Ciprofloxacin-Releasing ROS-Sensitive Nanoparticles Composed of Poly(Ethylene Glycol)/Poly(D,L-lactide-co-glycolide) for Antibacterial Treatment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
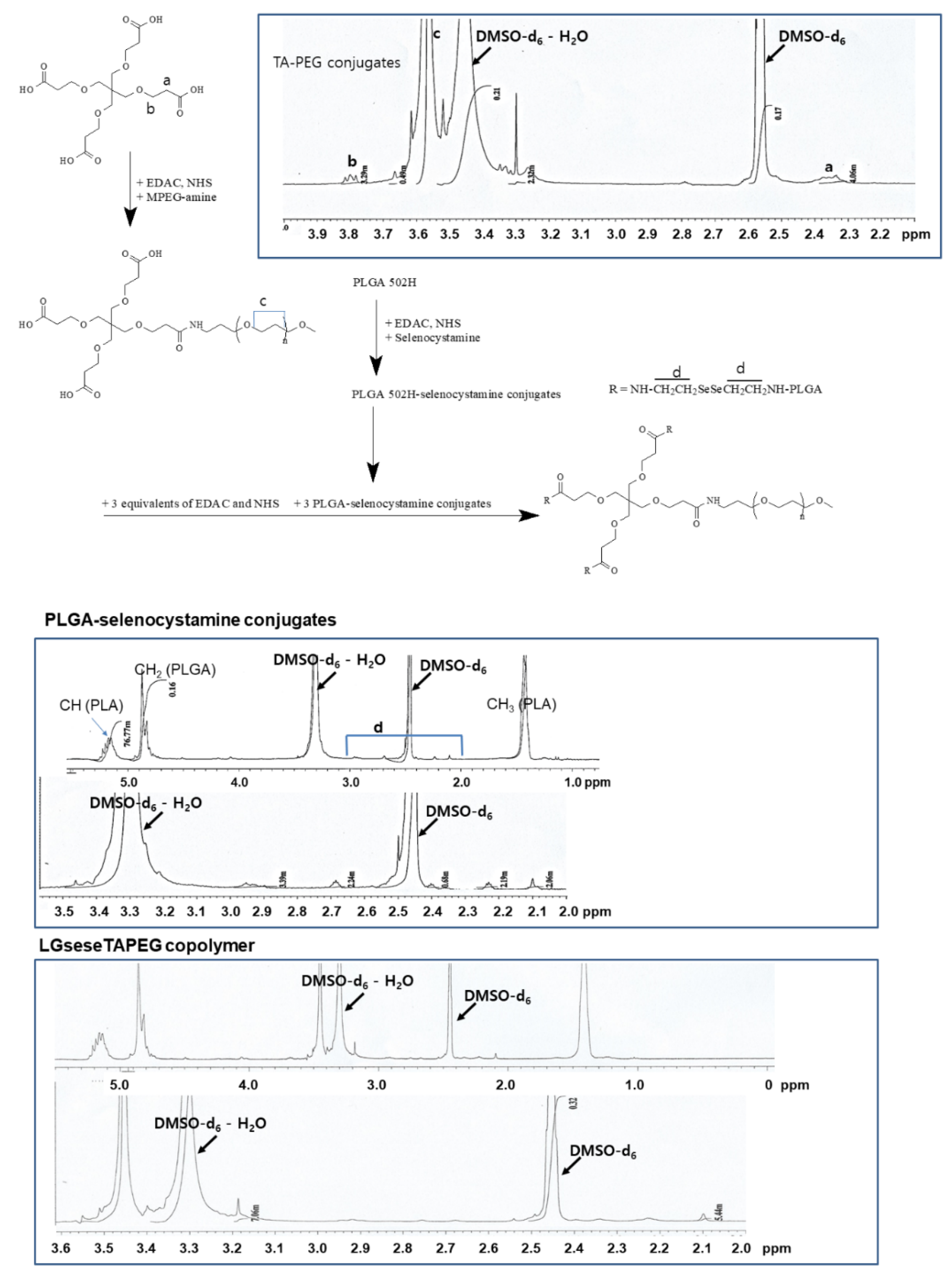
2.2. Synthesis of LGseseTAPEG Copolymer
2.2.1. TA-PEG Conjugates
2.2.2. PLGA-Selenocystamine Conjugates (LGsese)
2.2.3. LGseseTAPEG Copolymer
2.3. Analysis of LGseseTAPEG Copolymer
2.4. Preparation of CIP-Incorporated LGseseTAPEG Nanoparticles
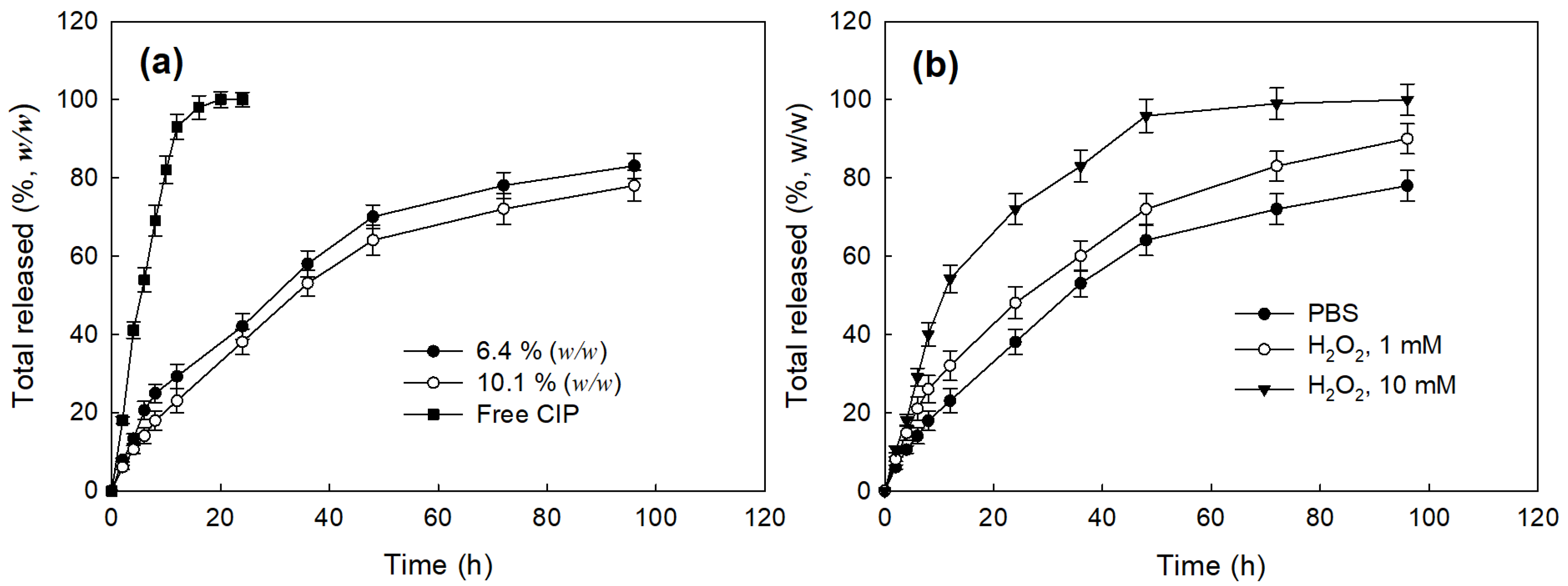
2.5. Drug Release from Nanoparticles
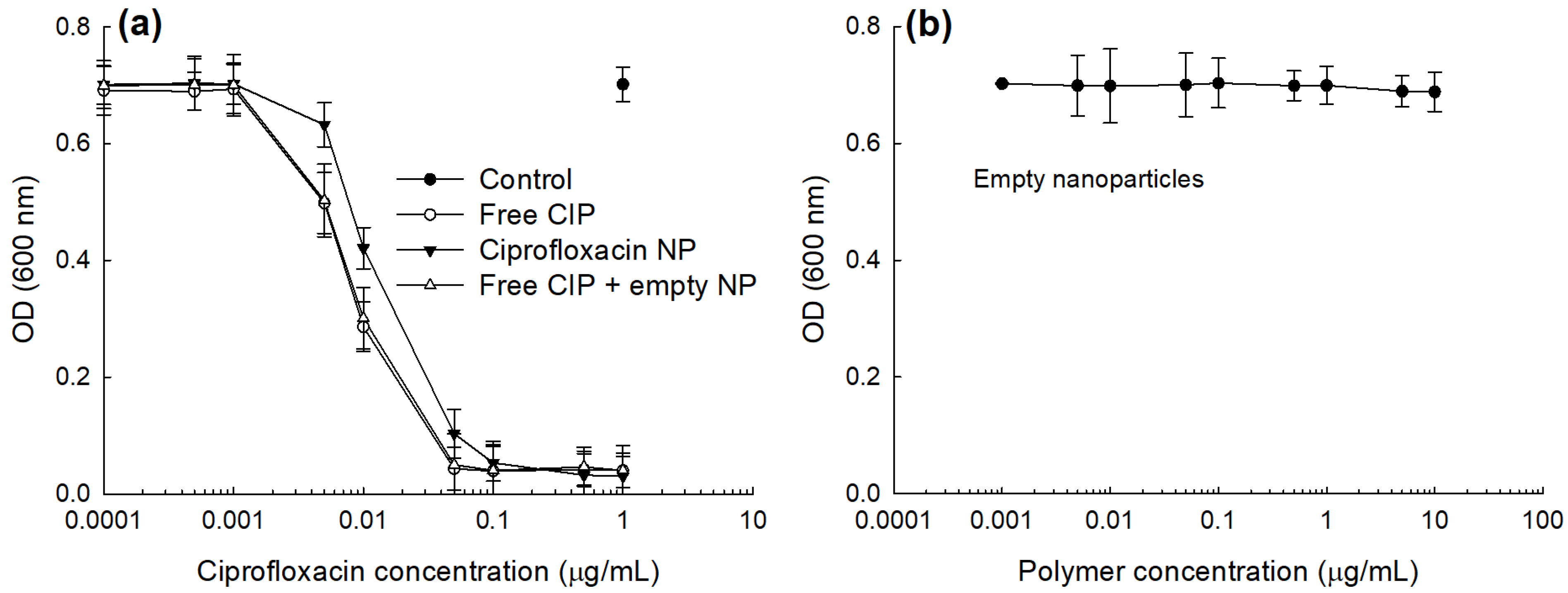
2.6. Antibacterial Activity of CIP-Incorporated Nanoparticles In Vitro
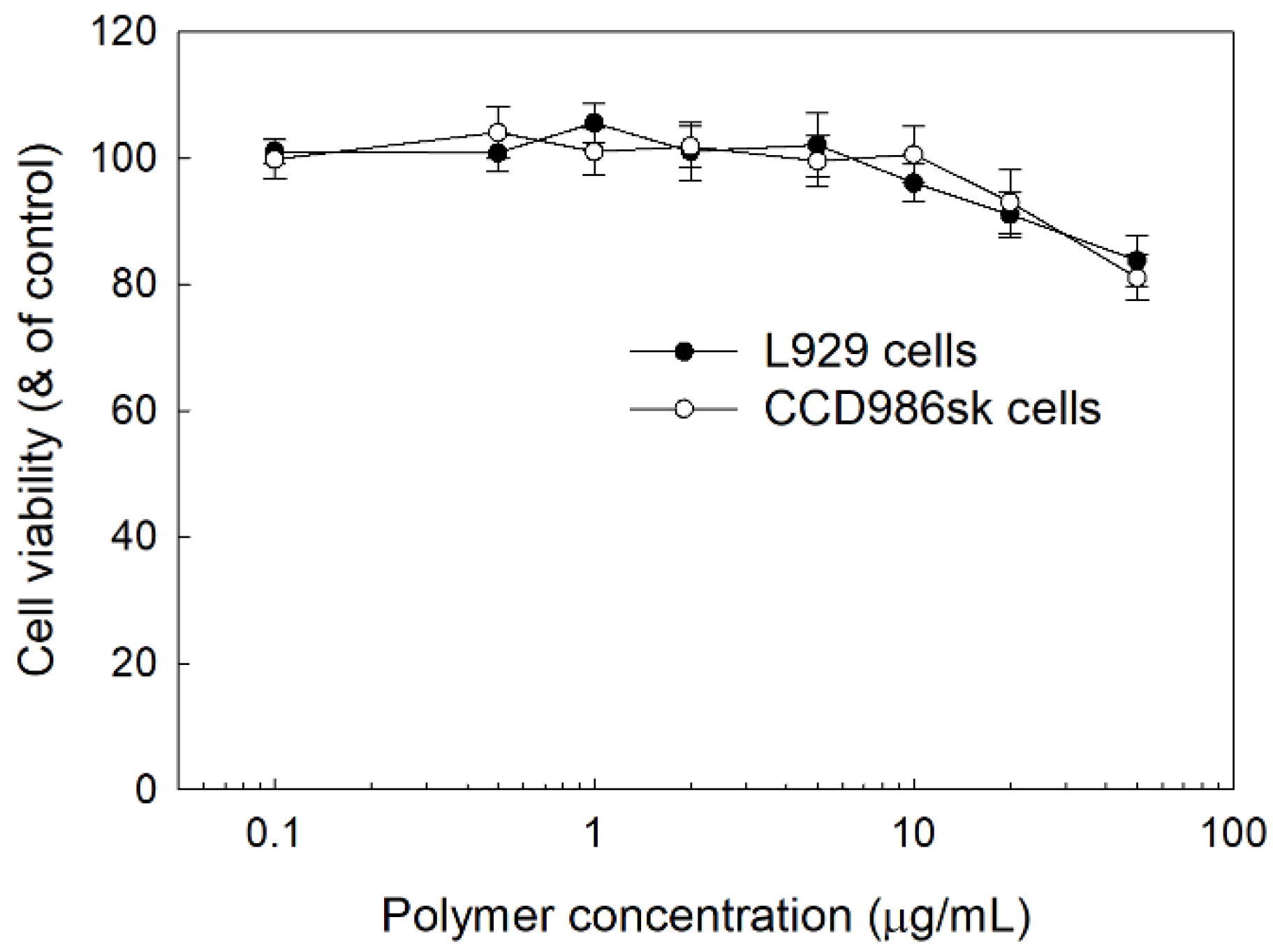
2.7. Cell Cytotoxcity of LGseseTAPEG Copolymer Nanoparticles In Vitro
2.8. Statistical Analysis
3. Results and Discussion
3.1. Synthesis of LGseseTAPEG Copolymer
3.2. Preparation and Characterization of CIP-Incorporated Nanoparticles
3.3. Antibacterial Activity of CIP-Incorporated Nanoparticles
3.4. Intrinsic Cytotoxicity of LGseseTAPEG Nanoparticles against L929 Mouse Fibroblast Cells and CCD986sk Human Skin Fibroblast Cells
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Flores-Mireles, A.L.; Walker, J.N.; Caparon, M.; Hultgren, S.J. Urinary tract infections: Epidemiology, mechanisms of infection and treatment options. Nat. Rev. Microbiol. 2015, 13, 269–284. [Google Scholar] [CrossRef] [PubMed]
- Millner, R.; Becknell, B. Urinary tract infections. Pediatr. Clin. North. Am. 2019, 66, 1–13. [Google Scholar] [CrossRef]
- Warren, J.W.; Platt, R.; Thomas, R.J.; Rosner, B.; Kass, E.H. Antibiotic irrigation and catheter-associated urinary-tract infections. N. Engl. J. Med. 1978, 299, 570–573. [Google Scholar] [CrossRef]
