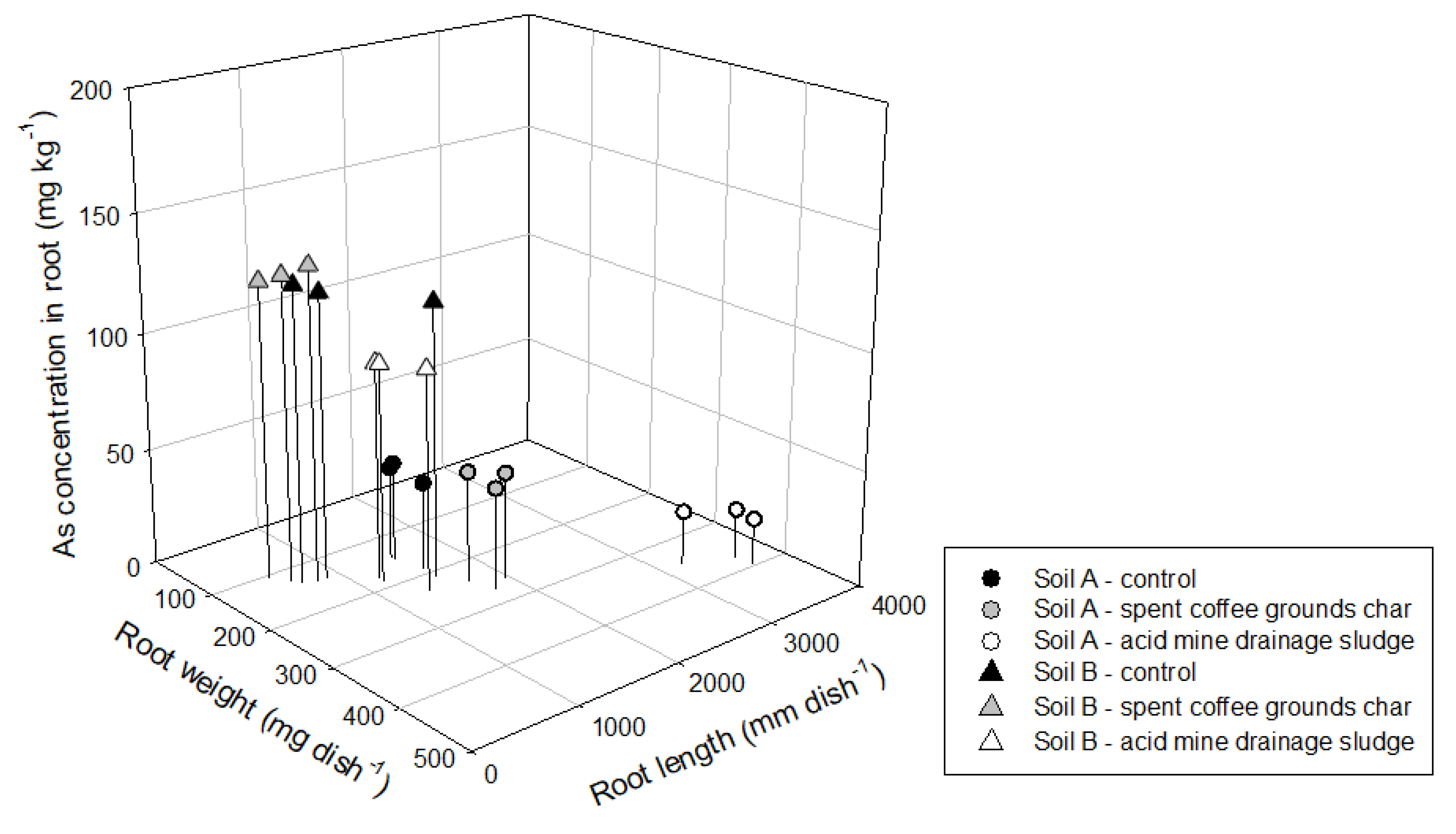
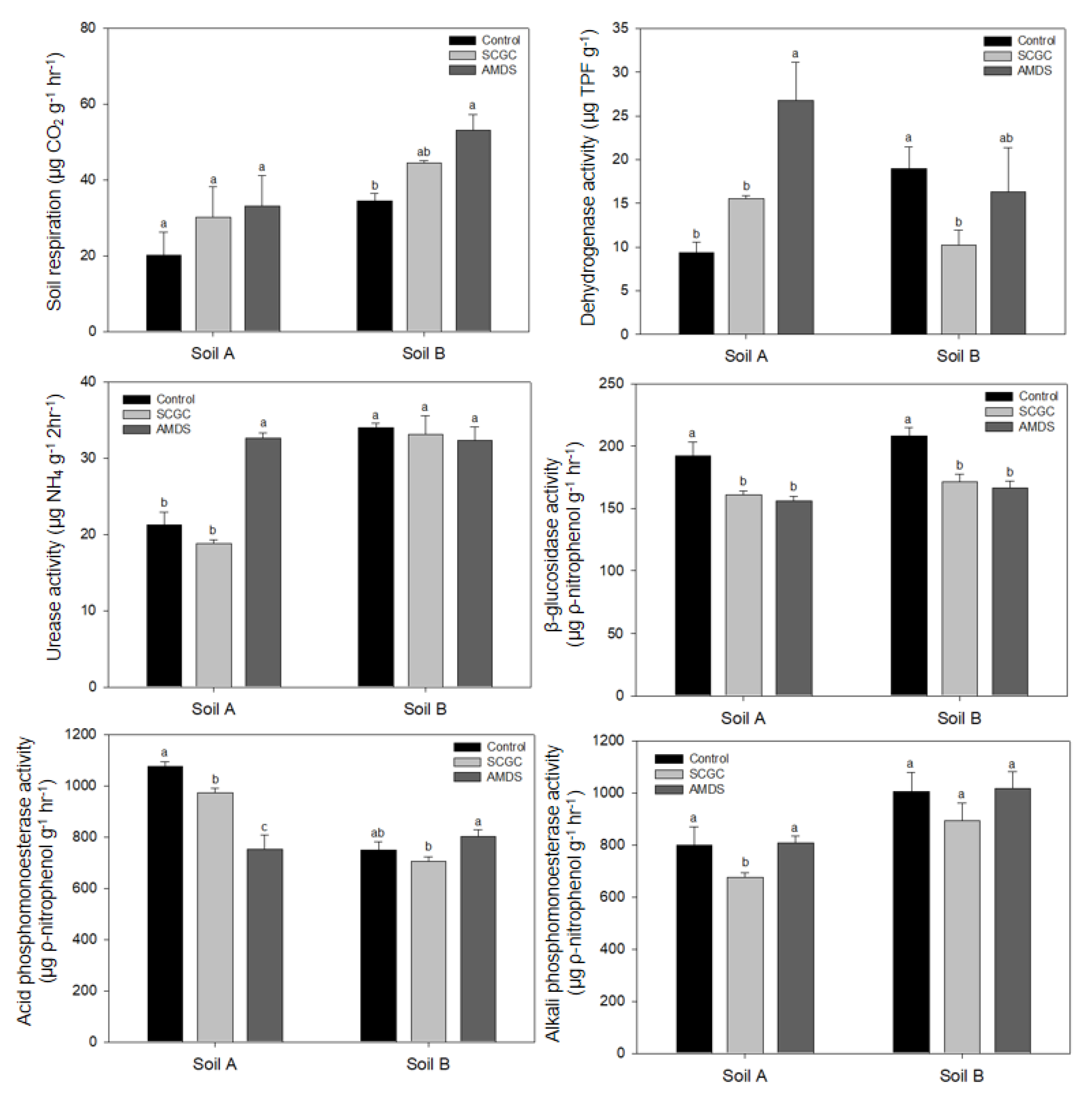


| Soil Property | Soil A | Soil B |
|---|---|---|
| pH | 5.8 ± 0.3 | 8.3 ± 0.3 |
| EC 1 (us cm−1) | 35 ± 2.6 | 75 ± 1.5 |
| LOI 2 (%) | 4.9 ± 0.3 | 4.5 ± 0.1 |
| Sand (%) | 80.3 ± 1.2 | 64.6 ± 1.1 |
| Silt (%) | 2.5 ± 0.1 | 11.2 ± 3.0 |
| Clay (%) | 17.2 ± 0.6 | 24.2 ± 2.2 |
| Soil texture 3 | sandy loam | sandy clay loam |
| Total As (mg kg−1) | 784 ± 12 | 2999 ± 124 |
| Wenzel Sequential extraction 4 | ||
| Fraction 1 (mg kg−1) | 2.4 ± 0.4 | 46.5 ± 1.3 |
| Fraction 2 (mg kg−1) | 107.2 ± 2.7 | 262.8 ± 11.0 |
| Fraction 3 (mg kg−1) | 395.8 ± 6.4 | 1931.4 ± 129.0 |
| Fraction 4 (mg kg−1) | 221.3 ± 10.7 | 523.8 ± 92.6 |
| Fraction 5 (mg kg−1) | 78.2 ± 3.2 | 234.0 ± 12.4 |
| Sum | 804.9 | 2998.4 |
| Recovery (%) | 102.6 | 99.9 |
| Treatment | pH | EC 1 | DOC 2 | Extractable As 3 | |
|---|---|---|---|---|---|