- Chenoweth, C.E.; Gould, C.V.; Saint, S. Diagnosis, management, and prevention of catheter-associated urinary tract infections. Infect. Dis. Clin. North. Am. 2014, 28, 105–119. [Google Scholar] [CrossRef] [PubMed]
- Nicolle, L.E. Catheter associated urinary tract infections. Antimicrob Resist. Infect Control 2014, 3, 23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kurutas, E.B.; Ciragil, P.; Gul, M.; Kilinc, M. The effects of oxidative stress in urinary tract infection. Mediat. Inflamm 2005, 2005, 242–244. [Google Scholar] [CrossRef]
- Miyata, Y.; Matsuo, T.; Mitsunari, K.; Asai, A.; Ohba, K.; Sakai, H. A Review of oxidative stress and urinary dysfunction caused by bladder outlet obstruction and treatments using antioxidants. Antioxidants 2019, 8, 132. [Google Scholar] [CrossRef] [Green Version]
- Andersson, K.E. Oxidative stress and its possible relation to lower urinary tract functional pathology. BJU Int. 2018, 121, 527–533. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wise, R.; Hart, T.; Cars, O.; Streulens, M.; Helmuth, R.; Huovinen, P.; Sprenger, M. Antimicrobial resistance. Is a major threat to public health. BMJ 1998, 317, 609–610. [Google Scholar] [CrossRef] [Green Version]
- Bader, M.S.; Loeb, M.; Leto, D.; Brooks, A.A. Treatment of urinary tract infections in the era of antimicrobial resistance and new antimicrobial agents. Postgrad. Med. 2020, 132, 234–250. [Google Scholar] [CrossRef]
- Zhang, G.F.; Liu, X.; Zhang, S.; Pan, B.; Liu, M.L. Ciprofloxacin derivatives and their antibacterial activities. Eur. J. Med. Chem. 2018, 146, 599–612. [Google Scholar] [CrossRef]
- Gutiérrez-Castrellón, P.; Díaz-García, L.; de Colsa-Ranero, A.; Cuevas-Alpuche, J.; Jiménez-Escobar, I. Efficacy and safety of ciprofloxacin treatment in urinary tract infections (UTIs) in adults: A systematic review with meta-analysis. Gac. Med. Mex. 2015, 151, 225–244. [Google Scholar]
- Henry, D.C., Jr.; Bettis, R.B.; Riffer, E.; Haverstock, D.C.; Kowalsky, S.F.; Manning, K.; Hamed, K.A.; Church, D.A. Comparison of once-daily extended-release ciprofloxacin and conventional twice-daily ciprofloxacin for the treatment of uncomplicated urinary tract infection in women. Clin. Ther. 2002, 24, 2088–2104. [Google Scholar] [CrossRef]
- Edlund, C.; Nord, C.E. Effect on the human normal microflora of oral antibiotics for treatment of urinary tract infections. J. Antimicrob. Chemotherapy 2000, 46 (Suppl. A), 41–48. [Google Scholar] [CrossRef] [Green Version]
- Jeong, Y.I.; Kim, Y.W.; Jung, S.; Pei, J.; Wen, M.; Li, S.Y.; Ryu, H.H.; Lim, J.C.; Jang, W.Y.; Kim, I.Y.; et al. Delivery of transferrin-conjugated polysaccharide nanoparticles in 9L gliosacoma cells. J. Nanosci. Nanotechnol. 2015, 15, 125–129. [Google Scholar] [CrossRef]
- Kwak, T.W.; Kim, D.H.; Jeong, Y.I.; Kang, D.H. Antitumor activity of vorinostat-incorporated nanoparticles against human cholangiocarcinoma cells. J. Nanobiotechnology 2015, 13, 60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sánchez, S.V.; Navarro, N.; Catalán-Figueroa, J.; Morales, J.O. Nanoparticles as potential novel therapies for urinary tract infections. Front. Cell Infect. Microbiol. 2021, 11, 656496. [Google Scholar] [CrossRef]
- Choi, G.E.; Kang, M.S.; Kim, Y.J.; Yoon, J.J.; Jeong, Y.I. Magnetically responsive drug delivery using doxorubicin and iron oxide Nanoparticle-Incorporated Lipocomplexes. J. Nanosci. Nanotechnol. 2019, 19, 675–679. [Google Scholar] [CrossRef]