| Soil A | Control | 5.78 ± 0.03 c | 35 ± 2.6 b | 109.0 ± 5.3 c | 0.14 ± 0.01 a |
| SCGC 4 | 6.20 ± 0.05 b | 36 ± 2.1 b | 126.2 ± 0.1 b | 0.06 ± 0.00 b | |
| AMDS 5 | 8.06 ± 0.02 a | 111 ± 3.6 a | 186.5 ± 6.4 a | 0.08 ± 0.02 b | |
| Soil B | Control | 8.30 ± 0.03 b | 75 ± 1.5 b | 136.5 ± 3.5 b | 1.57 ± 0.06 b |
| SCGC | 8.36 ± 0.06 ab | 119 ± 4.2 a | 184.6 ± 7.2 a | 1.91 ± 0.01 a | |
| AMDS | 8.45 ± 0.03 a | 119 ± 6.0 a | 179.6 ± 9.9 a | 1.08 ± 0.04 c | |
| Treatment | OTU | Chao 1 | Shannon | Simpson | Good’s Coverage | |
|---|---|---|---|---|---|---|
| Soil A | Control | 2299 | 14,349.01 | 7.538 | 0.9692 | 0.7974 |
| SCGC 1 | 3138 | 9827.65 | 10.038 | 0.9968 | 0.7702 | |
| AMDS 2 | 3263 | 10,061.11 | 9.763 | 0.9914 | 0.7566 | |
| Soil B | Control | 4265 | 15,885.57 | 10.259 | 0.9951 | 0.6642 |
| SCGC | 5451 | 18,111.69 | 11.667 | 0.9992 | 0.5879 | |
| AMDS | 5366 | 16,197.01 | 11.705 | 0.9993 | 0.6059 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, M.-S.; Lee, S.-H.; Park, H.; Kim, J.-G. Evaluation of Two Amendments (Biochar and Acid Mine Drainage Sludge) on Arsenic Contaminated Soil Using Chemical, Biological, and Ecological Assessments. Materials 2021, 14, 4111. https://doi.org/10.3390/ma14154111
Kim M-S, Lee S-H, Park H, Kim J-G. Evaluation of Two Amendments (Biochar and Acid Mine Drainage Sludge) on Arsenic Contaminated Soil Using Chemical, Biological, and Ecological Assessments. Materials. 2021; 14(15):4111. https://doi.org/10.3390/ma14154111
Chicago/Turabian StyleKim, Min-Suk, Sang-Hwan Lee, Hyun Park, and Jeong-Gyu Kim. 2021. "Evaluation of Two Amendments (Biochar and Acid Mine Drainage Sludge) on Arsenic Contaminated Soil Using Chemical, Biological, and Ecological Assessments" Materials 14, no. 15: 4111. https://doi.org/10.3390/ma14154111