- Lee, S.J.; Jeong, Y.I. Hybrid nanoparticles based on chlorin e6-conjugated hyaluronic acid/poly(l-histidine) copolymer for theranostic application to tumors. J. Mater. Chem. B 2018, 6, 2851–2859. [Google Scholar] [CrossRef]
- Dou, Y.; Li, C.; Li, L.; Guo, J.; Zhang, J. Bioresponsive drug delivery systems for the treatment of inflammatory diseases. J. Control. Release 2020, 327, 641–666. [Google Scholar] [CrossRef] [PubMed]
- Cho, B.; Lee, S.H.; Song, J.; Bhattacharjee, S.; Feng, J.; Hong, S.; Song, M.; Kim, W.; Lee, J.; Bang, D.; et al. Nanophotonic cell lysis and polymerase chain reaction with gravity-driven cell enrichment for rapid detection of pathogens. ACS Nano 2019, 13, 13866–13874. [Google Scholar] [CrossRef]
- Zhang, C.Y.; Gao, J.; Wang, Z. Bioresponsive nanoparticles targeted to infectious microenvironments for sepsis management. Adv. Mater. 2018, 30, e1803618. [Google Scholar] [CrossRef]
- Qindeel, M.; Barani, M.; Rahdar, A.; Arshad, R.; Cucchiarini, M. Nanomaterials for the diagnosis and treatment of urinary tract infections. Nanomaterials 2021, 11, 546. [Google Scholar] [CrossRef] [PubMed]
- Alomary, M.N.; Ansari, M.A. Proanthocyanin-capped biogenic TiO2 nanoparticles with enhanced penetration, antibacterial and ROS mediated inhibition of bacteria proliferation and biofilm formation: A comparative approach. Chemistry 2021, 27, 5817–5829. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Gu, W.; Gao, Y.; Ma, N.; Fan, C.; Ci, X. Daphnetin ameliorated GM-induced renal injury through the suppression of oxidative stress and apoptosis in mice. Int. Immunopharmacol. 2021, 96, 107601. [Google Scholar] [CrossRef] [PubMed]
- Gul, M.; Kurutas, E.; Ciragil, P.; Cetinkaya, A.; Kilinc, M.; Aral, M.; Buyukbese, M.A. Urinary tract infection aggravates oxidative stress in diabetic patients. Tohoku J. Exp. Med. 2005, 206, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.M.; Shim, Y.H.; Kwon, H.; Kim, J.P.; Park, J.I.; Kim, D.H.; Kim, D.H.; Kim, J.H.; Jeong, Y.I. CD44 Receptor-specific and redox-sensitive nanophotosensitizers of hyaluronic acid-chlorin e6 tetramer having diselenide linkages for photodynamic treatment of cancer cells. J. Pharm. Sci. 2019, 108, 3713–3722. [Google Scholar] [CrossRef]
- Chowdhury, F.A.; Mahboob, S.; Saha, A.; Jahan, A.; Islam, M.N. Effect of oxidative stress on glutathione reductase activity of Escherichia coli clinical isolates from patients with urinary tract infection. J. Immunol. Clin. Microbiol. 2017, 2, 68–75. [Google Scholar] [CrossRef] [Green Version]
- Hincal, F.; Gürbay, A.; Favier, A. Biphasic response of ciprofloxacin in human fibroblast cell cultures. Nonlinearity Biol. Toxicol. Med. 2003, 1, 481–492. [Google Scholar] [CrossRef] [Green Version]
- Ma, N.; Xu, H.; An, L.; Li, J.; Sun, Z.; Zhang, X. Radiation-sensitive diselenide block co-polymer micellar aggregates: Toward the combination of radiotherapy and chemotherapy. Langmuir 2011, 27, 5874–5878. [Google Scholar] [CrossRef]
- Jang, H.H.; Park, S.B.; Hong, J.S.; Lee, H.L.; Song, Y.H.; Kim, J.; Jung, Y.H.; Kim, C.; Kim, D.M.; Lee, S.E.; et al. Piperlongumine-eluting gastrointestinal stent using reactive oxygen species-sensitive nanofiber mats for inhibition of cholangiocarcinoma cells. Nanoscale Res. Lett. 2019, 14, 58. [Google Scholar] [CrossRef] [Green Version]
- Fan, X.X.; Xu, M.Z.; Leung, E.L.; Jun, C.; Yuan, Z.; Liu, L. ROS-responsive berberine polymeric micelles effectively suppressed the inflammation of rheumatoid arthritis by targeting mitochondria. Nanomicro. Lett. 2020, 12, 76. [Google Scholar] [CrossRef] [Green Version]
- Wu, X.; Li, P.; Cheng, J.; Xu, Q.; Lu, B.; Han, C.; Huo, W. ROS-sensitive nanoparticles co-delivering dexamethasone and CDMP-1 for the treatment of osteoarthritis through chondrogenic differentiation induction and inflammation inhibition. Front. Bioeng. Biotechnol. 2021, 9, 608150. [Google Scholar] [CrossRef]
- Jeong, Y.I.; Na, H.S.; Seo, D.H.; Kim, D.G.; Lee, H.C.; Jang, M.K.; Na, S.K.; Roh, S.H.; Kim, S.I.; Nah, J.W. Ciprofloxacin-encapsulated poly(DL-lactide-co-glycolide) nanoparticles and its antibacterial activity. Int. J. Pharm. 2008, 352, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Mitragotri, S.; Stayton, P. Organic nanoparticles for drug delivery and imaging. MRS Bull. 2014, 39, 219–223. [Google Scholar] [CrossRef] [Green Version]
- Mittal, R.; Pan, D.R.; Parrish, J.M.; Huang, E.H.; Yang, Y.; Patel, A.P.; Malhotra, A.K.; Mittal, J.; Chhibber, S.; Harjai, K. Local drug delivery in the urinary tract: Current challenges and opportunities. J. Drug Target. 2018, 26, 658–669. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, M.M.; Ivanova, K.; Francesko, A.; Mendoza, E.; Tzanov, T. Immobilization of antimicrobial core-shell nanospheres onto silicone for prevention of Escherichia coli biofilm formation. Process. Biochem. 2017, 59, 116–122. [Google Scholar] [CrossRef] [Green Version]
- Francesko, A.; Fernandes, M.M.; Ivanova, K.; Amorim, S.; Reis, R.L.; Pashkuleva, I.; Mendoza, E.; Pfeifer, A.; Heinze, T.; Tzanov, T. Bacteria-responsive multilayer coatings comprising polycationic nanospheres for bacteria biofilm prevention on urinary catheters. Acta Biomater. 2016, 33, 203–212. [Google Scholar] [CrossRef]



| Polymer/Ciprofloxacin (mg/mg) | Drug Contents (%, w/w) | Loading Efficiency (%, w/w) a | Particle Size (nm) b | ||
|---|---|---|---|---|---|
| Theoretical a | Experimental a | Average Diameter ± S.D. | PDI c | ||
| 100/0 | - | - | - | 153 ± 7.94 | 0.065 |
| 100/10 | 9.1 | 6.4 | 68.4 | 238 ± 15.6 | 0.125 |
| 100/20 | 16.7 | 10.1 | 56.1 | 318.4 ± 22.5 | 0.158 |
| Drug or NP Treatment a | IC50 (μg CIP/mL) |
|---|---|
| Free CIP | 0.008 |
| CIP-incorporated NP | 0.019 |
| Free CIP + empty NP | 0.009 |
| Empty NP b | >10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, J.; Kook, M.-S.; Kim, B.-H.; Jeong, Y.-I.; Oh, K.-J. Ciprofloxacin-Releasing ROS-Sensitive Nanoparticles Composed of Poly(Ethylene Glycol)/Poly(D,L-lactide-co-glycolide) for Antibacterial Treatment. Materials 2021, 14, 4125. https://doi.org/10.3390/ma14154125
Song J, Kook M-S, Kim B-H, Jeong Y-I, Oh K-J. Ciprofloxacin-Releasing ROS-Sensitive Nanoparticles Composed of Poly(Ethylene Glycol)/Poly(D,L-lactide-co-glycolide) for Antibacterial Treatment. Materials. 2021; 14(15):4125. https://doi.org/10.3390/ma14154125
Chicago/Turabian StyleSong, Jaeik, Min-Suk Kook, Byung-Hoon Kim, Young-IL Jeong, and Kyung-Jin Oh. 2021. "Ciprofloxacin-Releasing ROS-Sensitive Nanoparticles Composed of Poly(Ethylene Glycol)/Poly(D,L-lactide-co-glycolide) for Antibacterial Treatment" Materials 14, no. 15: 4125. https://doi.org/10.3390/ma14154125





